Abstract
This study examined the effects of mindfulness-based stress reduction (MBSR) on the brain-behavior mechanisms of self-referential processing in patients with social anxiety disorder (SAD). Sixteen patients underwent functional magnetic resonance imaging while encoding self-referential, valence, and orthographic features of social trait adjectives. Post-MBSR, 14 patients completed neuroimaging. Compared to baseline, MBSR completers showed (a) increased self-esteem and decreased anxiety, (b) increased positive and decreased negative self-endorsement, (c) increased activity in a brain network related to attention regulation, and (d) reduced activity in brain systems implicated in conceptual-linguistic self-view. MBSR-related changes in maladaptive or distorted social self-view in adults diagnosed with SAD may be related to modulation of conceptual self-processing and attention regulation. Self-referential processing may serve as a functional biobehavioral target to measure the effects of mindfulness training.
Keywords: social anxiety disorder; fMRI; mindfulness, self; clinical intervention
Social anxiety disorder (SAD) is a common and frequently debilitating condition characterized by intense fear of evaluation in social or performance situations (Jefferys, 1997; Kessler et al., 1994). SAD has an early onset (Otto et al., 2001) and regularly precedes other anxiety, mood, and substance abuse/dependence disorders (Lampe, Salde, Issakidis, & Andrews, 2003; Matza, Revicki, Davidson, & Stewart, 2003).
SAD is associated with significant distress and functional impairment in both work and social domains (Lochner et al., 2003; Rapee, 1995; Schneier et al., 1994) and typically persists unless treated (Clark & Wells, 1995). The early onset of SAD magnifies its impact, including increased school dropout (Van Ameringen, Mancini, & Farvolden, 2003), poor social integration, and increased comorbid psychopathology (Lampe et al., 2003; Matza et al., 2003; Randall, Thomas, & Thevos, 2001).
Self-Referential Processing in SAD
Cognitive models of social anxiety (Clark & McManus, 2002; Clark & Wells, 1995; Rapee & Heimberg, 1997) suggest that during social situations several psychological processes characterize SAD, including fear of negative evaluation, maladaptive cognitions regarding self (e.g., as socially incompetent) and others (e.g., as critical judges), and an exaggerated self-focus. Self-referential processing (SRP) is heightened during social and performance situations in patients with SAD (Spurr & Stopa, 2002). Exaggerated SRP in patients with SAD distorts interpretations of social cues and maintains social fears (Bogels & Mansell, 2004). SRP is thought to contribute to (a) negative emotional reactivity, (b) deficits in cognitive regulation of emotion, and (c) interference with interpersonal functioning. Thus, clinical interventions that influence SRP may modulate a core mechanism related to SAD.
Mindfulness-Based Stress Reduction and Self-Referential Processing
Mindfulness meditation, as taught in mindfulness-based stress reduction (MBSR), involves the cultivation of present moment focus without distorted evaluation (Kabat-Zinn, 1990). Mindfulness practice is conceptualized as directly reducing the habitual tendency to automatically engage in and react to evaluative mental states (Teasdale et al., 2000).
MBSR has been shown to be an effective intervention for reducing the symptoms of stress, depression, and anxiety across a wide range of clinical populations (Bishop, 2002). One mechanism by which MBSR may produce these reductions in clinical symptoms is through its effect on SRP, including reduction in negative self-rumination (Ramel, Goldin, Carmona, & McQuaid, 2004) and enhancement of experiential and visceral modes of SRP (Farb et al., 2007).
MBSR and SAD
It is hypothesized that mindfulness training may diminish SRP in patients with SAD, specifically reducing habitual tendencies to engage in hypercritical social self-view (self-evaluation) and to react in an exaggerated manner to beliefs about how others might view oneself (other evaluation). Thus, for patients with SAD, mindfulness training may lead to a shift from cognitive distortions of the social self toward a more adaptive (i.e., less distorted) mode of SRP.
Although the proposed mechanisms of MBSR appear to match the self–other evaluative distortions at the core of SAD, to date there have been only three studies that have examined the effect of mindfulness training as a clinical intervention for SAD. Patients with generalized SAD treated with either 8-week MBSR or 12-week cognitive-behavioral group therapy (CBGT) showed equivalent improvement on mood, functionality, and quality of life; however, clinician- and patient-rated measures of social anxiety indicated greater effectiveness of CBGT compared to MBSR (Koszycki, Benger, Shlik, & Bradwejn, 2007). Online delivery of MBSR was found to reduce shyness, anxiety, and social anxiety (Arana, 2006). An intervention that combined mindfulness and task concentration training reduced fear of negative evaluation and self-ideal discrepancy (Bogels, Sijbers, & Voncken, 2006).
Thus, there is preliminary evidence that mindfulness training may impact self-processing and reduce social anxiety. An important gap in the literature, however, is the lack of an understanding of how MBSR impacts the neural bases of self-referential processes.
SRP, Neural Circuitry, and MBSR
Neuroimaging investigations using different stimulus presentations, including judgments of visually presented trait adjectives (Kelley et al., 2002), aurally presented statements (Johnson et al., 2002), or mental reflection on self-traits (Kjaer, Nowak, & Lou, 2002), have identified a brain network associated with SRP. A recent meta-analysis of neuroimaging investigations has identified three cortical midline brain regions that are reliably activated as a network during SRP: ventral medial prefrontal cortex (MPFC), dorsal MPFC, and a region spanning posterior cingulate cortex (PCC) and precuneus (Northoff et al., 2006).
