Abstract
Objectives
Emergency department (ED) management of mild traumatic brain injury (TBI) patients with any form of traumatic intracranial hemorrhage (ICH) is variable. Since 2000, our center’s standard practice has been to obtain a repeat head computed tomography (CT) at least 6 hours after initial imaging. Patients are eligible for discharge if clinical and CT findings are stable. Whether this practice is safe is unknown. This study characterized clinical outcomes in mild TBI patients with acute traumatic ICH seen on initial ED neuroimaging.
Methods
This retrospective cohort study included patients presenting to the ED with blunt mild TBI with Glasgow Coma Scale (GCS) scores of 14 or 15 and stable vital signs, during the period from January 2001 to January 2010. Patients with any ICH on initial head CT and repeat head CT within 24 hours were eligible. Cases were excluded for initial GCS < 14, injury > 24 hours old, pregnancy, concomitant nonminor injuries, and coagulopathy. A single investigator abstracted data from records using a standardized case report form and data dictionary. Primary endpoints included death, neurosurgical procedures, and for discharged patients, return to the ED within 7 days. Differences in proportions were computed with 95% confidence intervals (CIs).
Results
Of 1,011 patients who presented to the ED and had two head CTs within 24 hours, 323 (32%) met inclusion criteria. The median time between CT scans was 6 hours (interquartile range = 5 to 7 hours). A total of 153 (47%) patients had subarachnoid hemorrhage, 132 (41%) patients had subdural hemorrhage, 11 (3%) patients had epidural hemorrhage, 78 (24%) patients had cerebral contusions, and 59 (18%) patients had intraparenchymal hemorrhage. Four of 323 (1.2%, 95% CI = 0.3% to 3.2%) patients died within 2 weeks of injury. Three of the patients who died had been admitted from the ED on their initial visits, and one had been discharged home. There were 206 patients (64%) discharged from the ED, 28 (13.6%) of whom returned to the ED within 1 week. Of the 92 who were hospitalized, three (0.9%, 95% CI = 0.2% to 2.7%) required neurosurgical intervention.
Conclusion
Discharge after a repeat head CT and brief period of observation in the ED allowed early discharge of a cohort of mild TBI patients with traumatic ICH without delayed adverse outcomes. Whether this justifies the cost and radiation exposure involved with this pattern of practice requires further study.
Every year, 1.5 million people suffer traumatic brain injury (TBI) in the United States,1 with approximately 10% of these demonstrating positive findings (intracranial hemorrhage [ICH]) on computed tomography (CT).2 The emergency department (ED) provides evaluation and treatment in almost 80% of these cases.3 Most TBI is in the mild category, defined as a Glasgow Coma Scale (GCS) score of 13 or greater.4 Mortality and neurosurgical intervention are infrequent in mild TBI, regardless of cranial CT findings (0.1 and 0.9%, respectively).5 Current disposition decisions for patients with mild TBI are broadly guided by neurologic examination and, increasingly, head CT results. ED discharge of a mild TBI patient with a GCS score of 14 or 15 and a normal head CT, particularly when there is no predisposition to bleeding, is a generally accepted practice.3,6,7 While mortality and neurosurgical intervention are infrequent in mild TBI without ICH on CT, the risk presumably increases if the initial CT scan reveals ICH of any type. To our knowledge, there are no national guidelines regarding management of patients with GCS scores of 14 or 15 with positive head CT findings. Current American College of Emergency Physicians clinical policy does not address patients with mild TBI and ICH on CT.8
The potential for poor or unexpected outcomes leads most institutions to admit any patient with traumatic ICH to the hospital for close monitoring,9 even if they otherwise fall within the mild TBI severity category. Beyond this, prognostic uncertainty leads to wide variation in management with respect to periods of observation, disposition, and intensity of repeat assessments, including need for repeat head CT scanning. For example, the Scandinavian Neurosurgical Society recommends that mild TBI patients with traumatic subarachnoid hemorrhage be observed for at least 12 hours.3 Some institutions observe patients for 24 hours after initial injury, while others have suggested that this might not be necessary.3 Being able to determine which mild TBI patients with traumatic ICH will not require intervention could help identify patients who may be safely discharged home directly from the ED and potentially guide the intensity of observation and repeat imaging.
