Abstract
Objective
To determine and compare outcomes with accepted benchmarks in burn care at six academic burn centers.
Background
Since the 1960s, U.S. morbidity and mortality rates have declined tremendously for burn patients, likely related to improvements in surgical and critical care treatment. We describe the baseline patient characteristics and well-defined outcomes for major burn injuries.
Methods
We followed 300 adults and 241 children from 2003–2009 through hospitalization using standard operating procedures developed at study onset. We created an extensive database on patient and injury characteristics, anatomic and physiological derangement, clinical treatment, and outcomes. These data were compared with existing benchmarks in burn care.
Results
Study patients were critically injured as demonstrated by mean %TBSA (41.2±18.3 for adults and 57.8±18.2 for children) and presence of inhalation injury in 38% of the adults and 54.8% of the children. Mortality in adults was 14.1% for those less than 55 years old and 38.5% for those age ≥55 years. Mortality in patients less than 17 years old was 7.9%. Overall, the multiple organ failure rate was 27%. When controlling for age and %TBSA, presence of inhalation injury was not significant.
Conclusions
This study provides the current benchmark for major burn patients. Mortality rates, notwithstanding significant % TBSA and presence of inhalation injury, have significantly declined compared to previous benchmarks. Modern day surgical and medically intensive management has markedly improved to the point where we can expect patients less than 55 years old with severe burn injuries and inhalation injury to survive these devastating conditions.
INTRODUCTION
Burn injuries accounted for an estimated 603,000 visits to U.S. emergency departments in 20101 and an estimated 50,000 hospital admissions.2 In those patients hospitalized for burn injury, the cost of fire and non-fire burn injuries totaled $1 billion.3 Survival from extensive burn injuries today is much higher than seen in the U.S. during the 1960s.4,5 At that time, it was common for patients with burns > 20% total body surface area (TBSA) to die early from the initial cutaneous burn injury, or later from infections or other complications related to the injury. With advances in surgical and critical care management and higher likelihood of treatment in centers specializing in burn care, survival after serious and life threatening burn injury has improved during the 1970’s and 80’s.6 Major burns like other significant traumatic injuries are complex to treat and often result in acute physiologic and metabolic derangements. Effective fluid resuscitation, control of infection and organ dysfunction, and management of complications are important tools to extenuate the life-threatening conditions associated with severe burn injury. This study was designed to collect outcomes data from patients with significant burn injury (burns over 20% TBSA) requiring operative treatment. It provides a homogenous, critically injured population in which to describe demographic injury and patient characteristics for major burn injuries, and to identify important clinical outcomes, including multiple organ failure (MOF), infectious complications, and death.
In burn injury studies, it is common to report relatively small patient populations or historical trends within a single institution that may have been influenced by changes in clinical burn care over time. The few multi-center studies available do provide analyses of larger numbers of patients; however, generalizing from these studies may be difficult because the data are collected under various local care protocols or no protocols at all, with little to no uniform categorization with regard to definitions for diagnosis and outcomes.7 Furthermore, burn injury databases used for clinical research typically have not been rich in physiologic data or outcomes beyond survival or death.
The Inflammation and Host Response to Injury is one of the National Institutes of Health – National Institute of General Medical Sciences large-scale, collaborative research projects (“Glue Grants”), which was funded to study the innate immune and metabolic to serious injury. By study design, we enrolled patients who sustained major burn injuries. Standard operating procedures (SOPs) were established and implemented to promote uniform high quality care in a number of clinically important areas.8 In this report, we provide clinical insight into the hospital course, compliance with SOPs, and patient outcomes in major acute burn injury patients admitted to U.S. burn centers. We compare this severely injured cohort to the current standards and benchmarks. Most importantly, the clinical physiologic, pathophysiologic, and outcomes data from this study are publicly accessible at http://www.gluegrant.org/ for other investigators to pursue hypotheses, correlations, or interventions of burn patient outcomes and recovery from major burn injury.
METHODS
Study Design
In this observational, prospective study conducted from May, 2003 to February, 2010 at six U.S. burn centers, patients were enrolled if they had thermal burns ≥ 20% TBSA, required operative treatment, and arrived at the participating burn center within 96 hours of injury. Patients with do-not-resuscitate orders on admission were not eligible for the study. See Supplemental Table 1 for the inclusion and exclusion criteria. SOPs for patient treatment were developed through a series of literature reviews and comprehensive, quarterly face-to-face meetings of the Glue Grant investigators.
Definition of Outcomes and Data Collection
In this study, we evaluated the relationship between baseline patient characteristics and injury severity with mortality. Eligibility screening was performed for all patients admitted to the participating burn centers. Demographic, clinical, physiological, pathophysiological, and outcomes data were abstracted through the hospitalization for burn injury and uploaded to an adaptation of the TrialDB9 web-based clinical data management system. The study investigators, relying on existing standards wherever possible, defined clinical data definitions prospectively. Trained data abstractors with a health care background participated in face-to-face meetings and conference calls to ensure uniformity of data abstraction across the centers. A dedicated data processing team was responsible for data management, curation, and storage. A clinical data manager performed ongoing quality monitoring to ensure data completeness and accuracy, which included computer-generated data queries submitted to the abstractors in the case of internal inconsistencies, and missing or implausible values. A 5% random sample of subject records was selected for review during each clinical site visit; the site visit team reviewed the records for study eligibility, cause of death (if applicable), and the presence or absence of infections, and other clinical complications. The Institutional Review Board of each participating center reviewed and approved the study.
Organ dysfunction and hospital mortality were analyzed as outcomes measures in this study. Organ dysfunction assessment was performed using a widely accepted post-injury multiple organ failure (MOF) score, the Denver MOF score10,11 (Supplemental Table 2). MOF was defined as a Denver MOF score ≥ 4.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) were defined according to the European-American consensus conference.12 Other non-infectious and infectious complications were assessed by objective criteria using standardized definitions. Definitions of nosocomial infections13 and surgical site infections14 were those definitions used by the Centers for Disease Control adapted such that with the exception of superficial surgical site infections, all infectious complications required the presence of a positive microbial culture.
Diagnosis of inhalation injury was determined by the standard practice of the participating institution (either by clinical history/physical examination or bronchoscopy). The diagnosis of pneumonia was based on quantitative culture when available or sputum samples with counts > 3+ of a single organism with radiographic evidence of pneumonia and leukocytosis.
Time to Recovery
A primary objective of this approach was to identify any organ dysfunction patterns and to identify time to recovery (TTR) using the Denver MOF score as a valid measure of morbidity. Organ recovery is relatively straightforward to identify when a failing organ requires support, but less intuitive for more subtle degrees of organ dysfunction. We considered a patient recovered when all organ failure component scores were zero.
Statistical Analyses
We compared injury characteristics and outcomes of patients enrolled in the Glue Grant study with a similar cohort of patients from the National Burn Repository (NBR), the largest database of burn-injured patients in the United States.15,16 These patients, excluding those from the Glue Grant clinical sites, sustained burn injuries during the period 2001–2009. Of the 150,709 inpatient records in the NBR database, we eliminated those records that did not match our inclusion and exclusion criteria (Supplemental Table 1). We excluded all patients with chemical and electrical burns or unknown etiology. Glue Grant patients were matched with the remaining 4,685 patients in the NBR based on the following criteria: inhalation injury (present or absent); age category (0–17, 18–54, and older than 55 years) and total body surface area burned category (20–39%, 40–59%, and ≥ 60%). A stratified logistic regression was performed with the resulting 4,640 patients to determine the difference in mortality risk between patients in the Glue study and patients in the NBR.