The selective engagement of this SRP-related brain network has been demonstrated for self versus situational focus (Ochsner et al., 2004) and for self versus perspective taking (D’Argembeau et al., 2007). The SRP-related brain network has been shown to vary with ratings of self-relatedness of stimuli recorded post–functional magnetic resonance imaging (fMRI) (Phan et al., 2004) and may be valence independent (Fossati et al., 2003).
An investigation of the effect of MBSR on the neural substrates of SRP (Farb et al., 2007) found that a narrative self-focus, characterized as a language-mediated conceptual-analytic mode of evaluation, resulted in activation of midline cortical regions implicated in conceptual self-representation (ventral and dorsal MPFC, PCC) and language processing (left inferior frontal gyrus, middle temporal gyrus). When cued to engage in an experiential self-focus, characterized as non–goal oriented, present-moment engagement with the contents of one’s thoughts, feelings, and body state, MBSR-trained participants had decreased brain responses implicated in conceptual self-representation and emotional reactivity (left dorsal amygdala) and increased right-lateralized brain responses linked to cognitive control (dorsal and ventrolateral PFC), visceral responses (posterior insular cortex, somatosensory cortex), and attention (inferior parietal lobule) (Gusnard, Akbudak, Shulman, & Raichle, 2001). This study, however, did not examine changes in neural responses from pre- to post-MBSR training, behavioral ratings of self-endorsement of traits, and differential positive and negative valence-related neural responses.
The Present Study
To investigate the effect of mindfulness training on the neural basis of self-processing in patients with SAD, the present study used fMRI to examine MBSR-related changes in clinical symptoms as well as behavioral and neural measures of self-processing. Clinically, we expected that MBSR would reduce symptoms of anxiety and depression. On the SRP task, we expected that mindfulness training would increase positive and decrease negative self-view as indexed by changes from pre- to post-MBSR in self-endorsement of social traits. Neurally, our analyses focused on a SRP-related network of cortical midline structures (ventral and dorsal MPFC, PCC). We expected that MBSR would result in reduced narrative/conceptual self-focus (i.e., midline cortical neural responses) and increased experiential self-focus (i.e., somatosensory and visceral neural responses) during both positive and negative SRP.
Methods
Participants
Participants included 16 (nine females) right-handed patients with SAD who met criteria of the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV) based on the Anxiety Disorders Interview Schedule—IV (ADIS-IV; DiNardo, Brown, & Barlow, 1994) for current primary generalized SAD. Participants had an average age of 35.2 (SD = 11.9) and 16.3 (SD = 3.5) years of education and included eight Anglo-Americans, five Asian Americans, two Latino Americans, and one Native American. Comorbid current diagnoses included three patients with generalized anxiety disorder, three with specific phobia, and one with panic disorder without agoraphobia. Comorbid past diagnoses included two with obsessive-compulsive disorder, three with dysthymia, and four with past major depressive disorder. All participants provided informed consent in accordance with Stanford University’s Human Subjects Committee guidelines for ethical research. Post-MBSR, two patients chose not to participate because of discomfort with MR scanning.
Exclusion Criteria
All participants passed a magnetic resonance scanning safety eligibility screen. Participants were excluded if they reported current use of any psychotropic medication, prior meditation training, or any history of neurological or cardiovascular disorders or met criteria for any current DSM-IV axis I psychiatric disorders other than social anxiety, generalized anxiety, agoraphobia, or specific phobia disorders.
Clinical Assessment
Clinical diagnostic assessments were conducted using the ADIS-IV (DiNardo et al., 1994) to diagnose psychiatric disorders. This structured clinical interview is based on the DSM-IV but has been extended to be more sensitive in differential diagnosis of anxiety disorders. We measured symptoms of social anxiety (Liebowitz, 1987), depression (Beck Depression Inventory—II; Beck, Steer, & Brown, 1996), rumination (Nolen-Hoeksema, 1991), state anxiety (Spielberger, Gorsuch, & Lushene, 1970), and self-esteem (Rosenberg, 1965).
Procedure
Participants were recruited through Web-based community listings and referrals from local mental health clinics. Following a phone screen to determine MR eligibility, potential participants were administered a structured clinical diagnostic interview. Eligible patients were administered a battery of questionnaires and, in a separate session, the fMRI SRP task. Prior to MR scanning, patients were instructed on the SRP task with six practice trials for each of the three trial types using word stimuli not presented during the fMRI task. Stimuli were presented visually with E-Prime software on a PC. Patients with SAD were scanned again after completion of a standard MBSR course.
MBSR
MBSR was delivered in an academic setting in a standard format based on the treatment protocol developed by Kabat-Zinn (1990). Participation included eight weekly 2.5-hour sessions, a half-day meditation retreat after session 6, daily home practice based on audiotapes produced by Kabat-Zinn, and daily monitoring of both formal and informal meditation practices. The course was led by a member of the team (P. G.) who was trained in Buddhist monastic settings in India and in the San Diego Veterans Hospital by a psychiatric nurse trained by Kabat-Zinn.
SRP Task
The SRP task is based on the self-referential encoding task (Derry & Kuiper, 1981), which is considered an information-processing measure of self-endorsement of self-schema. Stimuli consisted of 25 positive and 25 negative social trait adjectives from the Affective Norms of Emotion Words database (Bradley & Lang, 1999), balanced (all ps > .51) on word frequency (positive adjectives = 40.5, negative adjectives = 33.6), arousal (positive adjectives = 5.54, negative adjectives = 5.43), valence (deviation from neutral: positive adjectives = 2.66, negative adjectives = 2.58), and number of letters (positive adjectives = 6.9, negative adjectives = 7.2).