Sifri et al.10 showed that nearly one in 10 patients thought to have minor head injuries may have positive findings on head CT. Less than 1% of these require neurosurgical intervention,11 and the outcomes after disposition for patients with mild TBI and traumatic ICH are largely unknown.12 A meta-analysis by Wang et al.13 suggested a wide range of patients, between 8 and 67%, with mild TBI and traumatic ICH demonstrate radiologic progression on repeat head CT.
In 2000, our Level I trauma center adopted the practice of reevaluating and reimaging patients with mild TBI and traumatic ICH on initial head CT at least 6 hours after initial imaging, with ED discharge if both clinical and CT findings were stable. In actual practice, repeat scans ranged from 1 hour (required due to motion artifact) to 23 hours apart, with a median interval time between imaging of 6 hours. The safety of this approach in the management of ED mild TBI patients with traumatic ICH has not been rigorously studied. The purpose of this study was to characterize outcomes in mild TBI patients with acute traumatic ICH seen on initial neuroimaging.
METHODS
Study Design
This was a retrospective cohort study and was approved by the institutional review board.
Study Setting and Population
The University of Cincinnati Medical Center is an urban, academic medical center that serves as the region’s only Level I trauma center and is a quaternary care referral center offering trauma and neurosurgery services with separate dedicated surgical and neurosurgical intensive care units. The ED provides patient care for approximately 90,000 encounters annually and had the capability to place patients in ED observation throughout the study period. Patients presenting to the ED between January 2001 and December 2010 were identified for inclusion. A neurosurgery consultation was generated for all patients who were found to have ICH of any type.
Study Protocol
During the study period, our institution had neither standard discharge instructions for patients nor established patterns of follow-up for post–mild TBI symptoms, and discharge instructions were communicated according to each practitioner’s practice pattern. Patients were seen in follow-up at the discretion of the attending neurosurgeon evaluating the patient. While the Centers for Disease Control and Prevention has national guidelines recommending that a patient who sustains a head injury should not return to sports until seen by a primary care physician,14 there are no formal national guidelines dictating outpatient management postinjury, such as optimal blood pressure control in hypertensive patients, time frame for avoidance of anti-coagulation, or the timing of prophylactic antiepileptic use in patients with mild TBI who are found to have traumatic ICH on head CT.
Potential study subjects were identified from the cohort of all patients who underwent two head CTs in the ED within a 24-hour period between 2001 and 2010. A single data abstractor (NK) reviewed each identified case using explicitly defined inclusion and exclusion criteria. The CT scanner used during the study period, a third-generation eight-slice GE CT scanner, did not change.
Patients were included if they suffered blunt head injury, presented within 24 hours of injury with a GCS of 14 or 15, had evidence of traumatic ICH on initial head CT, and had a second head CT performed within 24 hours in the ED. Traumatic ICH was defined as epidural hemorrhage, subdural hemorrhage, traumatic subarachnoid hemorrhage, cerebral contusion, cerebral hematoma, or any combination thereof. The inclusion GCS score was of that documented by the clinical team. Retrospective calculation of GCS from chart data was not done.
Despite the fact that mild TBI is traditionally defined as GCS 13 to 15 following a traumatic disruption of brain function,15 we chose to limit our included patients to those with GCS scores of 14 or 15. This is because patients with GCS scores of 13 or lower have a greater chance of being admitted, compared to those patients with GCS scores of 14 or greater.16 Furthermore, a growing body of literature supports transitioning patients with GCS scores of 13 into the “moderate” category of TBI and that this should not be considered mild TBI.5
Patients were excluded if they had no documented GCS scores, unknown time of injury, or head CT scans performed or interpreted at outside hospitals; were pregnant; had penetrating head injury; were intubated prior to ED evaluation; had abnormal ED vital signs (systolic blood pressure [sBP] < 89 mm Hg, respiratory rate > 29 breaths/min, SO2 < 92% on room air) at any point during their ED visits; or had known coagulopathy, inherited or acquired. Inherited coagulopathies were defined as hemophilia A or B, von Willebrand disease, Bernard-Soulier syndrome, Wiskott-Aldrich syndrome, or Glanzmann’s thrombasthenia. Acquired coagulopathies were defined as liver failure, warfarin use, heparin product use, and disseminated intravascular coagulation as defined by laboratory measures of international normalized ratio (INR) ≥ 1.4, activated partial thromboplastin time > 39 seconds, or platelet count < 50 × 109/L. Patients with reported use of anticoagulant medications such as warfarin with INR < 1.4 or antiplatelet medications such as aspirin, clopidogrel (Plavix), prasugrel (Effient), ticlopidine (Ticlid), cilostazol (Pletal), or dipyridamole (Aggrenox) were not excluded. Prisoners were not excluded. Patients were excluded if they had other nonminor injuries, defined as injuries for which a patient would otherwise not be discharged home. Patients under the age of 18 years were not included, because there is a freestanding children’s hospital located less than 1 mile from our institution, so very few children present to our ED.