A previously published model6 for estimating the probability of death from burns was used to compare observed and predicted deaths using Flora’s Z-statistic.17 The probability of death was estimated logit = −5.89 + 2.58n where n is the number of risk factors (burn size of >40% TBSA, age of >60 years, or presence of inhalation injury) and logit is the natural logarithm of the ratio of the probability of dying to the probability of living. Logistic regression was used to compare mortality in our cohort with a subset of the NBR. Differences in baseline covariates between survivors and non-survivors for adults and children were assessed using a Wilcoxon test or Fisher’s exact test as appropriate. All analysis was done in SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA) and two sided p-value less than or equal to 0.05 were considered to be evidence of statistical significance.
RESULTS
Study Population
Five hundred and forty-one patients (300 adults and 241 children) were enrolled in the study. Table 1 shows the baseline and injury characteristics, resuscitation data, outcomes, and complications for the adults (age ≥17 years). In general, adult survivors were younger with smaller burn sizes. When comparing the two adult age categories, the mortality rate in those patients ≥55 years of age was greater (38.5 vs. 14.1%, p=0.0001). In these adults, survival was not affected by the sex, race, ethnicity, number of comorbidities, or mechanism of injury. Initial base deficit, lactate, and APACHE II scores were all significantly lower for those who survived. Fluid resuscitation volumes were greater than 6 ml/kg/% TBSA and comparable between survivors and non-survivors. Even at discharge, the adult patients remained seriously debilitated in that only 52% went directly home. Complications including pneumonia (47%), sepsis (10%), and nosocomial infections (70.7%) were common in these patients who experienced extended lengths of stay and numerous ventilator days. Presence of inhalation injury was associated with mortality (p=0.0023) in this univariate analysis.
Table 1.
Adult Patient Characteristics
| Baseline and Injury Characteristics | ||||
|---|---|---|---|---|
| Total n=300 |
Alive n=245 (81.7) |
Dead n=55 (18.3) |
Probability | |
| Age | 41.4±15.9 | 39.5±14.7 | 50.0±18.1 | 0.0001 |
| 17–55 | 248 | 213 (86.9) | 35 (63.6) | 0.0001 |
| ≥55 | 52 | 32 (13.1) | 20 (36.4) | |
| Sex | ||||
| Male | 225 | 186 (75.9) | 39 (70.9) | 0.491 |
| Female | 75 | 59 (24.1) | 16 (29.1) | |
| Race | ||||
| African American | 39 (13.0) | 30 (12.2) | 9 (16.4) | |
| Asian | 10 (3.3) | 10 (4.08) | 0 (0) | |
| Caucasian | 223 (74.3) | 184 (75.1) | 39 (70.9) | |
| Native American | 5 (1.7) | 4 (1.63) | 1 (1.8) | 0.396 |
| Pacific Islander | 1 (0.3) | 1 (0.41) | 0 (0) | |
| Other | 20 (6.7) | 15 (6.12) | 5 (9.1) | |
| Unknown | 2 (0.7) | 1 (0.41) | 1 (1.82) | |
| Hispanic Ethnicity | 46 (15.3) | 34 (13.9) | 12 (21.8) | 0.306 |
| Comorbidities | ||||
| None | 74 (24.7) | 62 (25.3) | 12 (21.8) | |
| 1 | 78 (26.0) | 64 (26.1) | 14 (25.5) | 0.838 |
| 2 or more | 148 (49.3) | 119 (48.6) | 29 (52.7) | |
| Burn Mechanism | ||||
| Flame | 250 (83.3) | 200 (81.6) | 50 (90.9) | |
| Flash | 21 (7.0) | 19 (7.8) | 2 (3.6) | 0.450 |
| Scald | 16 (5.3) | 15 (6.1) | 1 (1.8) | |
| Other | 13 (4.3) | 11 (4.5) | 2 (3.6) | |
| Burn Size | ||||
| % TBSA | 41.2±18.3 | 37.1±14.6 | 59.6±21.7 | <0.0001 |
| % Full Thickness | 31.3±19.3 | 27.4±15.5 | 48.3±24.3 | <0.0001 |
| Inhalation Injury Present | 114 (38.0) | 82 (33.5) | 32 (58.2) | 0.0023 |
| Resuscitation Data | ||||
| Initial Base Deficit | −5.1±4.9 | −4.6±4.3 | −7.0±6.4 | 0.0092 |
| Initial Lactate | 3.6±2.4 | 3.3±2.3 | 4.3±2.5 | 0.0253 |
| APACHE II Score | 20.8±9.1 | 19.4±9.0 | 27.0±6.5 | <0.0001 |
| Total Fluids* | 6.3±2.7 | 6.3±2.7 | 6.4±2.8 | 0.7563 |
| Total Crystalloid* | 6.2±2.7 | 6.2±2.7 | 6.1±2.8 | 0.7264 |
| Total 5% Albumin* | 0.04±0.1 | 0.04±0.1 | 0.05±0.2 | 0.7802 |
| Patient Outcomes and Complications | ||||
| Disposition Status | ||||
| Home | 156 (52.0) | |||
| Skilled Nursing/ Residential Facility | 24 (8.1) | |||
| Another Acute Care Facility | 10 (3.3) | |||
| Inpatient Rehabilitation Facility | 106 (35.4) | |||
| Other | 4 (1.2) | |||
| Length of Stay (days) † | 51.8±48.1 | |||
| LOS/%TBSA | 1.36±1.08 | |||
| LOS/% full thickness | 3.04±4.86 | |||
| Ventilator Days | 18.7±24.2 | |||
| Deep Vein Thrombosis | 10 (3.3) | |||
| Pulmonary Embolus | 7 (2.3) | |||
| Sepsis | 31 (10.3) | |||
| Nosocomial Infections | 212 (70.7) | |||
| Blood Stream Infection | 85 (28.3) | |||
| Pneumonia | 143 (47.7) | |||
| Burn Wound Infection | 157 (52.3) | |||
Values represent mean ± SD or N(%). Data were analyzed by Wilcoxon Signed Rank Test, Chi-Square Test, or Fisher’s Exact Test as appropriate.
Total over first 24 hours post injury including pre-hospital and referral hospital information (cc/kg/TBSA)
Length of stay (LOS) in days of survivors only
Table 2 shows the baseline and injury characteristics, resuscitation data, outcomes, and complications for children (age <17 years). There was a trend with females having a higher mortality rate (12.8 % vs. 5.5%, p=0.0712). There was a strong statistical difference in mortality related to race (p=0.0052). Ethnicity, comorbidities, and burn mechanism did not influence the likelihood of survival. Initial base deficit, lactate, and APACHE II scores were lower in those children who survived. As with the adults, average resuscitation fluid volumes exceeded 4 ml/kg/% TBSA and the presence of inhalation injury was associated with mortality in children (p=0.008).
Table 2.