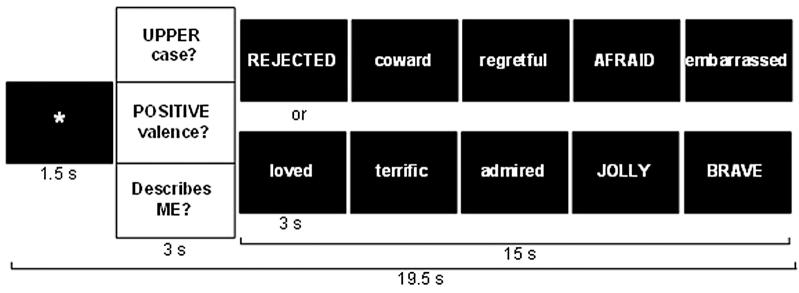
In a single functional run (9 minutes, 54 seconds), each of the 25 positive and 25 negative adjectives was presented three times, once in each of three conditions—self-referential (self-descriptive or not)—and two control conditions—valence identification (positive or negative affective meaning) and case identification (upper- or lowercase). Each of the six conditions (three instructions by two valences) included five blocks of five adjective stimuli. Each block was comprised of a fixation, question (one of three questions: “Describes ME?,” “POSITIVE valence?,” or “UPPERcase?”), and five adjectives of the same valence presented one at a time for 3 seconds each (Figure 1).
Figure 1.
Experimental design. Each block consists of (a) an asterisk fixation; (b) instruction question to cue self-referential, valence, or orthographic processing; and (c) a set of five different target words of a single valence.
Stimulus order was determined by a pseudorandom block sequence with no more than two blocks of the same condition in a row and a random sequence of words and upper-/lowercase within each block. Participants pressed one of two buttons to indicate whether a word was or was not self-descriptive (i.e., self-endorsement), positively valenced, or in uppercase letters. Behavioral responses were made using a custom button box and recorded using E-Prime software during the scan.
Image Acquisition
Imaging was performed on a General Electric 3 Tesla Signa magnet using a custom-built quadrature “dome” elliptical birdcage head coil and a T2*-weighted gradient echo spiral-in/out pulse sequence that used blood oxygenation level-dependent (BOLD) contrast. The spiral-in/out sequence results in increased signal-to-noise (Glover & Law, 2001) and has been shown to be effective in recovering BOLD signal in frontal cortex and temporal lobes (Preston, Thomason, Ochsner, Cooper, & Glover, 2004). Head movement was minimized using a bite bar, padding, and plungers. During a single run, 368 volumes (each consisting of 22 sequential axial slices) were obtained (TR = 1500 ms, TE = 30 ms, flip angle = 60, FOV = 22 cm, frequency encoding = 64, single shot, voxel resolution = 3.438 mm2 × 5 mm). A high-resolution anatomical scan was acquired using a fast spin echo spoiled grass pulse sequence (voxel resolution = .85942 × 1.2 mm; FOV = 22 cm, frequency encoding = 256).
fMRI Data Preprocessing
Analysis of functional neuroimages (Cox, 1996) was used for preprocessing and statistical analysis. Every volume was examined visually and computationally for MR signal artifacts and outliers related to head movement and magnetic field disturbances. The first four time points in each functional scan were eliminated to account for stabilization of the magnetic field. The optimal base image for realignment and calculation of six motion parameters (three translations and three rotations) was identified empirically based on an automated recursive analysis of the root mean square adjustment for motion correction at each time point. No brain volumes required greater than ±1.0 mm motion correction in the x, y, or z directions, and there was no evidence of stimulus-correlated motion for any of the six task conditions. MR signal in each voxel was subjected to a high-pass temporal filter (.011 Hz) to remove low-frequency oscillations and was converted to percent signal change based on the mean MR signal per voxel.
fMRI Statistical Analysis
A multiple regression model was implemented using the AFNI 3dDeconvolve program. The baseline model included parameters to remove variance in each voxel’s time series related to mean, linear and quadratic drifts, and the six motion correction parameters. Six reference vectors for each of the six conditions (positive and negative versions of self, valence, and case) were convolved with a gamma variate model (Cohen, 1997) of the hemodynamic response function to account for the hemodynamic delay to peak BOLD responses. Resultant statistical maps were spatially smoothed with a 4-mm3 isotropic Gaussian kernel, resampled to 3.438 mm3, and converted to Talairach (Talairach & Tournoux, 1988) atlas space. Second-level paired t tests were conducted according to a random-effects model. Neural results are reported for the contrast of self-processing versus case processing only in this article to reduce complexity.
To correct quantitatively for the multiple comparisons, AlphaSim, a Monte Carlo simulation bootstrapping program in the AFNI library, was employed to identify a joint-probability threshold consisting of a voxelwise threshold of p < .005 and minimum cluster-volume threshold ≥163 mm3 (4 voxels × 3.438 mm3) that resulted in protection against false-positive cluster detection at p < .01 in the whole-brain analyses.
Results
Clinical Results
Paired t tests showed that from baseline to post-MBSR, patients had decreased social anxiety, depression, rumination, and state anxiety as well as increased self-esteem (Table 1).
TABLE 1. Clinical Measures.
| Baseline Mean ± SD | Post-MBSR Mean ± SD | t Test, effect size | |
|---|---|---|---|
| LSAS | 68.7 ± 21.2 | 49.3 ± 17.0 | 4.3***, .59 |
| BDI-II | 8.7 ± 9.1 | 3.4 ± 3.2 | 2.2*, .27 |
| RSQ | 26.4 ± 6.5 | 19.3 ± 95.7 | 3.8**, .53 |
| STAI-State | 41.5 ± 9.3 | 29.6 ± 6.4 | 8.4***, .84 |
| RSES | 22.7 ± 4.6 | 27.2 ± 4.7 | 3.7*, .51 |
Note. LSAS = Liebowitz Social Anxiety Inventory; BDI-II = Beck Depression Inventory—II; RSQ = Rumination Style Questionnaire; STAI = Spielberg State Trait Anxiety Inventory; RSES = Rosenberg Self-Esteem Scale.
p < .05.
p < .01.
p < .001. Effect size = partial eta2.
