Abstract
Background
Recent studies have shown that BCCIP (BRCA2 & CDKN1A interacting protein) is essential for maintaining the transactivation activity of wild type p53. We analyzed the expression of BCCIP and p53 in a cohort of laryngeal cancer treated with radiotherapy and assessed whether BCCIP and p53, alone or in combination, would correlate with local control and overall survival.
Methods
One hundred twenty-three patients treated between 1975 and 2000 for early stage (stage I & II) squamous cell carcinoma of the larynx were included in the study. Treatment consisted of radiation therapy (RT) with standard fields and fractionation to a median dose of 66 GY. Tissue was collected from pre-RT biopsies and constructed in a tissue microarray and BCCIP and p53 expression was determined using immunohistochemistry.
Results
Loss of expression of BCCIP in combination with normal p53 (negative p53 staining) was associated with local recurrence (RR 2.04; 95% CI 0.99–4.56, p=0.05) and poor overall survival (RR 2.09; 95% CI 1.21–4.00, p=0.008) compared to patients who did express BCCIP. Expression of BCCIP or p53 alone was not found to be independently associated with benefits in local control or overall survival.
Conclusions
This study provides clinical evidence that BCCIP contributes to outcomes in patients with laryngeal cancer treated with RT. This benefit may be a result of increased radiosensitivity in patients who have functional BCCIP and p53. These data may be used to identify sub-groups of laryngeal cancer patients who are more likely to be cured with radiotherapy.
INTRODUCTION
In 2007, an estimated 11,300 new diagnosis and 3,660 deaths will occur from laryngeal cancer in the United States [1]. An analysis performed on the National Cancer Data Base (NCDB) for cases of head and neck cancer registered between 1985 and 1995 found the most common head and neck cancer reported in the United States was laryngeal cancer, accounting for 20.9% of the 295,022 total cases [2]. Definitive radiation therapy is the mainstay of treatment for early stage disease (T1-2N0), which accounts for 50–60% of reported cases of laryngeal cancer [3, 4]. The goals of treatment for early laryngeal cancer are cure and voice preservation [5]. Five-year local recurrence rates for T1 lesions are 5–20% – accounting for the primary cause of failure in these patients [4, 6]. These recurrences are generally treated with salvage laryngectomy, which has increased morbidity compared to primary surgery. Due to the prognostic limitations of current clinical markers, investigators are examining molecular markers as prognostic tools to guide clinical decision making [7].
The p53 status has been extensively used as a prognostic molecular marker. Mutations in p53 are found in approximately 50% of all human cancers, and inactivation of p53 leads to cancer predisposition in animal models [8]. Furthermore, p53 inactivation has been shown to be associated with decreased radiation sensitivity and apoptotic cell death [9]. A key element for p53’s tumor suppressor function is its transactivation activity [10,11]. Cancer bearing p53 mutations are often defective in its transcription activity [10,12,13], and mice expressing transactivation-deficient p53 are predisposed to cancer [10,13]. It has recently been shown that BCCIP, a BRCA2 and CDKN1A Interacting Protein, is required for the transactivation activity of wild type p53 [14]. In p53 wild type cells, BCCIP knockdown by RNAi diminished the transactivation activity of p53, inhibited the binding of p53 to promoters of p53 target genes p21 and HDM2, and reduced the tetrameric formation of p53 [14]. Thus, defects in BCCIP override the wild type p53 transactivation function, suggesting a critical role of BCCIP in maintaining critical functions of p53 in tumor suppression and response to therapy.
The primary purpose of this study was to analyze the expression of BCCIP and p53 in a large cohort of patients with T1-T2, N0 laryngeal cancer treated with primary radiation therapy and assess whether BCCIP and p53, alone or in combination, would correlate with local recurrence and overall survival.
PATIENTS AND METHODS
Patients
Patients diagnosed with T1-2 N0 squamous cell carcinoma of the glottic and supraglottic larynx and treated at the Department of Therapeutic Radiology, Yale University School of Medicine between 1975 and 2000 met the inclusion criteria for this study. Of these patients treated with primary radiation therapy, 123 had archived tumor specimens available for analysis. Patients’ charts were reviewed and information on demographics, radiation therapy parameters, and tumor staging was documented. Staging was done by the AJCC Staging Manual, 5th edition. Patients were followed for a median of 9.9 years. Each case was assigned a unique study identification number to delink patient identification and all analysis and reporting of data was performed using the unique study identification number. The protocol was reviewed and approved by the institutional review board.