Pediatric Patient Characteristics
| Baseline and Injury Characteristics | ||||
|---|---|---|---|---|
| Total n=241 |
Alive n=222 (92.1) |
Dead n=19 (7.9) |
Probability | |
| Age | 6.7±5.4 | 6.6±5.4 | 8.3±6.0 | 0.1788 |
| Sex | ||||
| Male | 163 (67.6) | 154 (69.4) | 9 (47.4) | 0.0712 |
| Female | 78 (32.4) | 68 (30.6) | 10 (52.6) | |
| Race | ||||
| African American | 12 (5.0) | 10 (4.5) | 2 (10.5) | |
| Asian | 0 | 0 (0) | 0 (0) | |
| Caucasian | 109 (45.2) | 106 (47.8) | 3 (15.8) | |
| Native American | 10 (4.2) | 9 (4.1) | 1 (5.3) | 0.0052 |
| Pacific Islander | 0 | 0 (0) | 0 (0) | |
| Other | 109 (45.2) | 97 (43.7) | 12 (63.2) | |
| Unknown | 1 (0.4) | 0 (0) | 1 (5.3) | |
| Hispanic Ethnicity | 189 (78.4) | 174 (78.4) | 15 (79.0) | 0.147 |
| Comorbidities | ||||
| None | 227 (94.2) | 208 (93.7) | 19 (100) | 1.0 |
| 1 | 11 (4.6) | 11 (5.0) | 0 (0) | |
| 2 or more | 3 (1.2) | 3 (1.4) | 0 (0) | |
| Burn Mechanism | ||||
| Flame | 183 (75.9) | 165 (74.3) | 18 (94.7) | |
| Flash | 4 (1.7) | 4 (1.8) | 0 (0) | 0.325 |
| Scald | 48 (19.9) | 47 (21.2) | 1 (5.3) | |
| Other | 6 (2.5) | 6 (2.7) | 0 (0) | |
| Burn Size | ||||
| % TBSA | 57.8±18.2 | 56.1±17.3 | 77.5±16.7 | <0.0001 |
| % Full Thickness | 48.0±24.1 | 45.8±23.2 | 70.4±22.9 | <0.0001 |
| Inhalation Injury Present | 132 (54.8) | 116 (52.3) | 16 (84.2) | 0.008 |
| Resuscitation Data | ||||
| Initial Base Deficit | −6.4±5.1 | −6.2±4.8 | −9.6±7.3 | 0.0048 |
| Initial Lactate | 2.6±2.6 | 2.3±2.3 | 4.3±3.8 | 0.0038 |
| APACHE II Score | 15.1±9.2 | 14.4±8.5 | 24.9±12.2 | 0.0012 |
| Total Fluids* | 6.6±3.7 | 6.7±3.7 | 5.6±3.8 | 0.3891 |
| Total Crystalloid* | 6.8+4.4 | 6.9±4.5 | 5.5±3.4 | 0.4103 |
| Total 5% Albumin* | 0.06±0.2 | 0.06±0.2 | 0.12±0.1 | 0.0490 |
| Patient Outcomes and Complications | ||||
| Disposition Status | ||||
| Home | 5 (2.2) | |||
| Skilled Nursing/ Residential Facility | 18 (7.6) | |||
| Another Acute Care Facility | 12 (5.0) | |||
| Inpatient Rehabilitation Facility | 196 (81.1) | |||
| Other | 10 (4.1) | |||
| Length of Stay (days) † | 32.3±25.1 | |||
| LOS/%TBSA | 0.55±0.33 | |||
| LOS/% full thickness | 1.03±1.31 | |||
| Ventilator Days | 8.6±14.1 | |||
| Deep Venous Thrombosis | 6 (2.5) | |||
| Pulmonary Embolus | 0 | |||
| Sepsis | 7 (2.9) | |||
| Nosocomial Infections | 163 (67.6) | |||
| Blood Stream Infection | 38 (15.8) | |||
| Pneumonia | 25 (10.4) | |||
| Burn Wound Infection | 226 (93.8) | |||
Values represent mean ± SD or N(%). Data were analyzed by Wilcoxon Signed Rank Test, Chi-Square Test, or Fisher’s Exact Test as appropriate.
Total over first 24 hours post injury including pre-hospital and referral hospital information (cc/kg/TBSA)
Length of stay (LOS) in days of survivors only
As expected, there were multiple differences observed when comparing children and adults. When compared to adults, mortality (7.9% in children vs. 18.3% in adults, frequency of complications, and frequency of comorbidities were all substantially lower despite a larger average burn size for the children 57.8±18.2 versus 41.2±18.3 % TBSA. Compared with adults, children had a shorter length of stay (32.2±25.1 vs. 51.8±48.1 days) and fewer ventilator days (8.6±14.1 vs. 18.7±24.2). The volumes of resuscitation fluid on a 1-ml/kg/%TBSA basis were comparable between children and adults and were not related to the mortality rates (Tables 1 and 2).
SOP Compliance
Regarding minimal urinary output, the fluid resuscitation SOP requires that average urinary output be ≥0.3 ml/kg/h. To be compliant, patients with urinary outputs of <0.3 ml/kg/h should have received ≥2 ml/kg/% TBSA over the first 24 hours. Compliance was excellent in that 97% of patients met the protocol criterion for both the first and second 24 hour time periods. However, compliance with the requirement of total fluid resuscitation volumes of 2–4 ml/kg/% TBSA was poor. Patients received more than the recommended fluid volumes for resuscitation with 46% of patients receiving >4 ml/kg/% TBSA over the first 24 hours. These compliance values remained stable over the course of the study.
Only 1% of the patients had a recorded MAP ≤60 mmHg at any time during the first 24 hours post-injury in the settings of the pre-hospital setting, emergency department(s), transferring facility, or in the participating burn center.
With respect to the protocol for prevention of hyperglycemia, blood glucose levels could have been on-target (<180 mg/dl) or off-target (≥180 mg/dl) if the patient was receiving insulin. Approximately 22.9% of the patients had recorded blood glucose values off-target (≥180 mg/dl) and were not receiving insulin. Compliance with this SOP did not appear to change over the course of the study.
