The Children’s Oncology Group (COG) AALL0031 study included very high-risk (VHR) pediatric acute lymphoblastic leukemia (ALL) patients who had an expected 5-year event-free survival ≤45%. The chemotherapy regimen was based on previous strategies; eligible patients received 4 weeks of standard induction chemotherapy and then were enrolled on AALL0031, which included an intensive consolidation followed by a continuation regimen (Supplementary Figure 1).1 COG AALL0031 enrolled patients aged 1–21 years with VHR ALL from 14 October 2002 to 20 October 2006. Induction therapy was limited to a combination of vincristine, prednisone or dexamethasone, and asparaginase with or without daunomycin. VHR features included the following: (a) Philadelphia chromosome [t(9;22)(q34;q11.2)]; (b) hypodiploidy: defined as ≤44 chromosomes or DNA index <0.81; (c) any rearrangement of the MLL gene in conjunction with a slow early response ≥5% marrow blasts at day 15 and/or ≥0.1% minimal residual disease (MRD) at the end of induction as detected by multiparameter flow cytometry;2,3 and (d) induction failure (IF) defined as either >25% blasts (M3 marrow status) by histology at the end of 4 weeks of induction therapy or an M2 marrow (5–25% blasts) or MRD ≥1% by flow cytometry at the end of induction followed by an M2 (or M3) marrow or MRD≥1% after receiving two additional weeks of induction therapy (M2/M2 IFs). The therapy was identical to that presented in a previous publication on outcomes for Ph+ ALL patients,1 except that the Ph− patients received no imatinib (see Supplementary Figure 1).
Prior approval was obtained from the National Cancer Institute and the Institutional Review Boards of the COG member institutions. Informed consent was obtained in accordance with the Federal guidelines. Sixty-three hypodiploid (41) and IF (22) patients were enrolled in AALL0031 after 4 weeks of a three- or four-drug induction regimen for National Cancer Institute standard and high-risk ALL, respectively. Data on adverse events and clinically significant abnormal laboratory findings were collected using National Cancer Institute Common Terminology Criteria version 2.0. MRD was assessed by multiparameter flow cytometry.2 Samples were available from 46 of 63 (73%) patients at study entry. MRD high was defined as >0.01% and low as ≤0.01%.
The primary outcome in this report is disease-free survival (DFS). Overall survival (OS), DFS and event-free survival were all defined as the time from the end of consolidation to the first event or last contact. An event was defined as relapse at any site, secondary malignancy or death in remission. A historical control data set of hypodiploid patients included patients enrolled on the Pediatric Oncology Group 8602, 9005, 9006, 9201, 9405, 9406 and 9605 protocols for B-ALL (January 1986–November 1999).3 The percentage of patients undergoing bone marrow transplant (BMT) in these comparator studies is unknown. IF patients were excluded from post-induction therapy in the historical control trial. Estimates of DFS, event-free survival and OS were computed using the Kaplan–Meier method4 and s.e. of the estimates according to Peto and Peto.5 The log-rank test was used for comparison of survival curves between groups. Comparison of MRD rates and toxicity rates between groups used the χ2- and Fisher’s exact tests. AALL0031 study data were frozen as of 30 September 2010 for these analyses.
AALL0031 enrolled 160 patients; 2 were ineligible because of invalid consents (1 Ph+, 1 Ph−). The 158 eligible patients included 93 Ph+ patients (excluded from these analyses), 41 with hypodiploidy, 22 Ph− IFs and 2 with MLL rearrangement and a slow early response (excluded owing to small numbers). Of the 22 IF patients, 9 had an M3 marrow at day 29 of induction and 13 were M2/M2 at days 29 and 43. Of the M2/M2 population, four had histological M2/M2 with between 5 and 25% blasts at days 29 and 43, and eight were M2/M2 because of ≥1% MRD at both days 29 and 43. For the M2/M2 population, 5 out of 12 received BMT and 7 out of 12 chemotherapy. For the M3 group, four out of nine received BMT, four out of nine chemotherapy and one came off protocol for not achieving remission. Of the 22 IFs, one was inevaluable because of missing marrow status at the end of consolidation and the other was excluded from analyses (except OS) for a major therapy protocol violation and two additional patients had an M3 marrow after consolidation and were removed from analysis as off study. Thus, 18 patients with IF were evaluable.