Radiation treatment parameters included opposed lateral fields using beam energies ranging from 2 to 6 MeV, five fractions per week, without planned treatment breaks. Patients were treated with a median daily fraction of 200 cGy (range, 180–255 cGy) to a total median dose of 66 Gy (range, 49.5–79 Gy) over 47 days (range, 27–78 days) and 109 patients received at least 60 Gy.
Immunohistochemical Analysis of Tissue Microarray for Expression of BCCIP and p53
A tissue microarray was constructed for this analysis. A pathologist examined hematoxylin and eosin–stained slides of the archived paraffin blocks and circled representative tumor sections. From these tumor sections, two 0.6 mm cores were extracted using a Tissue Microarrayer (Beecher Instruments, Silver Spring, MD, U.S.A.). The minimal percentage of the total specimen represented by the cores varied from 10% for the larger supraglottic specimens to 90% for the smaller glottic specimens. Sections of the microarrays 5 μm thick were cut with a tape-based tissue transfer system (Intrumedics, Hackensack, NJ, U.S.A.) and processed as described previously [15]. The reproducibility of the tissue microarray biopsies have been previous validated in a variety of tumors, including head and neck, colorectal, and lung [16–18].
Immunohistochemical analysis was performed on 5-μm-thick tissue sections prepared from formalin-fixed, paraffin-embedded archival tissue from the resected primary tumor. These slides were immunostained with a standard immunocytochemical protocol, using an affinity-purified rabbit poly-colonial anti-BCCIP antibody that was reported previously [19]. Briefly, slides were de-paraffinized with xylene. The antigen was retrieved in citrate acid buffer (pH6.5) by steaming in a rice cooker for 20 min. Slides were blocked with 5% milk in TBS-T (25mM Tris-HCl pH 7.5, 150mM NaCl, 0.1% Tween20) for 30 min at room temperature, and incubated with BCCIP antibody (1:100 dilution) for 3 hrs at room temperature. Following 3 times wash with TBS-T, the slides were incubated for 1 hr at room temperature with anti-Rabbit IgG secondary antibody (1:100) that was conjugated with horseradish peroxidase. The BCCIP positive stain (in brown color) was visualized by incubate the slides with chromogenic substrate diaminobenzidine (DAB), followed by 10 second contrast staining (in blue color) with haematoxylin for the nuclei. Then the slides were dehydrated and mounted.
For p53 staining, the tissue sections were pretreated to promote antigen retrieval with the DAKO Target Retrieval Solution (DAKO, Carpinteria, CA, U.S.A.). After antigen retrieval, a 3% solution of hydrogen peroxide was used for endogenous peroxidase blocking. Slides were then incubated overnight with monoclonal antibody p53 (DO-8; DAKO; dilution 1:3200). After overnight incubation, the slides were washed in phosphate buffered saline and a biotinylated secondary antibody was applied. Samples were then labeled with DAKO streptavidin-horseradish peroxidase, and DAKO Antibody Diluent was applied. The slides were then counterstained with hematoxylin and mounted.
Assessment of BCCIP and p53 staining was qualitative and done by a single independent pathologist who was blinded to patient outcome. BCCIP positive epithelial cells were stained brown in nuclei, but negative cells were blue. For each core, the region of predominant staining intensity was scored. For p53, less than 20% nuclear reactivity in tumor cells was scored as negative (intact) and 20% or greater nuclear reactivity was scored as positive (overexpression). BCCIP tumor staining intensity was scored 0 for nuclear staining < 10% of tumor, 1+ for faint or moderate nuclear staining of >10% of tumor, and 2+ for strong and intense membrane staining of >10% of the tumor. Samples staining as moderate, strong, or intense (1+ and 2+) were considered positive in the statistical analysis.
The study endpoints were local relapse and overall survival, including all deaths. Time to local relapse was defined by biopsy-proven relapses in the larynx. Both endpoints were calculated from the date of radiation therapy completion, as this represents the start of continuous risk for relapse. Additionally, data were censored after 10 years to calculate 10-year statistics and to minimize random censoring due to losses of follow-up. The last recorded follow-up date was February 11, 2003. Median follow-up was calculated by the reverse Kaplan-Meier method. The independent variables for this analysis included age, sex, race, T stage, tumor subsite, BCCIP expression, and p53 status. Age was treated as continuous variables after assessing for linearity.
Statistical Analysis
BCCIP status and relevant covariables were assembled in a database and analyzed using SAS User’s Guide, Version 9.1 (SAS Institute, Cary, NC). All tests of statistical significance were two-sided. P-values less than 0.05 were considered statistically significant. Follow-up time and time to recurrence were calculated from the date of radiation to the date of the relevant outcome. Disease-free survival was calculated as the interval between the date of radiation and the date of the first recurrence of disease.