Comparative Outcomes
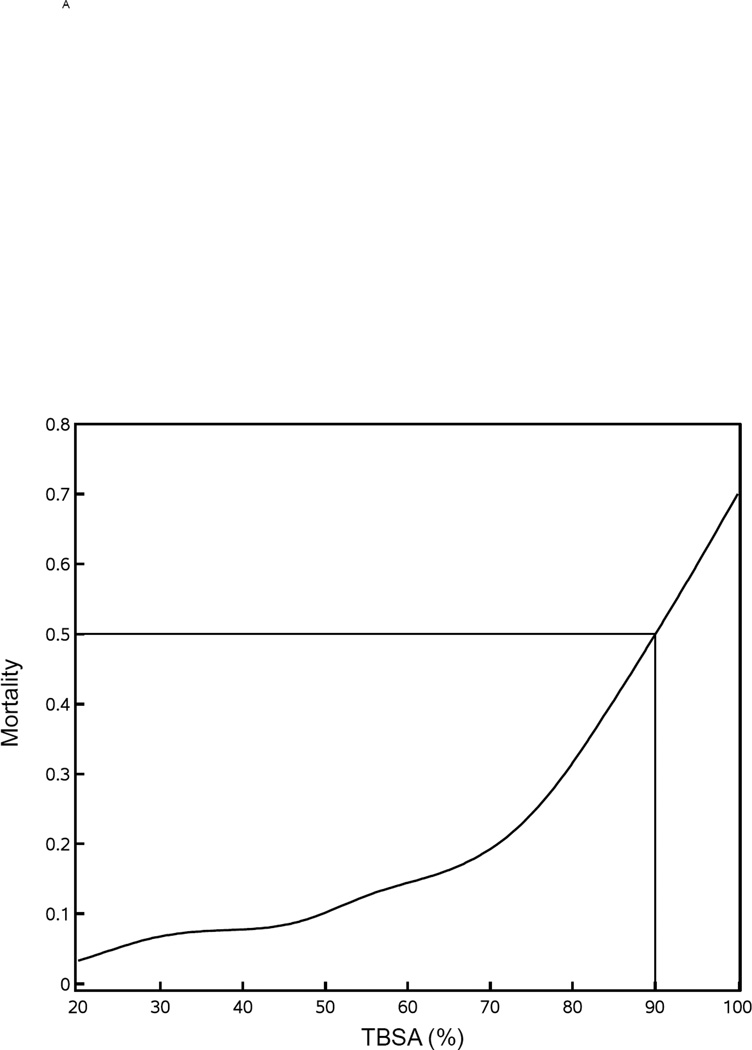
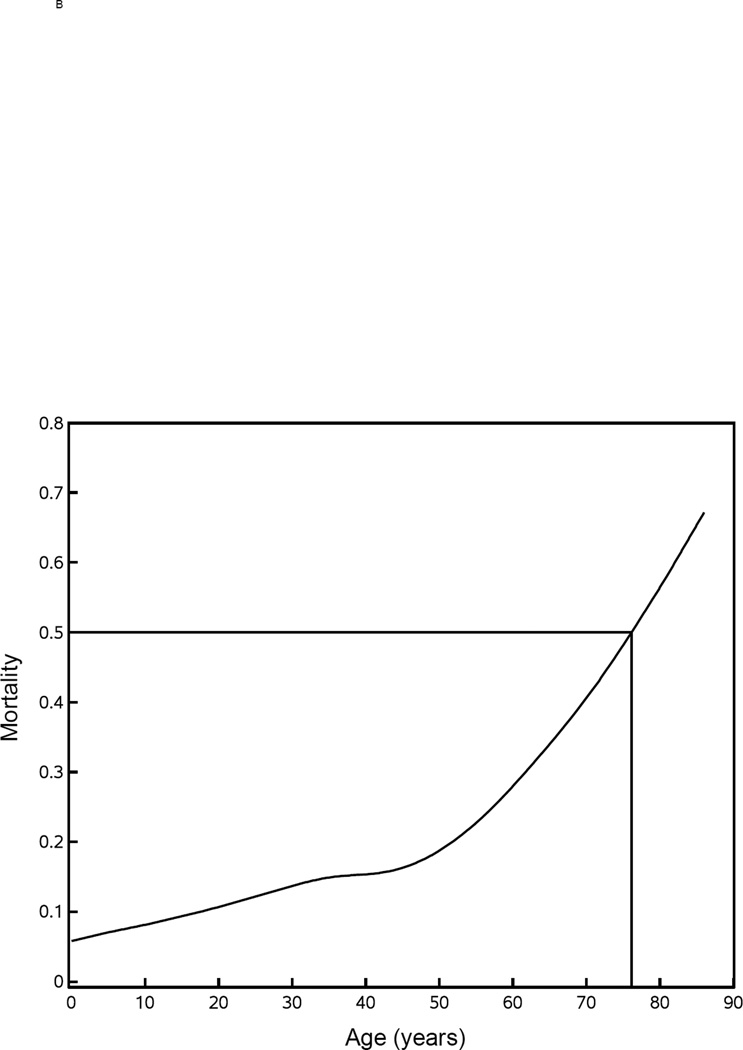
It has been well described that three factors strongly affect the probability of death from burn injuries – age, overall burn size, and presence of inhalation injury. Figure 1A shows a gradual, monotonic effect of age up to approximately 50 years with an inflection point between 50 and 60 indicating that the affect of age becomes more dominant. Similarly, Figure 1B shows a gradual, monotonic effect of burn size of 20% up to approximately 70% TBSA beyond which burn size appears to provide an even stronger effect. With respect to burn size, the LD50 (Lethal Dose 50, burn size with a lethality of 50% of patients) is approximately 90% TBSA. When the adult and pediatric databases were combined, mortality was 19.5% for those with inhalation injury and 8.9% for those without. This difference in mortality has a calculated p<0.0001 that is unadjusted for potential imbalances other than inhalation injury.
Figure 1. Mortality versus age and % TBSA.
Mortality rates were plotted versus age (panel A) and % TBSA (panel B). The Lethal Dose 50 (LD50) is shown for each.
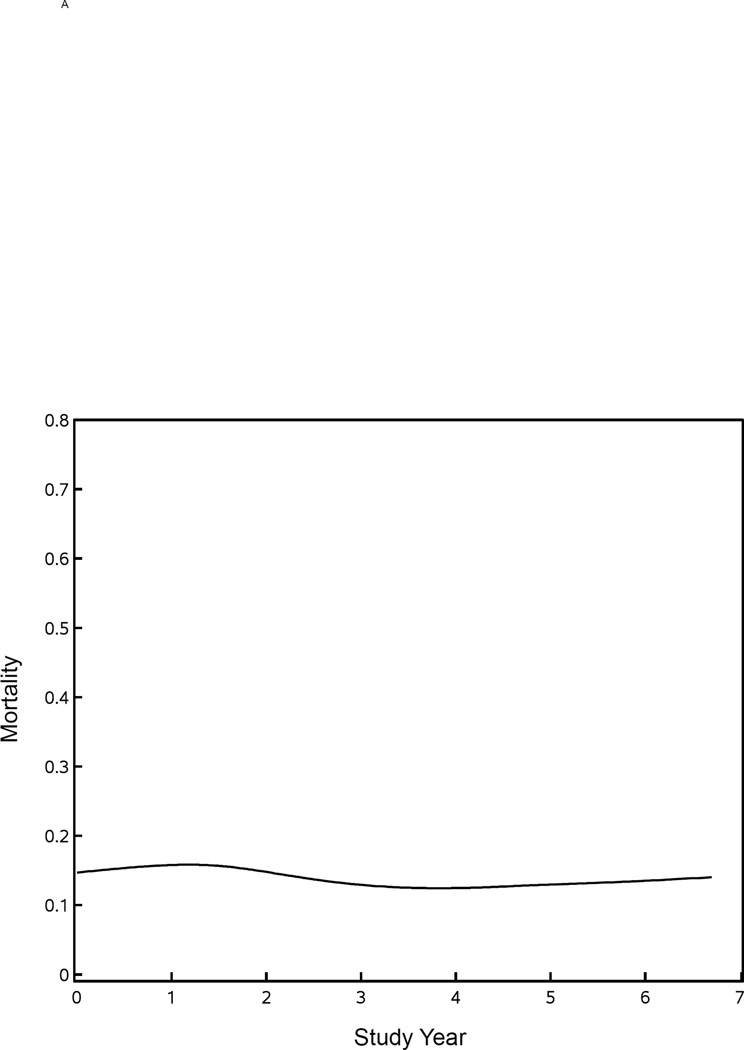
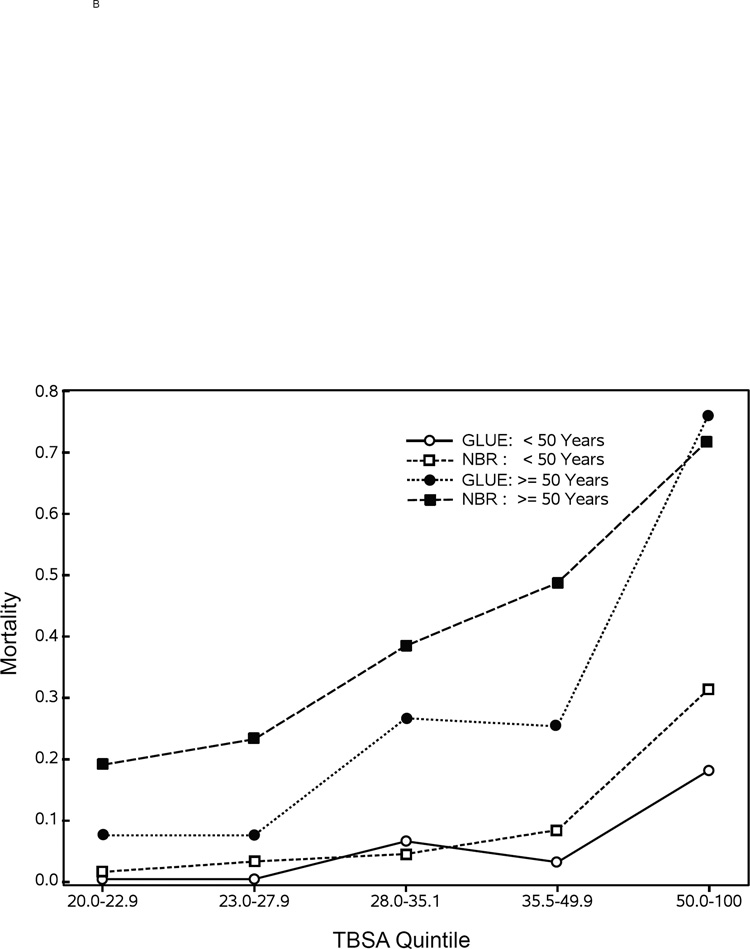
Figure 2A shows an invariant mortality rate of approximately 14% over the course of our seven-year study. Figure 2B shows the comparison of mortality in TBSA quintiles of burn size for patients below versus above the age of 50 years in the NBR versus the current study, which suggests that there might be survival improvements in patients <50 years old with larger burns. In patients ≥50 years old, there might be survival improvements in the smaller burns, but there does not appear to be any survival differences in older patients with very large burn injuries.
Figure 2. Mortality for Glue Grant study patients compared with the NBR.
Panel A shows the mortality rate by year for those patients in the Glue Grant study. Comparisons of mortality rates by TBSA quintile and age group (<50 years old versus ≥50 years old) for those patients in the Glue Grant with those in the NBR are shown in panel B.
In a comparison of the data in the current study versus patients in the NBR after excluding those with burn sizes <20% TBSA, a logistic regression stratified on age, burn size, and presence of inhalation injury, was performed. Following these adjustments, there was a lower risk of mortality for patients in our current study [OR=0.71 (95% CI: 0.53–0.97), p=0.03]. An analysis of the mortality rate in the Glue cohort alone was based upon a model that predicts expected mortality rate in patients admitted to the Massachusetts General Hospital and Shriners Hospitals for Children – Boston from 1990–1994.6 The result of this model was highly consistent with the outcomes in the current study and predicted that 74·09 deaths would occur versus the 74 deaths that actually occurred (Flora’s score Z=−0.01, p=0.9895).
