Abstract
There is widespread recognition that consistency between research centres in the ways that patients with tinnitus are assessed and outcomes following interventions are measured would facilitate more effective co-operation and more meaningful evaluations and comparisons of outcomes. At the first Tinnitus Research Initiative meeting held in Regensburg in July 2006 an attempt was made through workshops to gain a consensus both for patient assessments and for outcome measurements. It is hoped that this will contribute towards better cooperation between research centres in finding and evaluating treatments for tinnitus by allowing better comparability between studies.
Keywords: tinnitus, standards, assessment, questionnaires, treatment, outcome, case history
Introduction
Chronic tinnitus, the phantom perception of sound, can be a debilitating and life-altering experience. It affects millions of people in western countries. Despite the enormous social and economic burden tinnitus causes, no well-established specific treatment for this disorder is available. Among the reasons for this unsatisfactory situation are the difficulties in assessing tinnitus as it is a purely self-report subjective phenomenon.
There is widespread recognition that consistency between research centres in the ways that patients with tinnitus are assessed and outcomes following interventions are measured would facilitate more effective co-operation and more meaningful evaluations and comparisons of outcomes. On the other hand most research centres already have long established systems for collecting and assessing data and hence are unable or unwilling to completely change to a different system because so much would be lost.
At the recent (July, 2006) Tinnitus Research Initiative meeting in Regensburg, Germany an attempt was made through workshops to gain a consensus both for patient assessments and for outcome measurements.
It is hoped that this consensus will facilitate cooperation between tinnitus research centres in finding and evaluating treatments for tinnitus and will help achieve more meaningful comparisons between studies.
Consensus for diagnosis and assessment
General statements
There is a need for consensus on assessment and outcome measurement
There is an urgent need for a set of assessment methods to be agreed and utilised by the international tinnitus research community. This includes assessment of patients with tinnitus and subsequent measurement of outcomes following intervention.
Consistency in assessment of patients with tinnitus will advance the characterisation of their tinnitus into subtypes. As results for most known therapeutic interventions are very inconsistent, the identification of predictors for the effectiveness of various treatments is essential. Characterization of patients with tinnitus into subgroups would be greatly facilitated by consistent assessment of potentially relevant data and may contribute to the development of treatment plans individualised for patients within a subgroup.
An agreement on outcome measurements is a pre-requisite for comparison between treatment studies. This in turn will greatly increase the efficiency of tinnitus research by facilitating meta-analysis and by facilitating communication amongst disciplines using such different treatment interventions as acoustic stimulation, hearing aids, counselling, cognitive behavioural therapy, drug treatments, electrical and magnetic stimulation. Furthermore agreement on outcome measures is required for the engagement of the pharmaceutical industry in tinnitus research.
Any proposed standards must take into account cultural differences, language differences, different health systems, existing databases and existing routines
Even though a high degree of conformity is desirable, recognition has to be given to intrinsic limitations to achieving this. Comparability of questionnaires is limited by the sensitivity of some items to cultural differences and language. Other factors such as costs and the organisational structures of health care systems and facilities have a marked impact on routines used in the assessment of tinnitus patients. Some tinnitus centres already have very large databases built up through their existing systems. Such limitations by established practice have to be accommodated. There was consensus that wide acceptance can only be achieved if any proposed new instrument is as compatible as possible with existing routines.
The consensus achieved at Regensburg is an agreement on minimum requirements
It was agreed that a “Consensus for Patient Assessment and Treatment Outcome Measurement” had to be limited to a set of core items and could only represent a minimum requirement. A consensus agreement cannot claim to meet optimum expectations or to represent an ideal. It documents what all participants agree is necessary. Many of the participants considered that additional information is required about patients and will collect and assess this additional information. However because of the limitations identified in the previous section there is no consensus about such additional information.
Agreement was achieved on which aspects should be assessed
Consensus was achieved on which aspects of tinnitus and related items should be assessed while retaining flexibility on how they can be assessed. This strategy allows both consistency and compatibility with routines already existing in different centres.
Prioritisation of items
Prioritisation of items was agreed as a further strategy to affect a compromise between consistency and feasibility. Items were prioritised according to their level of importance. Items assessed as level A are considered essential, items assessed as level B are highly recommended and items assessed as level C might be of interest in some contexts. This prioritisation of items is based on the understanding that the consensus represents an agreement on minimum requirements and not on optimal expectations.
Applicability
These consensus agreements for patient assessment and outcome measurements should be applicable and useful to all clinicians and researchers dealing with patients with tinnitus. They are a step towards more comprehensive agreements. They may also be helpful to those advising Tinnitus Research Initiative and other funding organisations about research applications.