Behavioral Results
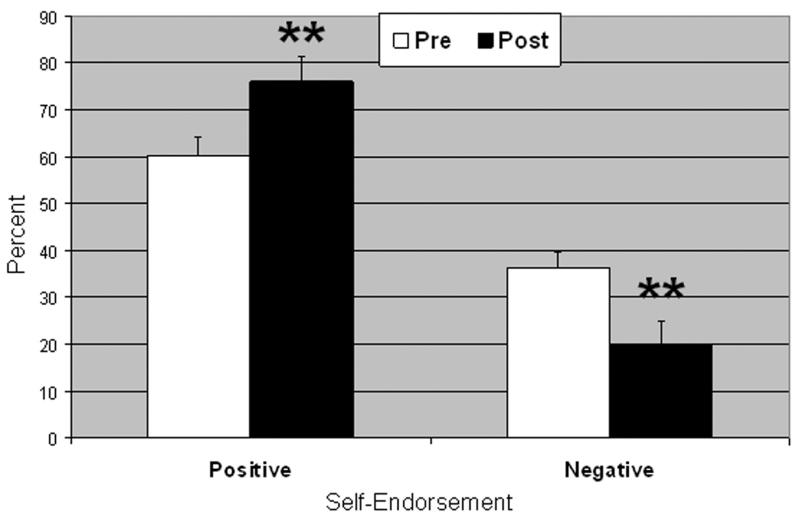
A Time (baseline, post-MBSR) × Word Valence (negative, positive) repeated-measures analysis of variance resulted in a significant interaction of Time × Word Valence on self-endorsement, F(1, 14) = 19.91, p < .001, ηp2 = .60. Follow-up paired t tests showed that from baseline to post-MBSR, patients had reduced negative, t(1, 14) = 3.39, p < .005, and increased positive, t(1, 14) = 4.04, p < .005, self-endorsement (Figure 2).
Figure 2.
Percent self-endorsement for negative and positive social trait words pre- and post-MBSR. Error bars = SEM. **p < .005.
Baseline Neural Results
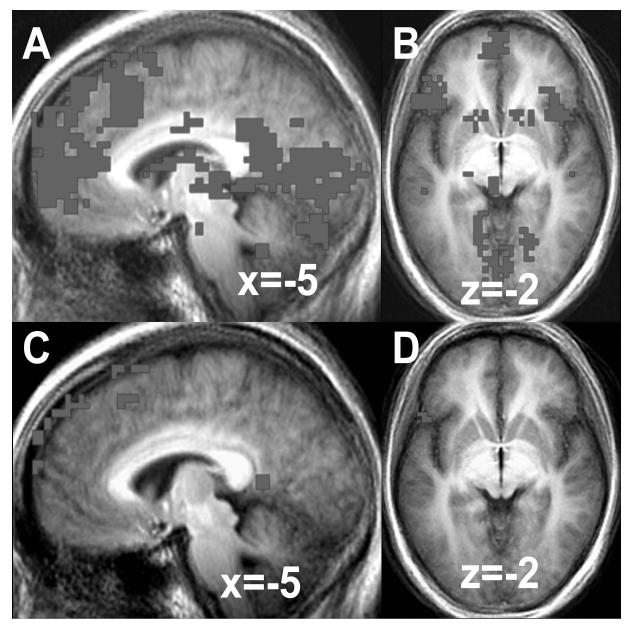
At baseline, a one-sample t test of the contrast of positive SRP versus positive case yielded greater BOLD responses for positive SRP in brain regions implicated in self-processing (medial and dorsomedial PFC, medial precuneus), language processing (left ventrolateral PFC, posterior superior temporal gyrus), memory (parahippocampal gyrus), and visual processing (fusiform, cuneus, lingual gyri) (Table 2 and Figure 3).
TABLE 2. Baseline Bold Responses: Positive Self > Case.