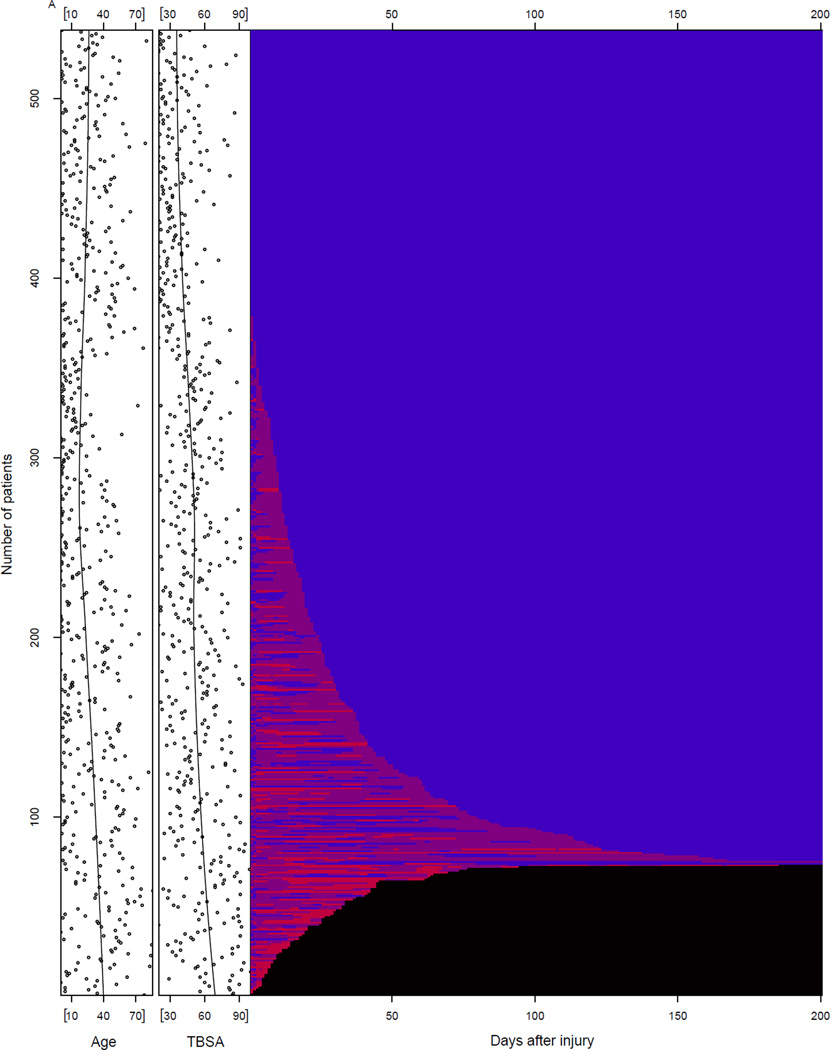
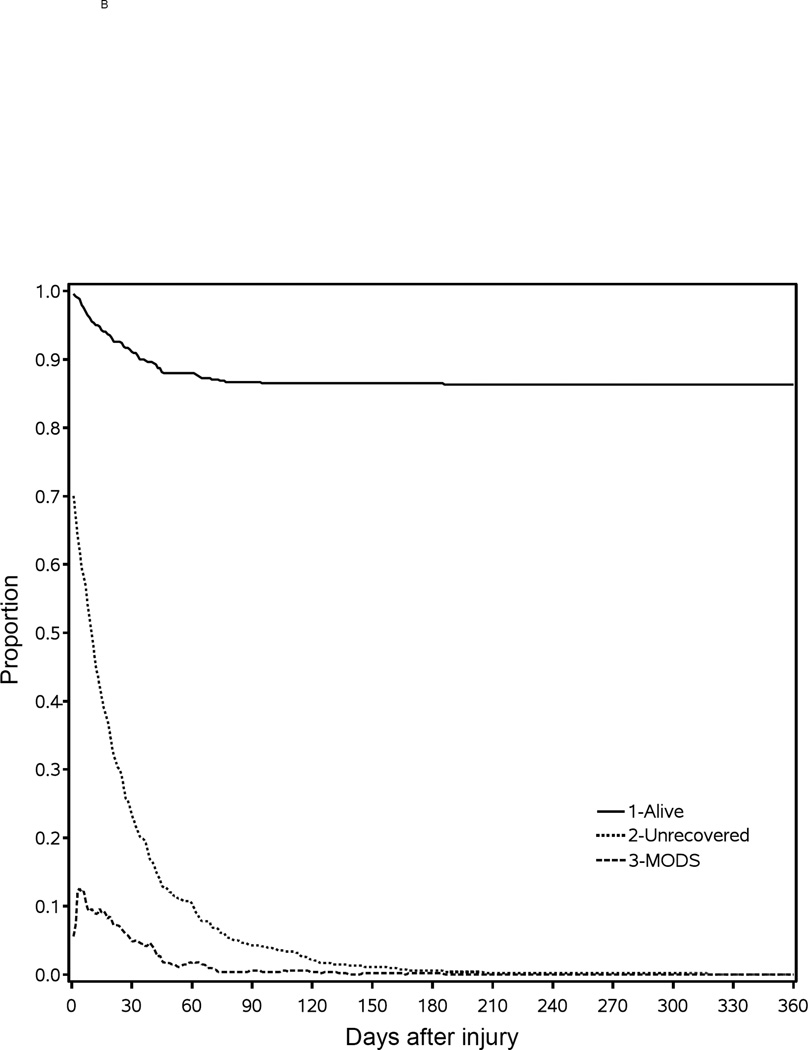
Figure 3A shows four groups of patients categorized by Denver MOF score: 0 (blue), 1–3 (purple), >=4 (light red), and non-survival (black). Note that approximately 30% of the patients demonstrated a score of 0 for all components of the Denver score throughout their entire hospital course. Most patients showed waxing and waning of component Denver scores with occasional scores ≥4, consistent with MOF, for periods up to 180 days post-injury. Deaths occurred soon after admission, but continued to occur as many as 100 days post-admission. To the left of the Figure 3A, there is a scatter plot of both age and burn size for each of the patients shown top to bottom. A smooth curve plots the centroids for each. Figure 3A shows that the average age increased from approximately 25 to 40 years and burn size increased from 40 to 70 % TBSA from top to bottom of the figure. These findings are consistent with the finding that those patients with organ failure scores of 0 are younger and with more moderate sized injuries. Figure 3B identifies four categories of patients that shows there were deaths occurring up to 90 to 100 days post-injury. There was gradual recovery for those patients with Denver scores of 1–3 and ≥4 (MOF) over the 180-day period. All patients had died or were recovered from organ failure or MOF by 180 days.
Figure 3. Time to Recovery.
(A) To the left of panel A, the age and burn size were plotted for each of the 541 patients in the Glue Grant study. From top to bottom, the centroid was plotted for age and burn size. To the right of panel B, the Denver organ failure scores were plotted for each time period up to 200 days. The patients can be considered as having no organ failure, in organ failure, or dead. There are four categories shown by Denver organ failure scores: no organ failure – 0 (blue), organ failure – 1–3 (purple), multiple organ failure – ≥4 (red), and dead (black). (B) The Kaplan-Meyer mortality curves and the organ recovery curves over the year post-injury are shown. At each time point, there are four patient possibilities plotted as the proportion of the total population: multiple organ failure (below dashed curve), organ failure (above dashed curve and below dotted curve), fully recovered (above dotted curve and below solid curve), or dead (above solid curve).