Consensus for patient assessments and treatment outcome measurements (TRI workshop 2006)
This consensus was developed during workshops at the TRI meeting in Regensburg July 2006. A provisional consensus summary was then sent to all workshop participants giving them the opportunity to check whether this summary accurately reflected the agreements reached during the workshops. Feedback was received from all authors and the consensus summary modified slightly as a result (see Table 1).
Table 1.
Consensus for patient assessment and outcome measurements (TRI workshop 2006)
| In each category recommendations are ordered according to their level of significance |
| A: Essential B: Highly recommended C: Might be of interest |
| Patient Assessment |
| Physical examination |
| A: Otologic examination by a specialist |
| A: Examination of the neck (range of motion, tenderness, muscle tension…) |
| B: Examination of the temporomandibular function |
| Audiologic assessment |
| A: Audiometry (pure tone threshold; up to 8 kHz) |
| B: Immitance audiometry |
| B: High-frequency audiometry (at least up to 12 kHz) |
| B: Otoacoustic emissions |
| B: Loudness discomfort level |
| C: Auditory evoked potentials |
| Psychophysic measures of tinnitus |
| B: Loudness match |
| B: Pitch match |
| B: Maskability (MML) |
| B: Residual inhibition |
| Case history |
| A majority of participants preferred a questionnaire to be filled in by the patient (with access to someone for clarification) rather than at a structured interview. This was not a consensus. It was agreed that as a first step towards consensus a list of those items common to most existing questionnaires should be made. A first attempt to extract such a list is attached. |
| Questionnaires |
| A: Validated questionnaire for the assessment of tinnitus severity, which at present can be THI, THQ, TRQ or TQ (it was agreed that in the future a better and more widely validated questionnaire was required) |
| B: Assessment of tinnitus severity by additional questionnaires, and especially by the THI because it is believed that THI is validated in most languages |
| C: Assessment of depressive symptoms (e.g. BDI) |
| C: Assessment of anxiety (e.g. STAI) |
| C: Assessment of quality of life (e.g. WHODAS II) |
| C: Assessment of insomnia (e.g. PSQI) |
| Outcome Measurements |
| A: Validated questionnaire for the assessment of tinnitus severity, which at present can be THI, THQ, TRQ or TQ (it was agreed that in the future a better and more widely validated questionnaire was required) |
| B: Assessment of tinnitus severity by additional questionnaires, and especially by the THI because it is believed that THI is validated in most languages |
| C: Assessment of depressive symptoms (e.g. BDI) |
| C: Assessment of anxiety (e.g. STAI) |
| C: Assessment of quality of life (e.g. WHODAS II) |
| C: Assessment of insomnia (e.g. PSQI) |
| C: Tinnitus loudness match |
| C: Maskability (MML) |
| C: Objective measurement of brain function (functional imaging, electrophysiology) |
| Abbreviations: KHz, Kilohertz; dB, Decibel; SL, Sensation level; MML, Minimal masking level; THI, Tinnitus Handicap Inventory (Newman et al., 1998); THQ, Tinnitus Handicap Questionnaire (Kuk et al., 1990); TRQ, Tinnitus Reaction Questionnaire (Wilson et al., 1991); TQ, Tinnitus Questionnaire (Hallam et al., 1988); BDI, Beck Depression Inventory (Beck and Steer, 1984); STAI, State Trait Anxiety Inventory (Spielberger et al., 1970); WHODAS, WHO Disability Assessment Schedule (McArdle et al., 2005); PSQI, Pittsburgh Sleep Quality Index (Buysse et al., 1989); |
Physical examination
There was agreement that an otologic examination by a specialist is an essential part of tinnitus patient assessment. Otologic examinations are performed by otolaryngologists and also by a variety of other health professionals depending on the health system. The profession of the specialist has not been specified.
For assessment of potential somatosensory components of a patient’s tinnitus examination of the neck (including range of motion, tenderness and muscle tension) is considered essential. Examination of temporomandibular function (including dental problems) is highly recommended.
Audiologic assessment
Diagnostic pure tone audiometry (up to 8 kHz) for the assessment of hearing loss is considered necessary in every patient. High-frequency audiometry up to 12 kHz is highly recommended, as are immitance audiometry, assessment of otoacoustic emissions and loudness discomfort levels. The measurement of auditory evoked potentials might be of interest, especially for excluding vestibular schwannoma if appropriate organ imaging is not available.