| Brain Regions | BA | x | y | za | % Signal Change |
Vol (voxels) | t Value |
|---|---|---|---|---|---|---|---|
| Positive self | |||||||
| Frontal cortex | |||||||
| Medial PFC 2 | 10 | 0 | 45 | 56 | .53 | 862 | 3.60 |
| R dorsolateral PFC 8 | 46 | 48 | 21 | 22 | .14 | 24 | 3.87 |
| Dorsomedial PFC | |||||||
| L inferior frontal gyrus 3 | 47 | −52 | 25 | −5 | .44 | 264 | 5.89 |
| R inferior frontal gyrus 5 | 47 | 48 | 25 | −12 | .26 | 132 | 3.43 |
| Middle cingulate cortex 14 | 23 | 0 | −13 | 32 | .16 | 12 | 3.42 |
| L middle frontal gyrus 16 | 6 | −38 | 11 | 50 | .11 | 8 | 3.63 |
| L middle frontal gyrus 17 | 6 | −45 | 11 | 46 | .15 | 7 | 3.47 |
| L middle frontal gyrus 17 | 6 | −48 | 4 | 53 | .30 | 7 | 3.58 |
| Temporal cortex | |||||||
| L posterior superior temporal gyrus 7 |
22 | −52 | −27 | 1 | .14 | 26 | 3.52 |
| R posterior superior temporal gyrus 11 |
22 | 48 | −27 | 5 | .13 | 15 | 3.39 |
| L anterior temporal pole 10 | 38 | −48 | 14 | −30 | .16 | 18 | 3.38 |
| R anterior temporal pole 21 | 38 | 41 | 21 | −30 | .14 | 4 | 3.83 |
| Parietal cortex | |||||||
| Medial precuneus 13 | 7 | 0 | −72 | 36 | .22 | 12 | 3.38 |
| Occipital cortex | |||||||
| Bilateral fusiform, lingual gyrus, declive 1 |
19 | 24 | −86 | −19 | .42 | 946 | 4.15 |
| L fusiform gyrus 6 | 19 | −38 | −75 | −16 | .20 | 36 | 3.95 |
| R cuneus 25 | 18 | 14 | −92 | 12 | .10 | 4 | 4.42 |
| Subcortical | |||||||
| Thalamus 4 | −3 | −13 | 15 | .26 | 241 | 4.47 | |
| Tonsil 9 | −3 | −51 | −30 | .12 | 23 | 4.15 | |
| L declive 12 | −45 | −65 | −19 | .22 | 14 | 3.30 | |
| R declive 15 | 34 | −61 | −19 | .18 | 9 | 3.73 | |
| R lentifom nucleus 20 | 17 | −6 | 8 | .07 | 5 | 3.40 | |
| L parahippocampal gyrus 24 | −21 | −20 | −9 | .14 | 4 | 3.48 | |
| Positive case: none |
Note. t value threshold ≥ 3.29, voxel p < .005, minimum cluster volume threshold ≥ 163 mm3 (4 voxels × 3.438 mm3), cluster p < .01. BA = Brodmann area; L = left; PFC = prefrontal cortex; R = right.
Talairach and Tournoux coordinates of maximum BOLD signal intensity voxel.
Figure 3.
Baseline self-referential processing BOLD responses for positive SRP in (A) medial prefrontal cortex and (B) ventrolateral prefrontal cortex and negative SRP in (C) medial prefrontal cortex and (D) ventrolateral prefrontal cortex.
For the baseline contrast of negative SRP versus negative case, a one-sample t test yielded greater BOLD responses for negative SRP in brain regions implicated in self-processing (medial and dorsomedial PFC, posterior cingulate), language processing (left ventrolateral PFC), affective processing (subgenual ACC), and visual processing (fusiform gyrus, lingual gyrus) (Table 3 and Figure 3C and D).
TABLE 3.
Baseline BOLD Responses: Negative Self > Case
| Brain Regions | BA | x | y | za | % Signal Change |
Vol (voxels) | t Value |
|---|---|---|---|---|---|---|---|
| Negative self | |||||||
| Frontal cortex | |||||||
| Medial PFC 3 | 9 | −10 | 62 | 32 | .49 | 529 | 3.56 |
| Medial PFC 9 | 10 | −3 | 66 | 19 | .29 | 244 | 3.98 |
| Dorsomedial PFC 7 | 6,8 | −3 | 18 | 63 | .51 | 285 | 3.37 |
| Dorsomedial PFC 5 | 8 | −7 | 28 | 60 | .42 | 326 | 4.22 |
| Dorsomedial PFC 2 | 8 | −7 | 52 | 46 | .55 | 1,547 | 3.67 |
| Dorsomedial PFC 10 | 8 | −3 | 25 | 50 | .31 | 204 | 3.36 |
| L inferior frontal gyrus 6 | 47 | −55 | 21 | −2 | .38 | 285 | 3.85 |
| Posterior cingulate 4 | 29 | −3 | −48 | 8 | .33 | 326 | 3.42 |
| R subgenual ACC 11 | 25 | 13 | 11 | −9 | .17 | 163 | 3.52 |
| Occipital Cortex | |||||||
| R fusiform gyrus 1 | 19 | 41 | −72 | −19 | .39 | 1,913 | 3.59 |
| R lingual gyrus 12 | 18 | 3 | −96 | −9 | .26 | 163 | 3.60 |
| Subcortical | |||||||
| Declive 8 | 7 | −82 | −19 | .26 | 244 | 3.47 | |
| Negative case: none |
Note. t value threshold ≥ 3.29, voxel p < .005, minimum cluster volume threshold ≥ 163 mm3 (4 voxels × 3.438 mm3), cluster p < .01. ACC = anterior cingulate cortex; BA = Brodmann area; L = left; PFC = prefrontal cortex.
Talairach and Tournoux coordinates of maximum BOLD signal intensity voxel.