Table 3 tabulates the values for organ component scores. Overall rate for MOF was substantial with 27% of patients demonstrating MOF. Pulmonary dysfunction was the most common organ failure following burn injury with nearly 63% of patients demonstrating some degree of pulmonary failure within the first three weeks (early organ failure) following admission and 31.0% after three weeks (late organ failure). During the early organ failure phase, cardiac failure was the second most common organ failure with 29.9% of patients showing abnormal values. Hepatic and renal dysfunctions were uncommon with only 13% (hepatic) and 16% (renal) of the patients demonstrating any abnormal values during the first three weeks. In the comparison between adults and children, organ function values were different for each of the organs evaluated. There appeared to be greater cardiac (p=0.0003), pulmonary (p=0.0189), and hepatic (p=0.00002) organ dysfunction and less renal (p=0.0001) dysfunction in children than adults. Given the greater degree of organ failure in children, it is surprising that the mortality rate was significantly different compared with adults. ARDS was diagnosed far more commonly in adults (p<0.0001).
Table 3.
Organ Failure
| Total 0–21days n=541 |
Total >21 days§ n=416 |
Adults n=300 |
Pediatric n=241 |
Probability | |
|---|---|---|---|---|---|
| Total (%) | Total (%) | ||||
| Organ Failure Scores* | |||||
| Maximum Cardiac Score: | |||||
| 0 | 379 (70.1) | 325 (78.1) | 217 (72.3) | 137 (56.9) | |
| 1 | 20 (3.7) | 14 (3.4) | 10 (3.3) | 13 (5.4) | 0.000296† |
| 2 | 62 (11.5) | 37 (8.9) | 21 (7.0) | 41 (17.0) | |
| 3 | 80 (14.8) | 40 (9.6) | 52 (17.3) | 50 (20.8) | |
| Maximum Pulmonary Score: | |||||
| 0 | 202 (37.3) | 287 (69.0) | 112 (37.3) | 85 (35.3) | |
| 1 | 61 (11.3) | 43 (10.3) | 20 (6.7) | 35 (14.5) | 0.0189† |
| 2 | 124 (22.9) | 48 (11.5) | 72 (24.0) | 45 (18.7) | |
| 3 | 154 (28.5) | 38 (9.1) | 96 (32.0) | 76 (31.5) | |
| Maximum Hepatic Score: | |||||
| 0 | 469 (86.7) | 377 (90.6) | 269 (89.7) | 180 (74.7) | |
| 1 | 51 (9.4) | 21 (5.1) | 17 (5.7) | 40 (16.6) | 0.0000183† |
| 2 | 15 (2.8) | 8 (1.9) | 6 (2.0) | 14 (5.8) | |
| 3 | 6 (1.1) | 10 (2.4) | 8 (2.7) | 7 (2.9) | |
| Maximum Renal Score: | |||||
| 0 | 453 (83.7) | 359 (86.3) | 220 (73.3) | 212 (88.0) | |
| 1 | 30 (5.6) | 18 (4.3) | 25 (8.3) | 9 (3.7) | 0.000102† |
| 2 | 35 (6.5) | 22 (5.3) | 29 (9.7) | 15 (6.2) | |
| 3 | 23 (4.3) | 17 (4.1) | 26 (8.7) | 5 (2.1) | |
| ARDS | 109 (20.0) | 97 (32.3) | 12 (5.0) | 1.15E-16 | |
| Multiorgan Failure | 147 (27.0) | 80 (26.7) | 67 (27.8) | 0.772 | |
Values represent mean ± SD or N(%). Data were analyzed by Wilcoxon Signed Rank Test, Chi-Square Test, or Fisher’s Exact Test as appropriate.