Bivariate analyses for the association between co-variables and BCCIP positivity included the chi2 test and the Fisher’s exact test. Univariate survival analysis was done with the Cox proportional hazards model, which was used to calculate unadjusted relative risks for 5-year local recurrence and 5-year poor overall survival. In univariate analysis, BCCIP and p53 status were assessed both independently and in combination with each other to determine 5-year local recurrence and 5-year poor overall survival. Linear trends in the associations between predictor variables and 10-year local recurrence-free and overall survival were conducted using the chi2 test and the Kaplan Meier log-rank test.
Multivariate analysis with the Cox proportional hazards model was used to calculate the adjusted relative risks for 5-year local recurrence and 5-year poor overall survival in the subset of patients who did not overexpress p53.
RESULTS
Descriptive statistics
With a median follow-up of 9.9 years, a total of 32 patients experienced local relapse, for a 5-year actuarial local recurrence-free rate of 70.4%. The 5-year actuarial overall survival rate for the entire cohort was 60.2% with 44 events. Table 1 summarizes the demographics, staging, and molecular marker status. Median treatment doses received by the BCCIP and p53 subgroups were comparable.
Table 1.
Patient Characteristics
| Characteristics | No. (%) |
|---|---|
| Age (years) | |
| Median ± SD | 64.0 ± 10.9 |
| Range | 38–90 |
| Sex | |
| Male | 106 (86.2) |
| Female | 17 (13.8) |
| Race | |
| White | 111 (90.2) |
| Black | 12 (9.80) |
| T Stage | |
| T1 | 84 (68.3) |
| T2 | 39 (31.7) |
| Tumor Subsite | |
| Glottic | 98 (79.7) |
| Supraglottic | 25 (20.3) |
| BCCIP Status | |
| Positive | 62 (59.0) |
| Negative | 43 (41.0) |
| p53 Status | |
| Positive | 75 (63.6) |
| Negative (intact) | 43 (36.4) |
For BCCIP and p53, nuclear localization of immunoreactivity was evaluated. Overexpression of p53 was defined as 20% or greater nuclear reactivity with 75 (63.6%) of 118 cores positive. The BCCIP phenotype was defined as 10% nuclear reactivity or greater with 62 (59%) of 105 positive.
Chi2 analysis
Using chi2 analysis and Fisher’s exact test, loss of BCCIP expression was significantly associated with T stage, with 67% of T2 tumors having loss of BCCIP compared to only 35% of T1 tumors with loss of BCCIP expression (p = 0.01). As seen in Table 2, BCCIP expression did not correlate with any other molecular marker status, including p53 expression. Other prognostic variables, such as sex, race, and tumor subsite, were not significantly associated with the BCCIP.
Table 2.
Association between BCCIP Status and Prognostic Factors
| Prognostic Factor | BCCIP Status
|
p-value | |
|---|---|---|---|
| Positive | Negative | ||
| Sex | |||
| Male | 35 | 26 | 0.46 |
| Female | 5 | 6 | |
| Race | |||
| White | 34 | 30 | 0.35 |
| Black | 5 | 2 | |
| T Stage | |||
| T1 | 45 | 25 | 0.01* |
| T2 | 8 | 16 | |
| Tumor Subsite | |||
| Glottic | 33 | 45 | 0.57 |
| Supraglottic | 8 | 8 | |
| p53 Status | |||