Two of the 41 hypodiploid patients were removed from protocol therapy before the end of consolidation 1: 1 withdrawn by the family and 1 taken off the study owing to inappropriate chemotherapy. The distribution of AALL0031 patients in the 44, 40–43, 30–39 and <30 chromosome ranges was 5, 0, 15 and 19, respectively. Analysis for other major toxicities revealed no major differences between patients receiving chemotherapy versus related donor BMT.
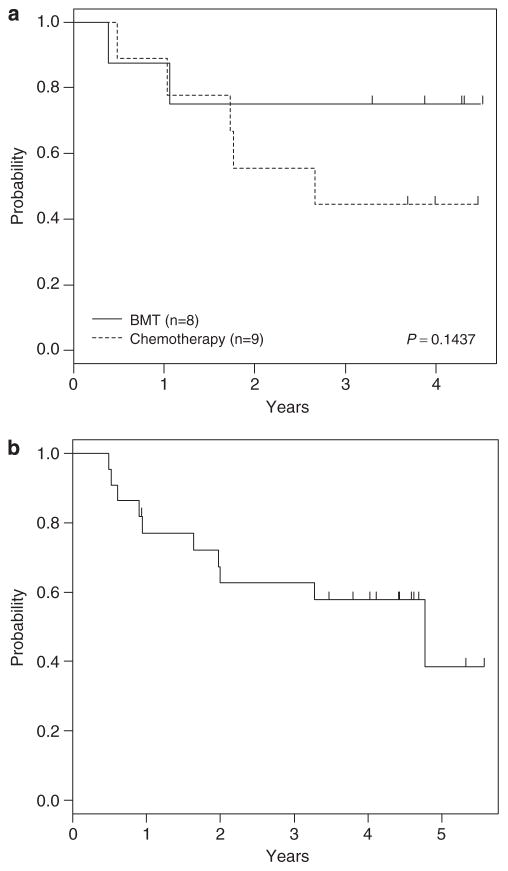
Seventeen of 18 evaluable (94%) IF patients achieved a complete remission after two 3-week cycles of consolidation therapy. Of these 17 patients, 9 continued chemotherapy, 2 underwent an human leukocyte antigen-identical sibling allogeneic BMT and 6 were removed from protocol therapy and underwent unrelated donor BMT. Overall, 4-year DFS for IF patients was 59±15% (N = 17): 44±23% for chemotherapy and 75±19% for related/unrelated BMT (P = 0.14; Figure 1a). No historical comparison was performed as the COG lacks historical controls for a comparison with IF patients as defined in this study. The 4-year OS was 58±12% for the 22 IF patients (Figure 1b).
Figure 1.
Outcomes for IF patients. (a) DFS curves by chemotherapy versus BMT. (b) OS curve for all IF patients.