Study Protocol
Patients meeting study criteria underwent a detailed chart review, including review of ED visit dictations, ED nursing notes, laboratory results, and radiology reports. All emergency physician, consultant, and radiology dictations were contained in the electronic medical record. Nursing notes that were originally handwritten and then scanned into the electronic medical record were also reviewed.
A standardized case report form with explicit data dictionary was used for abstraction by a single investigator (NK). The primary abstractor was an emergency medicine resident at the time of the study. The first 30 charts were reviewed in duplicate by two separate reviewers (NK and a research assistant) to ensure accuracy. An additional 50 charts were selected for duplicate abstraction sporadically throughout the study period. Discrepancies were resolved by investigator adjudication but did not lead to changes in definitions or procedures during the study. These discrepancies were largely related to information that was not found in the charts by one investigator, but was found by the primary investigator (NK). The adjudication process entailed the primary investigator reviewing the abstracted charts thoroughly and correcting errors or information that had not been found initially. Data were entered into the database by a single abstractor; therefore, a measure of agreement could not be obtained.
Information regarding head CT findings was obtained from attending radiology reports. Our institution has ACGME-accredited radiology residency and fellowship programs. The initial head CT was generally interpreted by radiology residents, supervised by attending radiology faculty. Overnight radiology resident interpretations were overread by attendings in the morning. There were instances in which the preliminary CT interpretation was reported as normal and the patient was discharged, but subsequent overreads by the attending radiologist identified an abnormality. By standing ED policy, in these cases, patients were instructed to return to the ED. These events were rare, although we do not know precisely how frequent, as our review methods did not capture this detail. Identified patients from this category who returned to the ED and were discharged following second head CT scans are considered for analysis to be in the “discharged” category.
Primary outcomes included death within 30 days, neurosurgical intervention within 2 weeks, and for discharged patients, return to the ED within 7 days. We chose 7 days for return ED visit to increase the likelihood that these return visits would be related to the initial injuries. The follow-up period for mortality and neurosurgical procedure was 1 year after presentation to the ED. We did not calculate the median period of follow-up time if patients did not return within 1 week to the ED. When death occurred, a more extensive chart review was performed to describe the events surrounding this outcome. The Social Security Death Index Master File was searched for patients who did not return to the ED following their injuries to ensure that no deaths were undetected by chart review.
Data Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Cincinnati. REDCap (Research Electronic Data Capture) is a secure, Web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry, 2) audit trails for tracking data manipulation and export procedures, 3) automated export procedures for seamless data downloads to common statistical packages, and 4) procedures for importing data from external sources.17 The primary analysis estimated the proportion of patients with adverse outcomes with 95% confidence intervals (CIs). We also aimed to explore factors potentially associated with adverse outcomes in mild TBI patients (GCS 14 to 15) with traumatic ICH. Differences in proportions were computed with 95% CIs. All statistical analyses were conducted using SPSS 21.0. Missing data were minimal and were left missing. Missing data are noted where applicable.
RESULTS
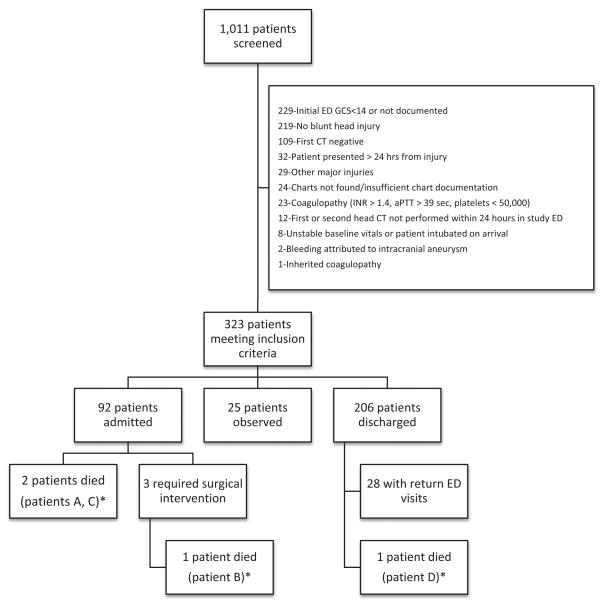
There were 1,011 patients identified for possible inclusion. Of these, 323 (32%) met all study criteria (Figure 1). Of those subjects excluded, the most commonly met exclusion criteria were initial GCS scores less than 14 (34%) and a reason other than trauma for repeat head CT within 24 hours, such as spontaneous ICH, spontaneous subarachnoid hemorrhage or stroke (33%) (Figure 1).