Psychophysical measures of tinnitus
Psychophysical measures are not considered essential, as neither the loudness match nor other psychoacoustic measures bear a consistent relationship to the severity or perceived loudness of tinnitus (Henry and Meikle, 2000). Nevertheless quantification can yield important information in tinnitus research and therefore the assessment of pitch match, loudness match, maskability and residual inhibition are highly recommended. Furthermore loudness match and maskability can be of interest as outcome measures in treatment trials.
Concerns were raised that results of psychoacoustic measurements depend on the protocols used for the assessments. Standardisation of assessment procedures, even if highly desirable, is very difficult to achieve, because it would require specialized instrumentation, which is not available to most audiologists. The workshop participants recommend thorough documentation of the techniques used for psychoacoustic measures.
Case history questionnaires
Information about the history and descriptive characteristics of the patient’s tinnitus or tinnitus related conditions could be obtained by questionnaires or by structured interviews. A large majority of the workshop participants agreed that these data are better obtained by a questionnaire both for logistical reasons and to achieve greater reliability. It was suggested that if a patient is uncertain how to answer a question this should be clarified during the subsequent clinical interview. Only a few case history questionnaires have been published (Newman and Sandridge, 2006). However, most clinicians and researchers have developed their own questionnaires (and/or structured interviews) in which they include those questions, which they consider important and relevant. Based on this information many centres have developed databases, which include large numbers of patients presenting at their clinics over several decades (e.g. Meikle, 1997). This approach does not facilitate comparison between centres.
In order to achieve comparability of data it was agreed that an item list for case history questionnaires should be developed which should include those items common to many of the questionnaires (and structured interviews) in current use. Due to time constraints these items were not identified during the workshops. Two of the authors (BL, RG) have sorted through a considerable variety of case history questionnaires and identified a set of items common to most of them, which might therefore be regarded as essential (level A) and could be expected to be included in all questionnaires. They also identified additional items common to many questionnaires, which could be regarded as highly desirable (level B). These items are listed as a starting point for further discussion and were sent to all workshop participants. Based on feedback from the participants the “Items list” for tinnitus case history questionnaires (see Table 2) has been compiled. This list consists of 14 essential (level A) items and 21 highly desirable (level B) items.
Table 2.
“Items list” for tinnitus case history questionnaires
| The items 1, 2, 4, 5, 6, 8, 9, 12, 16, 19, 21, 26, 27, 28 are considered as essential (Category A) all other items are regarded as highly desireable (Category B) |
Background
|
Tinnitus history
|
Modifying influences
|
Related conditions
|
As an example of how the above items can be expressed for patients to complete see Table 3.
These 35 items have also been shown in the form in which they appear in one particular case history questionnaire (Tinnitus Sample Case History Questionnaire, TSCHQ, Table 3). This could be used as a resource of how these items might be expressed.
Table 3.
Tinnitus Sample Case History Questionnaire (TSCHQ)
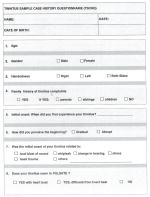




|
This approach of identifying items with high levels of use and hence of importance achieves compatibility with existing systems for case history data collection. Existing case history questionnaires can be modified by adding missing items. The TSCHQ (Table 3) could be used in its entirety or modified.
Questionnaires as instruments for measuring tinnitus and related disorders
Several questionnaires have been developed for the “measurement” of tinnitus severity. These were mainly designed for screening and diagnostic purposes. Most of these attempt to quantify a combination of tinnitus related distress, disability and handicap resulting in a large overlap of their items. Among the most widely used are the Tinnitus Handicap Inventory (THI) (Newman et al., 1998), the Tinnitus Handicap Questionnaire (THQ) (Kuk et al., 1990), the Tinnitus Questionnaire (Hallam et al., 1988) and the Tinnitus Reaction Questionnaire (TRQ) (Wilson et al., 1991). Even though these instruments were not specifically designed to be sensitive to treatment related changes, all of them have been used as outcome measurers in clinical trials.
It is unrealistic to expect everyone to agree to use the same one of these questionnaires as primary outcome measure in their clinical trials. First, each of these questionnaires has its strengths and weaknesses. Their sensitivities may vary depending on the therapy used and they sometimes produce results different from each other. Secondly, new questionnaires specifically designed to evaluate treatment related changes, will emerge in the near future. Thirdly, all of these questionnaires have been developed in the English language and only some of them have been translated and validated in some other languages.
It was generally agreed that a questionnaire is required which is specifically designed for the assessment of treatment outcomes, and which is validated in many languages and in many cultural and socio-economic groups.