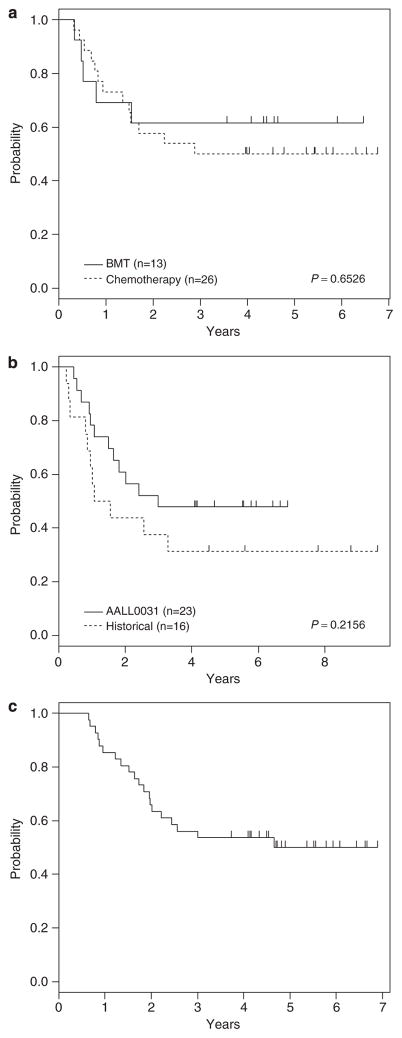
Twenty-eight hypodiploid patients received chemotherapy and 12 underwent related donor BMT. One patient was taken off the protocol for an unrelated donor BMT. One patient withdrew from protocol therapy during consolidation block 2 and the other patient was made inevaluable for receiving incorrect therapy. Four-year DFS for hypodiploid patients receiving chemotherapy (N = 26; 50±11%) was similar to that of patients who received related/unrelated donor BMT (N = 13; 62±14%; P = 0.65; Figure 2a). The event-free survival for the hypodiploid patients on this study was statistically not different from COG historical controls: (N = 23) 48±10% versus (N = 16) 31±12%; P = 0.22; Figure 2b). The comparison with historical controls excluded the following patients: those without chromosome counts, those with a chromosome count of 44 and those who received BMT. Four-year OS for all hypodiploid patients (N = 41) was 54±8% (Figure 2c).
Figure 2.
Outcomes for hypodiploid patients. (a) DFS curves by chemotherapy versus BMT. (b) Event-free survival (EFS) curves for AALL0031 patients who received chemotherapy versus COG historical controls. (c) OS curve for all AALL0031 hypodiploid patients.
In previous studies, MRD has been predictive of outcome both before BMT and as an early indicator of outcome in patients receiving chemotherapy.6–8 We evaluated the associations of MRD after consolidation cycle 2 with outcome combining the IF and hypodiploid populations. Four-year DFS rates for those with MRD<0.01% were 83±20% with BMT and 47±13% with chemotherapy, and 56±19% with BMT and 29±24% with chemotherapy for those with MRD >0.01% (P = 0.43; Supplementary Figure 2).
This report presents data on a cohort of VHR pediatric ALL patients who were treated in a consistent manner. Although the patient numbers in this study limited our statistical power, a number of important observations were made that may guide the design of future trials and decisions regarding clinical management of VHR ALL patients. Administration of the two blocks of ALL consolidation chemotherapy resulted in a very high complete remission rate (94%) for IF patients. This is higher than the previously reported complete remission rates of 50–80% for refractory and relapsed ALL in children,9–11 but the definition of IF used in this study is different from that used in many other studies.
The hypodiploid patients treated on AALL0031 had better, although not significant, outcomes than COG historical controls. This may be even more significant as the current data in AALL0031 were skewed to an increased proportion of patients with a lower modal chromosome number, which is associated with inferior outcome.12 The distribution in the CCG-1950/60 studies was 9 with 40–43 chromosomes, 2 with 30–39 chromosomes and 9 with <30 chromosomes (55% with <40 chromosomes).13 Given the association of low hypodiploidy with Li–Fraumeni syndrome, it will be important to develop therapy that is minimally genotoxic in this group of patients.14
There was a nonsignificantly better outcome for allogeneic BMT versus chemotherapy for IF patients and the Ponte di Legno group identified risk groups among IF patients that supported BMT, including the following: age 10 years or older, T-cell ALL, an 11q23 rearrangement and 25% or more blasts in the bone marrow at the end of 4–6 weeks of induction therapy.13 When selecting chemotherapy such as AAL0031, it should be noted, however, that the AALL0031 chemotherapy backbone included significant amounts of high-dose methotrexate, cranial irradiation and 11 gm/m2 of cyclophosphamide leading to potential risks of neurotoxicity and infertility that may be similar to those seen following BMT.