Figure 1.
Patient outcomes. *See Table 3 for patient cases. aPTT = activated partial thromboplastin time; GCS = Glasgow Coma Scale; INR = international normalized ratio.
The mean (±SD) age of the 323 included patients was 42 (±19) years. A total of 195 (60%) were white and 236 (73%) were male. The cohort, grouped by disposition, is described in Table 1. Symptoms and CT findings are detailed in Table 2. Study patients presented with a wide variety of symptoms, with the most frequent being headache (67%) and memory alteration (70%). A second head CT was normal in 32% of patients despite the presence of findings on initial head CT. Initial head CT findings included 153 (47%) patients with subarachnoid hemorrhage, 132 (41%) with subdural hemorrhage, 11 (3%) with epidural hemorrhage, 78 (24%) with cerebral contusions, and 59 (18%) with intraparenchymal hemorrhage. The most frequent findings on the second CT scans were subarachnoid hemorrhage (35%) and subdural hematomas (33%).
Table 1.
Demographics and Patient Characteristics by ED Discharge Status
| Characteristic | Discharged (n = 206) | Observation (n = 25) | Admitted (n = 92) | |||
|---|---|---|---|---|---|---|
| Age (yr), mean (±SD) | 39 | (±17) | 40 | (±17) | 48 | (±21) |
| Race: white | 129 | (62.6) | 14 | (56.0) | 52 | (56.5) |
| Sex: male | 148 | (71.8) | 18 | (72.0) | 70 | (76.1) |
| Medical history | ||||||
| Other traumatic injuries | 55 | (26.7) | 4 | (16.0) | 31 | (33.7) |
| Alcohol abuse | 55 | (26.7) | 6 | (24.0) | 25 | (27.2) |
| Medications that affect blood clotting | 11 | (5.3) | 1 | (4.0) | 17 | (18.5) |
| Neurosurgery or central nervous system | 11 | (5.3) | 4 | (16.0) | 8 | (8.7) |
| Liver disease | 6 | (2.9) | 0 | (0.0) | 2 | (2.2) |
| Renal dysfunction | 2 | (1.0) | 1 | (4.0) | 4 | (4.3) |
| Thrombotic disorder | 3 | (1.5) | 0 | (0.0) | 0 | (0.0) |
| Hematologic malignancy | 0 | (0.0) | 0 | (0.0) | 1 | (1.1) |
Data are reported as n (%) except where otherwise noted.
Table 2.