Post-MBSR Neural Results
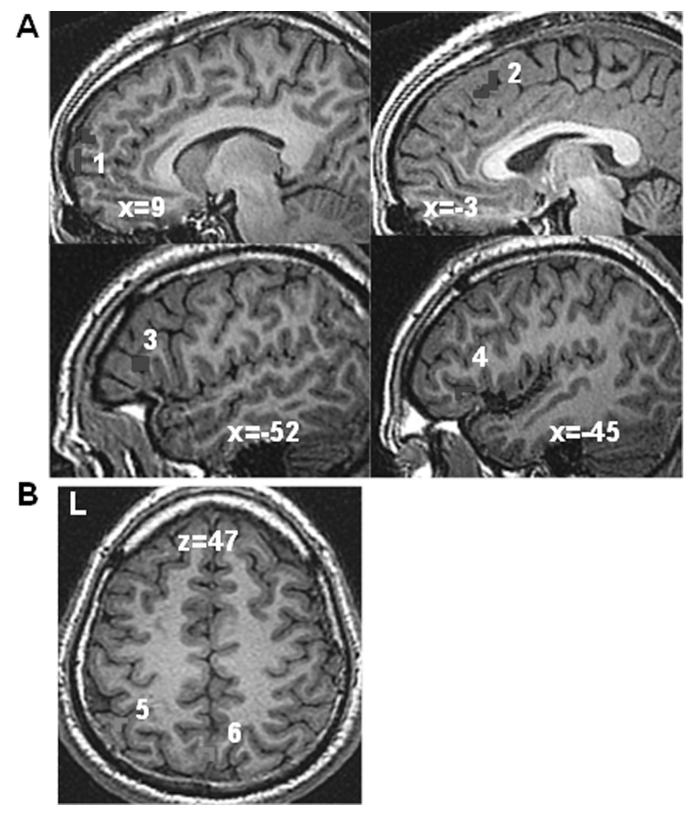
We conducted paired t tests to examine changes from pre- to post-MBSR for the contrast of positive SRP versus positive case and of negative SRP versus negative case. For positive SRP, there was evidence of decreased BOLD responses in brain regions related to self-processing (dorsomedial and medial PFC) and language processing (left inferior frontal gyrus) (Table 4 and Figure 4A). Negative SRP versus negative case processing resulted in increased brain responses in visual attention (left inferior parietal lobule and medial precuneus) (Table 4 and Figure 4B).
TABLE 4. Changes in BOLD Responses from Baseline to Post-MBSR.
| Brain Regions | BA | x | y | za | % Signal Change |
Vol (voxels) | t Value |
|---|---|---|---|---|---|---|---|
| Baseline > post-MBSR | |||||||
| Positive self | |||||||
| Frontal cortex | |||||||
| Medial PFC | 10 | 7 | 66 | 5 | −.33 | 7 | 3.83 |
| Medial PFC | 10 | 10 | 62 | 22 | −.22 | 4 | 3.35 |
| Dorsomedial PFC | 8 | −3 | 28 | 46 | −.35 | 6 | 3.23 |
| L inferior frontal gyrus | 47 | −45 | 25 | −2 | −.30 | 8 | 3.65 |
| L inferior frontal gyrus | 45 | −52 | 21 | 8 | −.23 | 5 | 4.13 |
| Subcortical | |||||||
| R uvula | 34 | −79 | −26 | −.32 | 5 | 3.65 | |
| Post-MBSR > baseline | |||||||
| Negative self | |||||||
| L inferior parietal lobule |
40 | −45 | −51 | 50 | .35 | 4 | 3.02 |
| Medial precuneus | 7 | 0 | −68 | 46 | .29 | 4 | 2.97 |
Note. t value threshold ≥ 3.21, voxel p < .005, minimum cluster volume threshold ≥ 163 mm3 (4 voxels × 3.438 mm3), cluster p < .01. BA = Brodmann area; L = left; PFC = prefrontal cortex.
Talairach and Tournoux coordinates of maximum BOLD signal intensity voxel.
Figure 4.
Changes in BOLD responses for self-referential processing from pre- to post-MBSR. (A) Decreased BOLD response during positive self-referential processing versus positive case—1 = medial PFC (coordinates of peak BOLD response: 7 66 5 and 10 62 22), 2 = dorsomedial PFC (-3 28 46), 3 = left ventrolateral PFC (-52 21 8), and 4 = left ventrolateral PFC (-45 25 -2)—and (B) increased BOLD response during negative self-referential processing—5 = left inferior parietal lobule (-45 -51 50), and 6 = medial precuneus (0 -68 46).
Discussion
The goal of this study was to investigate the effects of MBSR training on behavioral and neural bases of self-referential processing in patients with SAD. Mindfulness training was hypothesized to reduce symptoms of anxiety and depression, distorted social self-views, and activity in midline brain activations linked to a narrative/conceptual mode of self-referential processing.
Clinical Symptoms
MBSR resulted in moderate reduction of symptoms of social anxiety, depression, rumination, and state anxiety and increased self-esteem. These findings replicate previous findings in both healthy adults and clinical samples (Bishop, 2002) and preliminary evidence of clinical improvement in patients with SAD (Arana, 2006; Koszycki et al., 2007). While MBSR reduced social anxiety symptoms (Cohen, 1992), it did not match the effect of cognitive-behavioral therapy (cognitive restructuring plus exposure; d = 1.8) and pharmacological interventions (d = 1.5) on social anxiety symptoms (Fedoroff & Taylor, 2001). However, a direct comparison of the longer-term impact of these three clinical interventions for SAD has not been conducted.
SRP
MBSR resulted in decreased negative and increased positive self-views on the SRP task. This suggests that training in the mindfulness meditation skills may influence habitual distorted social self-views that are deeply entrenched in SAD. MBSR emphasizes cultivating an equanimous perspective toward mental and visceral experience. This perspective is described as a nonevaluative moment-to-moment awareness and a nonjudgmental metacognitive orientation.
Being able to shift from evaluative to nonevaluative awareness is a psychological skill that may be related to reductions in negative self-focused rumination. Decreased self-focused attention may account for the adaptive shift in social self-view in patients with SAD during MBSR. Changes in negative self-focused attention have been shown to vary as a function of social anxiety symptom reduction during from pre- to postexposure therapy (Hofmann, 2000). This pattern of results indicates that a modulation of both cognitive content (i.e., self-focused thoughts) and cognitive processes (i.e., attention and interpretative biases) may be core mechanisms underlying the effectiveness of psychosocial interventions for SAD.