The consensus agreement is that at the present time one validated questionnaire, which can be THI, THQ, TRQ or TQ, is an essential part of patient assessment. Therapeutic trials should use one of these questionnaires also as outcome measurement. Assessment of tinnitus severity with at least one additional questionnaire is highly recommended. A majority of the participants favoured the THI as an additional questionnaire because the THI is thought to be validated in the largest number of languages (Zachariae et al., 2000; Herraiz et al., 2001; Paula Erika et al., 2005; Kleinjung et al., 2007) and may thus facilitate comparability between studies.
Consensus was that assessments of tinnitus related disorders such as depression, anxiety and insomnia are of interest in some contexts. These can be assessed by specific validated instruments such as the Beck Depression Inventory (BDI) (Beck and Steer, 1984) for the quantification of depressive symptoms, the State and Trait Anxiety Inventory (STAI) (Spielberger et al., 1970) for anxiety and the Pittsburgh Sleep Quality Index (Buysse et al., 1989) for insomnia. To assess effects on quality of life the WHO Disability Assessment Scale (WHO DAS II) can be used. It was noted that this patient self-rating scale of functioning has been shown to be especially suitable as an outcome measure for the treatment of hearing disorders (McArdle et al., 2005).
Objective measures
An increasing amount of data from pilot studies indicates considerable potential for a variety of electrophysiologic and neuroimaging methods to assess alterations in brain structure and function in patients with tinnitus. None of the participants considered any of these to be an established diagnostic tool. However, there was recognition that behavioural tests are insufficient and that the identification of neurobiological changes that can be measured objectively is highly desirable.
Acknowledgments
The authors wish to thank Ulli Soltani for organisational assistance.
References
- Beck AT, Steer RA. Internal consistencies of the original and revised beck depression inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Hallam RS, Jakes SC, Hinchcliffe R. Cognitive variables in tinnitus annoyance. Br J Clin Psychol. 1988;27(Pt 3):213–222. doi: 10.1111/j.2044-8260.1988.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Henry JA, Meikle MB. Psychoacoustic measures of tinnitus. J Am Acad Audiol. 2000;11:138–155. [PubMed] [Google Scholar]
- Herraiz C, Hernandez CJ, Plaza G, Tapia MC, De los SG. Disability evaluation in patients with tinnitus. Acta Otorrinolaringol Esp. 2001;52:534–538. doi: 10.1016/s0001-6519(01)78247-7. [DOI] [PubMed] [Google Scholar]
- Kleinjung T, Fischer B, Langguth B, Sand PG, Hajak G, Dvorakova J, Eichhammer P. Validation of the German-version tinnitus handicap inventory (THI) Psychiatr Prax. 2007;34:140–142. [Google Scholar]
- Kuk FK, Tyler RS, Russell D, Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear Hear. 1990;11:434–445. doi: 10.1097/00003446-199012000-00005. [DOI] [PubMed] [Google Scholar]
- McArdle R, Chisolm TH, Abrams HB, Wilson RH, Doyle PJ. The WHO-DAS II: measuring outcomes of hearing aid intervention for adults. Trends Amplif. 2005;9:127–143. doi: 10.1177/108471380500900304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle MB. Electronic access to tinnitus data: the Oregon tinnitus data archive. Otolaryngol Head Neck Surg. 1997;117:698–700. doi: 10.1016/S0194-59989770055-X. [DOI] [PubMed] [Google Scholar]
- Newman CW, Sandridge SA. Tinnitus questionnaires. In: Snow JB, editor. Tinnitus Theory and Management. BC Decker; Hamilton, Ontario: 2006. pp. 237–254. [Google Scholar]
- Newman CW, Sandridge SA, Jacobson GP. Psychometric adequacy of the tinnitus handicap inventory (THI) for evaluating treatment outcome. J Am Acad Audiol. 1998;9:153–160. [PubMed] [Google Scholar]
- Paula Erika AF, Cunha F, Onishi ET, Branco-Barreiro FC, Gananca FF. Tinnitus handicap inventory: cross-cultural adaptation to Brazilian Portuguese. Pro Fono. 2005;17:303–310. doi: 10.1590/s0104-56872005000300004. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI, Manual for the State-Trait-Anxiety-Inventory. Consulting Psychologist Press; Palo Alto, CA: 1970. [Google Scholar]
- Wilson PH, Henry J, Bowen M, Haralambous G. Tinnitus reaction questionnaire: psychometric properties of a measure of distress associated with tinnitus. J Speech Hear Res. 1991;34:197–201. [PubMed] [Google Scholar]
- Zachariae R, Mirz F, Johansen LV, Andersen SE, Bjerring P, Pedersen CB. Reliability and validity of a Danish adaptation of the tinnitus handicap inventory. Scand Audiol. 2000;29:37–43. doi: 10.1080/010503900424589. [DOI] [PubMed] [Google Scholar]