Presenting Symptoms, Specialty Consultations, and CT Findings by ED Discharge Status
| Characteristic | Discharged (n = 206) | Observation (n = 25) | Admitted (n = 92) | |||
|---|---|---|---|---|---|---|
| Symptoms | ||||||
| Experiencing any symptoms | 176 | (85.4) | 24 | (96.0) | 80 | (87.0) |
| Headache | 123 | (69.9) | 18 | (75.0) | 47 | (58.8) |
| Memory alteration | 123 | (69.9) | 15 | (62.5) | 57 | (71.3) |
| Nausea | 23 | (13.1) | 4 | (16.7) | 11 | (13.8) |
| Confusion | 18 | (10.2) | 3 | (12.5) | 17 | (21.3) |
| Vomiting | 11 | (6.3) | 3 | (12.5) | 4 | (5.0) |
| Seizure | 11 | (6.3) | 4 | (16.7) | 4 | (5.0) |
| Focal neurologic deficit | 1 | (0.6) | 0 | (0.0) | 1 | (1.3) |
| Consultations | ||||||
| Specialty service consulted | 178 | (86.4) | 25 | (100.0) | 91 | (98.9) |
| Neurosurgical consult | 163 | (91.6) | 24 | (96.0) | 90 | (98.9) |
| Trauma consult | 19 | (10.8) | 7 | (28.0) | 43 | (47.3) |
| Time to repeat CT (hours), median (interquartile range) | 6 | (5–7) | 6 | (6–10) | 6 | (4–8) |
| CT one | ||||||
| Any finding* | 206 | (100.0) | 25 | (100.0) | 92 | (100.0) |
| Subarachnoid hemorrhage | 87 | (42.2) | 13 | (52.0) | 53 | (57.6) |
| Subdural hematoma | 70 | (34.0) | 13 | (52.0) | 49 | (53.3) |
| Epidural hematoma | 4 | (1.9) | 2 | (8.0) | 5 | (5.4) |
| Cerebral contusion | 42 | (20.4) | 8 | (32.0) | 28 | (30.4) |
| Intraparenchymal hematoma | 39 | (18.9) | 3 | (12.0) | 17 | (18.5) |
| CT two | ||||||
| Any finding* | 118 | (57.3) | 22 | (88.0) | 80 | (87.0) |
| Subarachnoid hemorrhage | 50 | (24.3) | 14 | (56.0) | 50 | (54.3) |
| Subdural hematoma | 49 | (23.8) | 12 | (48.0) | 47 | (51.1) |
| Epidural hematoma | 2 | (1.0) | 1 | (4.0) | 5 | (5.4) |
| Cerebral contusion | 23 | (11.2) | 8 | (32.0) | 32 | (34.8) |
| Intraparenchymal hematoma | 19 | (9.2) | 4 | (16.0) | 18 | (19.6) |
Data are reported as n (%) except where otherwise noted
Multiple findings are possible.
Patients were reimaged between 1 and 24 hours after their initial head CT scans. The median time to reimaging was 6 hours (interquartile range = 5 to 7 hours), and 80% of patients were reimaged within 9 hours of their initial head CT scans. These times did not vary between the admitted, observed, or discharged groups. For patients discharged from the ED, the mean (±SD) length of stay was 23 (±10) hours.
A total of 25 of 323 (8%) patients were placed into observation status in the ED. These 25 patients were all discharged home from the ED.
A total of four of 323 patients (1.2%, 95% CI = 0.3% to 3.2%) died following injury (at 3, 5, 11, and 12 days, respectively) as described in Table 3. Three of the 323 patients (0.9%, 95% CI = 0.2% to 2.7%) required neurosurgical intervention (Figure 1). One patient requiring neurosurgical intervention ultimately died. A total of 28 (8.7%, 95% CI = 5.8% to 12.5%) had return visits to the ED within 1 week. Twenty-one of 28 (75%) of these return visits were for reasons related to their initial injuries, such as headache, suture removal, and dizziness, but none of the patients presenting for return visits were admitted. Examination of the Social Security Death Index did not identify any deaths missed by chart review.
Table 3.
Description of Patients Who Died
| A | 66-year-old male with a past medical history of renal insufficiency, hypertension, coronary artery disease, prior nephrectomy, renal cell cancer, peripheral vascular disease, peptic ulcer disease, seizure disorder, and hyperlipidemia who fell from a roof and presented with an initial GCS score of 15. CT demonstrated intrafalcine subdural hematoma, left subdural hematoma, and intraparenchymal hemorrhage in the left lateral posterior temporal lobe, with diffuse left cerebral hemisphere sulcal effacement. Second head CT demonstrated a mild increase of the left temporal hemorrhage, but was otherwise unchanged. He remained in status epilepticus. He declined rapidly (less than 1 hour) after arrival to the ED and began seizing. After 11 days in status epilepticus the patient’s family withdrew care. |
| B | 68-year-old female with past medical history of ventriculoperitoneal shunt, meningioma resection, end-stage renal disease not on dialysis, coronary artery disease, depression, dyslipidemia, diabetes mellitus, and Menier’s disease who presented after a fall from standing height with an initial GCS score of 14. Pertinent medications included aspirin and clopidogrel. Her initial scan demonstrated subdural hemorrhage along the left lateral convexity, subfalcine subdural, subdural along tentorium bilaterally, subarachnoid hemorrhage within the bilateral sylvian fissures, and anterior interhemispheric fissure with effacement of lateral ventricles bilaterally. A second head CT obtained 3 hours later demonstrated worsening of all hemorrhages, as well as 8 mm of midline shift. She decompensated rapidly in the ED to a GCS score of 9 and was taken to the operating room for decompression, and the family withdrew care 3 days later. |
| C | 71-year-old male with a past medical history of hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, and chronic renal insufficiency who presented after a syncopal episode and had a concomitant urinary tract infection and pneumonia. His initial GCS score was 15, and he was found to have a small punctate cerebellar hemorrhage versus calcification that remained unchanged on subsequent head CTs obtained throughout his hospital course. He ultimately died of septic shock. |
| D | 79-year-old male with past medical history of coronary artery disease, diabetes mellitus, and pancreatic cancer who had been diagnosed with an unspecified movement disorder (likely Parkinson’s) previously, who presented after a fall from standing height. His initial GCS score was 15. His initial head CT demonstrated a small traumatic subarachnoid hemorrhage that had resolved by the second head CT. He was discharged home, but returned 2 days later after another witnessed fall from standing height. His head CT at that time demonstrated a large intraparenchymal hemorrhage, with left-sided parafalcine and left supratentorial subdural blood and a small amount of intraventricular extension. His family withdrew care. |
GCS = Glasgow Coma Scale.