Maximum Denver component scores within 21 days of admission (early organ failure)
Abbreviations: ARDS = acute respiratory distress syndrome
Multiple Organ Failure is defined as a maximum Denver MOF score of ≥ 4
Comparison of adults with children for early organ failure scores only
After 21 days (late organ failure)
DISCUSSION
This benchmark study, which includes a well-characterized cohort of 541 burn patients, represents one of the first comprehensive, multi-institutional reports of patient and injury characteristics, anatomic and physiologic derangements, and outcomes from patients with severe burn injuries involving greater than 20% TBSA. It is difficult for any single-institution to provide comparable data over a short time period due to the relative infrequency of larger burns managed with up-to-date evidence-based treatment protocols. Among other findings, this report challenges the relative importance of the three “traditional” risk factors for death from burn injury (burn size, age, and presence of inhalation injury). Furthermore, these data are web-accessible at http://www.gluegrant.org and may serve for future large-scale injury research studies.
First, we show that a patient is more likely to survive today what previously was considered a lethal injury. In this cohort of patients with TBSA greater than 20%, the LD50 is approximately 90% TBSA, which represents a tremendous advancement compared to the 1960–1980s. In the 1940s, the comparable LD50 was more closely considered to be 20% TBSA depending upon the ages of the underlying study population. These dramatic improvements in survival rates in the 1970s and 1980s were most likely related to advancements in surgical interventions and critical care support.
We have reached a plateau in survival rates until newer technologies or therapies become available to modify our current injury treatment paradigms. This is evident by the numbers of observed deaths in our current study comparing nearly identically with the predicted number of deaths from our previous 1998 report for patients admitted 1990–1994.6 Furthermore, mortality in our study patients was reduced relative to NBR patients when stratified by the presence of inhalation injury, age, and total burn size. Using multivariate stratified logistic regression, there was a statistically significant 29% survival benefit for our patients in comparison to patients in the NBR. This comparison might suggest that standardized protocols provide a survival benefit. If indeed there are improvements in survival, our younger patients (<50 years old), would be expected to survive larger burn injuries whereas those older patients (≥50 years old), would be expected to survive more modest sized injuries.
The NBR comparison suffers from at least one weakness, a significant reduction in the number of eligible NBR patients remaining for direct comparison with our Glue Grant patients. This huge reduction stems from the fact that the NBR records are reported from all-comers to the North American burn centers whereas the Glue Grant study selected only those patients at serious risk for organ dysfunction and potential death. The original contemporary NBR dataset contained 201,174 non-Glue Grant center records and after excluding 45,972 records for Emergency Department visits only, readmissions, or non-burn injuries and excluding 4,493 records missing essential patient data, the Glue Grant inclusion and exclusion criteria were applied to the remaining records, which reduced the NBR comparison set to fewer than 4,700 patients.
The very important effect of age on mortality, when burn size and presence of inhalation injury are controlled, was found to be less profound in those patients <55 years old. The average mortality for patients <17 years old was 7.9% and for those 17–55 was 18.3%, both of which appear lower than expected. The overall mortality for those patients with age ≥55 was 38.5%. The significant inflection point in which age becomes a dominant factor for survival lies between 50 and 60 years of age. This is an important finding in that individuals, demonstrating depressed organ reserves at the time of initial injury, can be at least partially compensated by supportive medical care. This compensation tends to become increasingly limited above 60 years of age. It is highly remarkable that when burn size and age are controlled, there is no residual statistical effect for the presence of inhalation injury, which is an important new finding. In our previous report,6 inhalation injury was identified as a significant risk predictor, however all burn sizes were included. Although the current study included only patients with burns greater than 20% TBSA, this finding about inhalation injury is highly significant and may reflect improved ventilation strategies. The certainty of this effect might be lessened by the fact that univariate analysis in the two separate adult and pediatric databases was statistically significant. Similar effects have recently been noted in the trauma literature as a decrease in the incidence, morbidity, and mortality of ARDS in the trauma population.18–20 Previous modes of ventilatory support were shown to incite barotrauma and accentuate the host inflammatory response, which likely compounded the initial damage caused by inhalation injury.
There is a growing trend in healthcare to develop evidence-based protocols to evaluate a variety of medical and surgical treatments and for measuring quality of care.21–22 Our study provided a modest step towards the ultimate goal of an evidence-based SOP for resuscitation. It is apparent that >4 ml/kg/% TBSA is routinely being administered to patients during the first 24 hours post-burn injury and indeed, that occurred both in our adults and children. This study provides no evidence that additional fluids either benefited or harmed the patient. At the very least, the additional fluids require more time to be reabsorbed in the days post-injury and most of the additional fluids reside in the lungs. Using a propensity score logistic model and an earlier subset of patients from our study, the increased fluids resulted in statistically important increases in the risk of pneumonia (odds ratio [OR] =1.92), blood stream infections (OR=2.33), ARDS (OR=1.44), MOF (OR=1.49), and death (OR=1.74).23 The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Group performed a randomized trial of conservative versus liberal fluid treatment for acute lung injury with the primary endpoint being death at 60 days. The secondary endpoints were ventilator-free days, organ-failure-free days, and measures of lung physiology.
In the ARDS Network study, there was no effect on 60-day mortality between the two fluid management strategies. However, the conservative strategy group did experience better lung function and fewer ventilator and intensive care days with no increase in nonpulmonary organ failures.24 Similar trials with lower volume resuscitation, possibly with more liberal use of vasopressors to compensate for the inflammatory mediator-induced vasodilation, are called for in patients with major burns.
The MOF rate of 27% in our study is comparable to the MOF rate of 35% seen in patients with major blunt trauma.25 This finding indicates that MOF remains a serious challenge in critically injured burn patients. The time required to recover (TTR) is potentially an important outcome measure. Given the overall high rate of MOF in children as well as adults, we suggest that a better description is required, not only to describe organ dysfunction, but also to quantify the dynamic nature for recovery from organ dysfunction.
The concept of TTR as an outcome benchmark is relatively new. We see at least two potential advantages: it has more clinical or bedside utility and in our view, it is easier to recognize signs of multi-system organ recovery than arbitrary grades of organ dysfunction. A third potential advantage is increased power to detect treatment effects in randomized trials. TTR is similar to ventilator-free days in that it combines information on both mortality and speed of recovery in survivors. Ventilator-free days have been shown to be a more powerful outcome measure than mortality alone under various plausible scenarios.