Our study was not powered adequately to assess the potential impact of MRD on outcome following either chemotherapy or BMT, but there was a clear trend toward better outcomes for patients with low MRD whether treated with chemotherapy or BMT. In conclusion, the outcome of IF and hypodiploid ALL patients treated in AALL0031 was substantially inferior to the 5-year OS rate of 90% obtained for all COG ALL patients treated in this era.12,15
Supplementary Material
Acknowledgments
This study was supported by grants to the COG including CA98543, CA98413 and CA29139. SPH is the Ergen Family Chair in Pediatric Cancer. We sincerely thank Doojduen Villaluna, MS for invaluable statistical support, Tammie Eslinger, CCRP for outstanding protocol development and performance support, Laura Francisco, for data management, and Bernice Pasut, RN for diligent and thorough protocol development support.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
List of where the study has been presented in part elsewhere: Schultz KR, Bowman P, Slayton WB, Aledo A, Devidas M, Sather H, Borowitz M, Davies S, Trigg M, Pasut B, Jorstad D, Eslinger T, Burden L, Wang CG, Rutledge R, Camitta B, Gaynon P, Carroll A, Heerema NA, Winick N, Hunger S, Carroll WL. Philadelphia chromosome-negative (Ph −) very high-risk (VHR) acute lymphoblastic leukemia (ALL) in children and adolescents: the impact of intensified chemotherapy on early event-free survival (EFS) in Children’s Oncology Group (COG) Study AALL0031. ASH 6–8 December 2008.
References
- 1.Schultz KR, Aledo A, Bowman WP, Slayton WB, Sather H, Devidas M, et al. Improved early event free survival (EFS) with tolerable toxicity in children with philadelphia chromosome positive (Ph +) acute lymphoblastic leukemia (ALL) with intensive imatinib mesylate with dose-intensive multiagent chemotherapy: Children’s Oncology Group (COG) Study AALL0031. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group Study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynon PS, Angiolillo AL, Carroll WL, Nachman JB, Trigg ME, Sather HN, et al. Children’s Oncology Group. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: a Children’s Oncology Group report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 5.Peto R, Peto J. Asymptotically efficient rank invariant test procedure. J R Stat Soc. 1972;135:185–198. [Google Scholar]
- 6.van van Dongen JJ, Seriu T, Panzer-Grümayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–1738. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- 7.Cazzaniga G, Gaipa G, Rossi V, Biondi A. Minimal residual disease as a surrogate marker for risk assignment to ALL patients. Rev Clin Exp Hematol. 2003;7:292–323. [PubMed] [Google Scholar]
- 8.Borowitz ML, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. the Children’s Oncology Group. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hijiya N, Gaynon P, Barry E, Silverman L, Thomson B, Chu R, et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23:2259–2264. doi: 10.1038/leu.2009.185. [DOI] [PubMed] [Google Scholar]
- 10.Steinherz PG, Shukla N, Kobos R, Steinherz L. Remission re-induction chemotherapy with clofarabine, topotecan, thiotepa, and vinorelbine for patients with relapsed or refractory leukemia. Pediatr Blood Cancer. 2010;54:687–693. doi: 10.1002/pbc.22321. [DOI] [PubMed] [Google Scholar]
- 11.Duval M, Weisdorf D, Klein JP, He W, Cahn J-Y, Cairo MS, et al. Hematopoietic stem cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nachman JB, Heerema NA, Sather H, Camitta B, Forestier E, Harrison CJ, et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood. 2007;110:1112–1115. doi: 10.1182/blood-2006-07-038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–1381. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L, et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet. 2013;45:242–252. doi: 10.1038/ng.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.