DISCUSSION
Our data suggest that for mild TBI patients with ICH on initial head CT, who subsequently undergo clinical observation and repeat head CT with stable or improved clinical examinations and CT findings, the probability of death is low (0.5%, 95% CI = 0.1% to 2.7%). In our cohort, two of the reported deaths, patients C and D, may likely not have been attributed to the sequelae of mild TBI with ICH (Table 3). These findings suggest that this practice may be safe in a very specific cohort of patients. This practice has the added advantage of potentially avoiding unnecessary admissions in mild TBI patients. Whether this justifies the corresponding increase in ED resource utilization and radiation exposure is unknown and deserves further exploration.
A key benefit of this study is its focus on patients with GCS scores of 14 or 15. Many studies have examined outcomes following traumatic ICH, but their findings tend to be dominated by moderate to severe TBI cases. While outcomes of mild TBI are generally less severe, the condition is far more prevalent. This suggests that safe and efficient ED evaluation and management strategies for mild TBI patients would be of great value.
Our study found that clinical deterioration was rare even with radiographic progression of ICH. However, there were no delayed adverse clinical outcomes detected that were not associated with radiographic progression. While preliminary, these findings suggest that optimal risk stratification may include radiographic phenotyping of the initial CT findings and a concurrent period of observation for clinical deterioration. These findings also suggest that repeat CT imaging in the absence of clinical deterioration for the mild TBI patient with GCS > 13 and traumatic ICH on initial CT may be unnecessary.
Thirty-two percent of our patients had positive findings on initial head CT, but had no abnormal findings on repeat head CT. This novel finding can be interpreted as an indication that 1) there is a subset of patients with small radiographic abnormalities which rapidly resolve, 2) there is a high rate of false-positive findings on initial CT imaging, or 3) the lack of findings on repeat CT imaging may represent a group of false negative scans. Regardless, this calls into question the benefit of initial and repeat head CT scans for this particular subset of patients. Whether there are viable methods to selectively reduce imaging for this group would require further study. The high rate of discrepancy found between initial and repeat CT imaging also suggests there may be many patients in the United States who receive extended medical evaluation as a result of small intracranial radiographic abnormalities in the setting of TBI and calls into question the accuracy of epidemiologic estimates of the rate of traumatic ICH in the setting of mild TBI. With respect to our study, the observed rate of illness severity among patients with true-positive findings on initial head CT is higher than the rate calculated for all patients in the cohort.
Determining the true balance of benefit (e.g., reduced cost or early intervention) and harm (increased cost or radiation) resulting from repeat CT imaging and/or extended observation will require further study. Our institutional practice allowed for many patients to be discharged who would otherwise have been hospitalized, and our results suggest that clinical deterioration in the absence of radiographic progression is highly unlikely. However, it remains unknown if: 1) these patients truly require or benefit from hospitalization, 2) they might be discharged without repeat CT imaging, or 3) serial clinical evaluation over time is preferable to serial radiographic imaging.