In summary, this study serves as a benchmark in burn care. Today, burn patients are expected to survive what might have been highly lethal injuries a few decades ago. Organ dysfunction and other detrimental sequelae of burn and inhalation injury, such as pneumonia and blood stream infection, are now preventable and better treated. The importance of inhalation injury as a significant risk factor for mortality appears to have been ameliorated, at least in patients with burns greater than 20% TBSA. We can certainly expect further improvements to come from, for example, better knowledge of resuscitation protocols as well as new surgical and critical care interventions. As more patients survive such devastating injuries and the concomitant conditions that often result, perhaps it is time to begin to focus more attention to the burn patient’s quality of life domains (physical, psychological, and family) for those many patients who do survive.
Supplementary Material
Acknowledgements
The Inflammation and the Host Response to Injury, Large Scale Collaborative Research Program also comprised of Lily Altstein, PhD, Henry V. Baker, PhD, Ulysses G.J. Balis, MD, Paul E. Bankey, MD, PhD, Timothy R. Billiar, MD, Bernard H. Brownstein, PhD, Steven E. Calvano, PhD, David G. Camp II, PhD, J. Perren Cobb, MD, Alex G. Cuenca, MD, Joseph Cuschieri, MD, Ronald W. Davis, PhD, Asit K. De, PhD, Philip A. Efron, MD, Brian G. Harbrecht, MD, Laura Hennessy, RN, Jeffrey L. Johnson, MD, Stephen F. Lowry, MD (deceased), Ronald V. Maier, MD, Bruce A. McKinley, PhD, Carol L. Miller-Graziano, PhD, Joseph P. Minei, MD, Lyle L. Moldawer, PhD, Ernest E. Moore, MD, Frederick A. Moore, MD, Avery B. Nathens, MD, PhD, MPH, Grant E. O'Keefe, MD, MPH, Laurence G. Rahme, PhD, Daniel G. Remick, MD, Michael B. Shapiro, MD, Richard D. Smith, PhD, Jason Sperry, MD, John D. Storey, PhD, Robert Tibshirani, PhD, Mehmet Toner, PhD, H. Shaw Warren, MD, Michael A. West, MD, PhD, Wing H. Wong, PhD, Yong-Ming Yu, MD, PhD
Source of Funding:
This study was supported by the Inflammation and the Host Response to Injury Large Scale Collaborative Research Grant from the National Institute of General Medical Sciences, 5U54GM062119.
Footnotes
Conflicts of Interest
For all authors, none were declared.
REFERENCES
- 1.National Hospital Ambulatory Medical Care Survey. [[cited July 24, 2013]];2010 Available from http://www.cdc.gov/nchs/ahcd/web_tables.htm#2010.
- 2.Guidelines for the Operation of Burn Centers. American Burn Association. 2006 Available from http://www.ameriburn.org/verification_about.php. [Google Scholar]
- 3.Finkelstein EA, Corso PS, Miller TR. Incidence and Economic Burden of Injuries in the United States. Oxford: Oxford University Press; 2006. [Google Scholar]
- 4.Tompkins RG, Burke JF, Schoenfeld DA, et al. Prompt eschar excision: a treatment system contributing to reduced burn mortality. A statistical evaluation of burn care at the Massachusetts General Hospital (1974–1984) Ann Surg. 1986;204:272–281. doi: 10.1097/00000658-198609000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tompkins RG, Remensnyder JP, Burke JF, et al. Significant reductions in mortality for children with burn injuries through the use of prompt eschar excision. Ann Surg. 1988;208:577–585. doi: 10.1097/00000658-198811000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan CM, Schoenfeld DA, Thorpe WP, et al. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998;338:362–366. doi: 10.1056/NEJM199802053380604. [DOI] [PubMed] [Google Scholar]
- 7.Thombs BD, Singh VA, Halonen J, et al. The effects of preexisting medical comorbidities on mortality and length of hospital stay in acute burn injury: evidence from a national sample of 31,338 adult patients. Ann Surg. 2007;245:629–634. doi: 10.1097/01.sla.0000250422.36168.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silver GM, Klein MB, Herndon DN, et al. Standard operating procedures for the clinical management of patients enrolled in a prospective study of Inflammation and the Host Response to Thermal Injury. J Burn Care Res. 2007;28:222–230. doi: 10.1097/BCR.0B013E318031AA44. [DOI] [PubMed] [Google Scholar]
- 9.Yale Center for Medical Informatics. TrialDB – A Clinical Studies Data Management System. Available from http://ycmi.med.yale.edu/trialdb/ [Google Scholar]
- 10.Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 11.Sauaia A, Moore EE, Johnson JL, et al. Validation of postinjury multiple organ failure scores. Shock. 2009;5:438–447. doi: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 13.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. pp. 1659–1702. [Google Scholar]
- 14.Mangram AJ, Horan TC, Pearson ML, et al. Guidelines for prevention of surgical site infections, 1999. Infect Control Hosp Epidemiol. 1999;20:248–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 15.McDonald-Smith GP. Progress report: 1998 NATIONAL TRACS/ABA Burn Registry and related activities. J Burn Care Rehabil. 1998;19:354–357. [PubMed] [Google Scholar]
- 16.Miller SF, Bessey P, Lentz CW, et al. National burn repository 2007 report: a synopsis of the 2007 call for data. J Burn Care Res. 2008;29:862–870. doi: 10.1097/BCR.0b013e31818cb046. discussion 71. [DOI] [PubMed] [Google Scholar]
- 17.Flora JD. A method for comparing survival of burn patients to a standard survival curve. J Trauma. 1978;18:701–705. doi: 10.1097/00005373-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Martin M, Salim A, Murray J, et al. The decreasing incidence and mortality of acute respiratory distress syndrome after injury: a 5-year observational study. J Trauma. 2005;59:1107–1113. doi: 10.1097/01.ta.0000188633.94766.d0. [DOI] [PubMed] [Google Scholar]
- 19.Rocco TR, Jr, Reinert SE, Cioffi W, et al. A 9-year, single-institution, retrospective review of death rate and prognostic factors in adult respiratory distress syndrome. Ann Surg. 2001;233:414–422. doi: 10.1097/00000658-200103000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salim A, Martin M, Constantinou C, et al. Acute respiratory distress syndrome in the trauma intensive care unit: Morbid but not mortal. Arch Surg. 2006;141:655–658. doi: 10.1001/archsurg.141.7.655. [DOI] [PubMed] [Google Scholar]
- 21.Khuri SF, Henderson WG, Daley J, et al. Successful implementation of the Department of Veterans Affairs' National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248:329–336. doi: 10.1097/SLA.0b013e3181823485. [DOI] [PubMed] [Google Scholar]
- 22.Brook RH, McGlynn EA, Cleary PD. Quality of health care. Part 2: measuring quality of care. N Engl J Med. 1996;335:966–970. doi: 10.1056/NEJM199609263351311. [DOI] [PubMed] [Google Scholar]
- 23.Klein MB, Hayden D, Elson C, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg. 2007;245:622–628. doi: 10.1097/01.sla.0000252572.50684.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of Two Fluid-Management Strategies in Acute Lung Injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 25.Cuschieri J, Johnson JL, Sperry J, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012;255:993–999. doi: 10.1097/SLA.0b013e31824f1ebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.