Neural Bases of SRP
Baseline neural results demonstrate that both positive and negative SRP in patients with SAD yielded robust activation of self-processing midline cortical brain regions (Northoff et al., 2006) and language processing areas (Iacoboni & Wilson, 2006). The apparent greater BOLD response for positive SRP compared to negative SRP may be due to either greater arousal for the positive SRP and/or reactivity to the negative social traits during the case condition, thereby resulting in a smaller difference in BOLD response in the contrast of negative SRP versus case. The similar pattern during positive and negative SRP at baseline in patients with SAD indicates that they automatically rely on a specific form of self-focus that recruits brain systems related to a language-mediated conceptual-analytic mode of evaluation (Farb et al., 2007).
Mindfulness Effects on Neural Bases of SRP
Behavioral ratings (i.e., self-endorsement) and neural responses during negative and positive SRP changed in opposite directions from pre- to post-MBSR. Thus, patients with SAD endorsed fewer negative social traits yet showed increased neural responses during negative SRP in brain regions implicated in attentional allocation (left inferior parietal lobule and medial precuneus) (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005). This overlaps with findings from Farb and colleagues, who found evidence of increased sensory and attentional brain systems in adults with mixed anxiety and depression symptoms after MBSR for experiential versus narrative/conceptual self-focus (Farb et al., 2007). Enhanced activation of attentional networks during negative self-processing may be related to greater attentional engagement toward negative social traits, as habitual automatic avoidance diminishes with mindfulness training (Allen, Chambers, & Knight, 2006).
For positive SRP, MBSR resulted in increased self-endorsement of positive social traits and decreased neural responses linked to self-referential and language processing. Similar to the Farb et al. (2007) study, the observed changes in neural patterns indicate that MBSR resulted in a shift away from the narrative/conceptual self-focus observed robustly at baseline. This suggests that mindfulness training reduces the prepotent conceptual-linguistic mode of self-processing and thus generates the possibility of utilizing other modes of self-processing. There was not, however, evidence of increased viscerosomatic activation indicative of embodied experiential self-focus. This may be due to specific features of social anxiety, including an exaggerated habitual tendency to experience interoceptive somatic indicators of anxiety (Edelmann & Baker, 2002) that trigger negative self-perception and self-beliefs (Wells & Papageorgiou, 2001). Thus, unlike the increase in embodied experiential self-focus observed in healthy adults who completed MBSR in the Farb et al. study, decreased emphasis on viscerosomatic interoceptive processes may be clinically beneficial in patients with SAD.
One of the potential effects of MBSR is a reduction in the habitual tendency to engage in a narrative/conceptual mode of self-processing. In terms of neural processes, this suggests that the default narrative/conceptual mode may be reduced (ventromedial PFC, dorsomedial PFC, posterior cingulate/precuneus), while other types of self-processing that rely less on conceptual-linguistic-narrative modalities are more easily accessed. If the prepotent response in adults with SAD is enhanced negative self-view with concomitant diminished positive self-view, then a training that reduces the habit of distorted conceptual self-view could very well result in increased positive self-endorsement together with decreased neural activity in brain systems that support conceptual self-view.
Clinical Implications
These results provide initial support for a specific neural mechanism of change related to mindfulness training for social anxiety. Furthermore, this study provides brain–behavior evidence for the cognitive model of social anxiety, specifically, abnormal behavioral and neural patterns of self-processing in SAD. Importantly, MBSR resulted in changes in both positive and negative self-view, both of which have been shown to be aberrant in patients with SAD (Weeks, Heimberg, Rodebaugh, & Norton, 2007). Future clinical intervention studies might consider how baseline SRP may moderate treatment adherence and how changes in SRP may predict treatment outcome status or mediate changes in social anxiety symptom severity, emotional reactivity, and affective dysregulation.
Limitations and Directions for Future Research
This study is limited by the lack of a control group or active comparison clinical intervention that would help delineate more clearly how SRP is modified by MBSR. Future research with control groups will be necessary in order to address potential factors that might be contributing to changes in self-view, such as practice effects and habituation to the scanner environment. It may be instructive to compare the effects of different clinical interventions with different mechanisms of change (e.g., cognitive disputation, acceptance, attention training) on the neural bases of SRP.
There is a need for better behavioral and neural assessment of different modes of SRP, including narrative/conceptual, experiential, and other forms. This study examined only experimenter-selected positive and negative social trait adjectives. Patient-generated stimuli may result in more robust brain–behavior responses in patients with SAD. This would provide a more ecologically valid test of the effects of clinical interventions on SRP.
In the present study, it was not possible to examine temporal dynamics of participants’ responses because word stimuli were presented for only 3 seconds each. Use of longer stimulus durations may allow for examination of sustained cognitive processes (e.g., negative rumination) and differential temporal features of BOLD responses in SRP midline structures.
Finally, the MBSR course was offered by the primary investigator of this study, and thus the course instructor was not blind to the study outcome measures. Future studies will benefit from having different individuals serve as the research investigator and the course instructor.
Acknowledgments
This research was supported by an NCCAM grant (R21 AT003644-01) awarded to James Gross, PhD, as well as an NIMH Postdoctoral Fellowship and a Mind and Life Summer Research Institute grant awarded to Philippe Goldin, PhD.