While reducing the number of CTs performed for mild TBI patients by 10% could result in more than $10 million in savings each year,18,19 a repeat CT scan showing lack of radiographic progression might avoid an otherwise expensive admission, especially recognizing that many hospitals currently admit these patients to an intensive care unit for intensive neurologic monitoring. Conversely, the risks of radiation exposure from CT scans is a growing concern, the true burden of which is largely unknown in the adult population; however, radiation exposure from diagnostic imaging has been correlated with increased cancer risks in children.20 It would seem the risks of cancer secondary to radiation exposure are lower than that of adverse outcomes in this population. For instance, Smith-Bindman and colleagues21 demonstrated that an estimated one in 8,100 women who undergo head CT scans at age 40 years will develop cancer from that scan.21 For a 20-year-old patient, those risks double, and for a 60-year-old patient, the risk is approximately 50% lower. It may also be that magnetic resonance imaging becomes increasingly used as a way to decrease radiation exposure. Although this would be more time-consuming, a recent study evaluating subarachnoid hemorrhage of all types (traumatic and nontraumatic) suggested that susceptibility weighted imaging combined with fluid attenuated inversion recovery sequencing would be 100% sensitive in detecting subarachnoid hemorrhage.22 Thus, the optimal imaging strategy for mild TBI patients with ICH remains unknown.
LIMITATIONS
The results should be interpreted in the context of the limitations of the study. As a retrospective chart review, the only information available was that recorded by the clinical care team. Missing data may not have been random and thus bias may have been introduced.
Of particular importance are the limitations associated with the outcome measures. Although the Social Security Death Index was examined to capture any patients who died outside of our institution, adverse outcomes other than death were missed if patients did not return to our institution for care. During the study period, there were nine other hospitals in the county. Thus, if patients did not return to our institution for care, we would have had an underestimation of minor adverse outcomes, especially return visits. However, the region’s other hospitals rarely accept neurotrauma patients; therefore, any significant consequences following the discharge of these patients would have likely been captured
Another limitation of our study is that data collection was primarily completed by a single chart abstractor. Due to time constraints, dual abstraction could not be performed on every chart. However, as a method to ensure accuracy, 50 charts were selected for duplicate abstraction throughout the study period. Discrepancies were not captured in these cases, and interobserver reliability was not calculated.
In addition, our outcome assessment was limited to mortality, surgical intervention, and repeat visits. Other sequelae such as functional outcome, the ability to perform activities of daily living, and symptoms related to the patients’ injuries could not be assessed. While our sample included patients presenting over a decade, our sample size was too small to estimate the adverse event rate with sufficient precision to broadly influence practice; prospective confirmatory studies are needed. Additionally, as more novel anticoagulants are being introduced, future practice patterns will be influenced in unpredictable ways. In future studies, factors such as cost and radiation exposure should be considered in evaluating the potential benefits of repeat imaging and disposition from the ED for mild TBI patients.
CONCLUSIONS
In ED patients with mild traumatic brain injury and traumatic intracranial hemorrhage, the lack of clinical and radiographic progression over a period of 6 to 24 hours portends a low probability of adverse outcomes. Discharge after repeat head computed tomography and brief observation in the ED allowed early discharge of a specific cohort of mild traumatic brain injury patients with traumatic intracranial hemorrhage without significant short-term adverse outcomes. Given the limitations of this study, these results provide a foundation for further investigation in this important area of high-risk ED patient management.
Acknowledgments
This project was supported in part by an Institutional Clinical and Translational Science Award, NIH/NCRR grant 5UL1RR026314-03.
Footnotes
Presented at the Neurocritical Care Society Conference, Philadelphia, PA, October 2013.
The authors have no relevant financial information or potential conflicts of interest to disclose.
References
- 1.Rutland-Brown W, Langlois J, et al. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehab. 2006;21:544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Kisat M, Zafar SN, Latif A, et al. Predictors of positive head CT scan and neurosurgical procedures after minor head trauma. J Surg Res. 2012;173:31–7. doi: 10.1016/j.jss.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armin SS, Colohan AT, Zhang JH. Traumatic subarachnoid hemorrhage: our current understanding and its evolution over the past half century. Neurol Res. 2006;28:445–52. doi: 10.1179/016164106X115053. [DOI] [PubMed] [Google Scholar]
- 4.Valadka AB. Injury to the cranium. In: Moore EJ, Feliciano DV, Mattox KL, editors. Trauma. New York: McGraw-Hill; 2004. pp. 385–406. [Google Scholar]
- 5.Shukla D, Devi BI. Mild traumatic brain injuries in adults. J Neurosci Rural Pract. 2010;1:82–8. doi: 10.4103/0976-3147.71723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fainardi E, Chieregato A, Antonelli V, Fagioli L, Servadei F. Time course of CT evolution in traumatic subarachnoid hemorrhage: a study of 141 patients. Acta Neurochir. 2004;146:257–63. doi: 10.1007/s00701-003-0207-y. [DOI] [PubMed] [Google Scholar]
- 7.Fortuna GR, Mueller EW, James LE, Shutter LA, Butler KL. The impact of preinjury antiplatelet and anticoagulant pharmacotherapy on outcomes in elderly patients with hemorrhagic brain injury. Surgery. 2008;144:598–605. doi: 10.1016/j.surg.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Jagoda AS, Bazarian JJ, Bruns JJ, Jr, et al. American College of Emergency Physicians; Centers for Disease Control and Prevention. Clinical policy: neuroimaging and decision making in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52:714–48. doi: 10.1016/j.annemergmed.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Bugaev N, Arabian S, Rabinovici R. Admission patterns of stable patients with isolated orthopedic or neurosurgical injuries. J Trauma Acute Care Surg. 2013;74:1151–5. doi: 10.1097/TA.0b013e3182827191. [DOI] [PubMed] [Google Scholar]
- 10.Sifri ZC, Livingston DH, Lavery RF, et al. Value of repeat cranial computed axial tomography scanning in patients with minimal head injury. Am J Surg. 2004;187:338–42. doi: 10.1016/j.amjsurg.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Jeret JS, Mandell M, Anziska B, et al. Clinical predictors of abnormality disclosed by computed tomography after mild head trauma. Neurosurgery. 1993;32:9–15. doi: 10.1227/00006123-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Almenawer SA, Bogza I, Yarascavitch B, et al. The value of scheduled repeat cranial computed tomography after mild head injury: single-center series and meta-analysis. Neurosurgery. 2013;72:56–64. doi: 10.1227/NEU.0b013e318276f899. [DOI] [PubMed] [Google Scholar]
- 13.Wang MC, Linnau KF, Tirschwell DL, Hollingworth W. Utility of repeat head computed tomography after blunt head trauma: a systematic review. J Trauma. 2006;61:226–33. doi: 10.1097/01.ta.0000197385.18452.89. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention, US. Department of Health and Human Services. [Accessed Jul 9, 2014];Heads Up: Facts for Physicians about Mild Traumatic Brain Injury (MTBI) Available at: http://www.cdc.gov/concussion/headsup/pdf/Facts_for_Physicians_booklet-a.pdf.
- 15.Ruff RM, Iverson GL, Barth JT, Bush SS, Broshek DK NAN Policy and Planning Committee. Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol. 2009;24:3–10. doi: 10.1093/arclin/acp006. [DOI] [PubMed] [Google Scholar]
- 16.Norwood SH, McAuley CE, Berne JD, Vallina VL, Creath RG, McLarty J. A prehospital Glasgow coma scale score < or = 14 accurately predicts the need for full trauma team activation and patient hospitalization after motor vehicle collisions. J Trauma. 2002;53:503–7. doi: 10.1097/00005373-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller EC, Holmes JF, Derlet RW. Utilizing clinical factors to reduce head CT scan ordering for minor head trauma patients. J Emerg Med. 1997;15:453–7. doi: 10.1016/s0736-4679(97)00071-1. [DOI] [PubMed] [Google Scholar]
- 19.Reinus WR, Wippold FJ, 2nd, Erickson KK. Practical selection criteria for noncontrast cranial computed tomography in patients with head trauma. Ann Emerg Med. 1993;22:1148–55. doi: 10.1016/s0196-0644(05)80981-3. [DOI] [PubMed] [Google Scholar]
- 20.Leiner T, deJong PA, Nievelstein R. CT scans in children and adolescents: only when appropriate and when optimized (Article in Dutch) Ned Tijdschr Geneeskd. 2013;157:A6711. [PubMed] [Google Scholar]
- 21.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated life-time attributable risk of cancer. Arch Intern Med. 2009;169:2078–86. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma RK, Kottke R, Andereggen L, et al. Detecting subarachnoid hemorrhage: comparison of combined FLAIR/SWI versus CT. Eur J Radiol. 2013;82:1539–45. doi: 10.1016/j.ejrad.2013.03.021. [DOI] [PubMed] [Google Scholar]