REFERENCES
- Allen NB, Chambers R, Knight W. Mindfulness-based psychotherapies: a review of conceptual foundations, empirical evidence and practical considerations. Australian and New Zealand Journal of Psychiatry. 2006;40:285–294. doi: 10.1080/j.1440-1614.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- Arana D. The practice of mindfulness meditation to alleviate the symptoms of chronic shyness and social anxiety. Dissertation Abstracts International: Section B. The Sciences and Engineering. 2006;67:2822. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory—Second edition manual. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bishop SR. What do we really know about mindfulness-based stress reduction? Psychosomatic Medicine. 2002;64:71–84. doi: 10.1097/00006842-200201000-00010. [DOI] [PubMed] [Google Scholar]
- Bogels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: Hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24:827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Bogels SM, Sijbers MA, Voncken M. Mindfulness and task concentration training for social phobia: A pilot study. Journal of Cognitive Psychotherapy. 2006;20:33–44. [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Technical manual and affective ratings. Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1999. [Google Scholar]
- Clark DM, McManus F. Information processing in social phobia. Biological Psychiatry. 2002;51:92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. Guilford Press; New York: 1995. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Derry PA, Kuiper NA. Schematic processing and self-reference in clinical depression. Journal of Abnormal Psychology. 1981;90:286–297. doi: 10.1037//0021-843x.90.4.286. [DOI] [PubMed] [Google Scholar]
- DiNardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime version (ADIS-IV-L) Oxford University Press; New York: 1994. [Google Scholar]
- Edelmann RJ, Baker SR. Self-reported and actual physiological responses in social phobia. British Journal of Clinical Psychology. 2002;41:1–14. doi: 10.1348/014466502163732. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, et al. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff IC, Taylor S. Psychological and pharmacological treatments of social phobia: A meta-analysis. Journal of Clinical Psychopharmacology. 2001;21:311–324. doi: 10.1097/00004714-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. In search of the emotional self: An FMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG. Self-focused attention before and after treatment of social phobia. Behavioral Research and Therapy. 2000;38:717–725. doi: 10.1016/s0005-7967(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Wilson SM. Beyond a single area: Motor control and language within a neural architecture encompassing Broca’s area. Cortex. 2006;42:503–506. doi: 10.1016/s0010-9452(08)70387-3. [DOI] [PubMed] [Google Scholar]
- Jefferys D. Social phobia: The most common anxiety disorder. Australian Family Physician. 1997;26:1061, 1064–1067. [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Dell; New York: 1990. [Google Scholar]
- Kelley WM, Macrae CL, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17:1080–1086. [PubMed] [Google Scholar]
- Koszycki D, Benger M, Shlik J, Bradwejn J. Randomized trial of a meditation-based stress reduction program and cognitive behavior therapy in generalized social anxiety disorder. Behavior Research and Therapy. 2007;45:2518–2526. doi: 10.1016/j.brat.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Lampe L, Slade T, Issakidis C, Andrews G. Social phobia in the Australian National Survey of Mental Health and Well-Being (NSMHWB) Psychological Medicine. 2003;33:637–646. doi: 10.1017/s0033291703007621. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems in Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Lochner C, Mogotsi M, du Toit PL, Kaminer D, Niehaus DJ, Stein DJ. Quality of life in anxiety disorders: A comparison of obsessive-compulsive disorder, social anxiety disorder, and panic disorder. Psychopathology. 2003;36:255–262. doi: 10.1159/000073451. [DOI] [PubMed] [Google Scholar]
- Matza LS, Revicki DA, Davidson JR, Stewart JW. Depression with atypical features in the National Comorbidity Survey: Classification, description, and consequences. Archives of General Psychiatry. 2003;60:817–826. doi: 10.1001/archpsyc.60.8.817. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Otto MW, Pollack MH, Maki KM, Gould RA, Worthington JJ, III, Smoller JW, et al. Childhood history of anxiety disorders among adults with social phobia: Rates, correlates, and comparisons with patients with panic disorder. Depression and Anxiety. 2001;14:209–213. doi: 10.1002/da.1068. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: A trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. Neuroimage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Carmona PE, McQuaid JR. The effects of mindfulness meditation on cognitive processes and affect in patients with past depression. Cognitive Therapy and Research. 2004;28:433. [Google Scholar]
- Randall CL, Thomas S, Thevos AK. Concurrent alcoholism and social anxiety disorder: A first step toward developing effective treatments. Alcoholism, Clinical and Experimental Research. 2001;25:210–220. [PubMed] [Google Scholar]
- Rapee RM. Descriptive psychopathology of social phobia. Guilford Press; New York: 1995. [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behavioral Research and Therapy. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the adolescent self-image. Princeton University Press; Princeton, NJ: 1965. [Google Scholar]
- Schneier FR, Heckelman LR, Garfinkel R, Campeas R, Fallon BA, Gitow A, et al. Functional impairment in social phobia. Journal of Clinical Psychiatry. 1994;55:322–331. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1970. [Google Scholar]
- Spurr JM, Stopa L. Self-focused attention in social phobia and social anxiety. Clinical Psychology Review. 2002;22:947–975. doi: 10.1016/s0272-7358(02)00107-1. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Thieme; New York: 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Teasdale JT, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Farvolden P. The impact of anxiety disorders on educational achievement. Journal of Anxiety Disorders. 2003;17:561–571. doi: 10.1016/s0887-6185(02)00228-1. [DOI] [PubMed] [Google Scholar]
- Weeks JW, Heimberg RG, Rodebaugh TL, Norton PJ. Exploring the relationship between fear of positive evaluation and social anxiety. Journal of Anxiety Disorders. 2007;22:386–400. doi: 10.1016/j.janxdis.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Wells A, Papageorgiou C. Social phobic interoception: Effects of bodily information on anxiety, beliefs and self-processing. Behavioral Research and Therapy. 2001;39:1–11. doi: 10.1016/s0005-7967(99)00146-1. [DOI] [PubMed] [Google Scholar]