| Positive | 35 | 28 | 0.91 |
| Negative (intact) | 17 | 13 | |
Statistically significant
Univariate Analysis
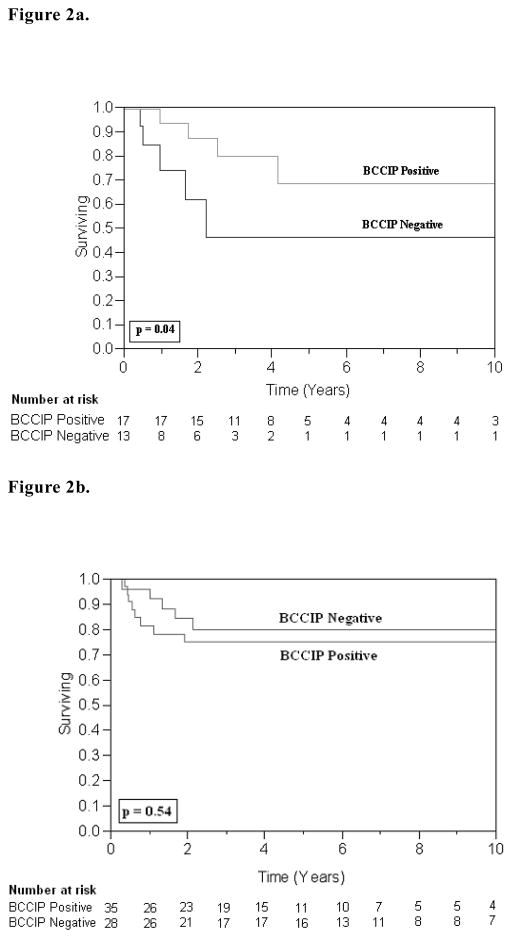
As depicted in the univariate analysis in Table 3, T2 stage was a significant predictor for 5-year local relapse (RR 1.71; 95% CI, 1.21–2.43, p=0.003). Table 4 illustrates that neither BCCIP expression, nor p53 expression was a statistically significant predictor for local control. However, in the sub-group of patients without overexpression of p53 (intact p53), those who lost expression of BCCIP showed over a two-fold increase in local recurrence that was statistically significant (RR 2.04; 95% CI 0.99–4.56, p=0.05). There was no statistically significant association between patients with overexpression of p53 and BCCIP status. Kaplan-Meier survival curves for 10-year local recurrence free survival are presented in Figure 2a and 2b.
Table 3.
Univariate Analysis of Prognostic Factors for 5-Year Local Recurrence and Poor Overall Survival
| Prognostic Factor | 5-Year LR | 5-Year Poor OS | ||
|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Sex | ||||
| Male | 1.00 (Ref.) | 0.63 | 1.00 | 0.21 |
| Female | 1.18 (0.63–2.97) | 1.50 (0.82–3.75) | ||
| Race | ||||
| White | 1.00 (Ref.) | 0.63 | 1.00 (Ref.) | 0.41 |
| Black | 0.85 (0.49–1.77) | 0.79 (0.48–1.46) | ||
| T Stage | ||||
| T1 | 1.00 (Ref.) | 0.003* | 1.00 (Ref.) | 0.001* |
| T2 | 1.71 (1.21–2.43) | 1.49 (1.10–2.00) | ||
| Tumor Subsite | ||||
| Glottic | 1.00 (Ref.) | 0.63 | 1.00 (Ref.) | 0.07 |
| Supraglottic | 1.11 (0.70–1.65) | 1.36 (0.97–1.86) | ||
RR = Risk ratio; CI = confidence intervals; LR = local recurrence; OS = overall survival; Ref. = reference group;
Statistically significant.
Table 4.
Univariate Analysis of BCCIP and p53 for 5-Year Local Recurrence and Poor Overall Survival
| Prognostic Factor | 5-Year LR | 5-Year Poor OS | ||
|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | |
| BCCIP Status | ||||
| Positive | 1.00 (Ref.) | 0.74 | 1.00 (Ref.) | 0.50 |
| Negative | 1.07 (0.69–1.63) | 1.01 (0.83–1.46) | ||
| P53 Status | ||||
| Positive | 1.00 (Ref.) | 0.41 | 1.00 (Ref.) | 0.86 |
| Negative (intact) | 1.16 (0.79–1.67) | 0.98 (0.74–1.25) | ||
| BCCIP Status in intact p53 sub-group | ||||
| Positive | 1.00 (Ref.) | 0.05* | 1.00 (Ref.) | 0.008* |
| Negative | 2.04 (0.99–4.56) | 2.09 (1.21–4.00) | ||
| BCCIP Status in p53 positive sub-group | ||||
| Positive | 1.00 (Ref.) | 0.53 | 1.00 (Ref.) | 0.41 |
| Negative | 0.84 (0.46–1.45) | 0.87 (0.61–1.22) | ||
RR = Risk ratio; CI = confidence intervals; LR = local recurrence; OS = overall survival; Ref. = reference group;
Statistically significant.
Figure 2.
Figure 2a. Local Recurrence in p53 Negative Patients: The 10-year LR-free survival was 68% for BCCIP-positive tumors (n = 17) and 46% for BCCIP-negative tumors (n = 13); (p = 0.04, log-rank test).
Figure 2b. Local Recurrence in p53-Positive Patients: The 10-year LR-free survival was 75% for BCCIP-positive tumors (n = 35) and 80% for BCCIP-negative tumors (n = 28); (p = 0.54, log-rank test).
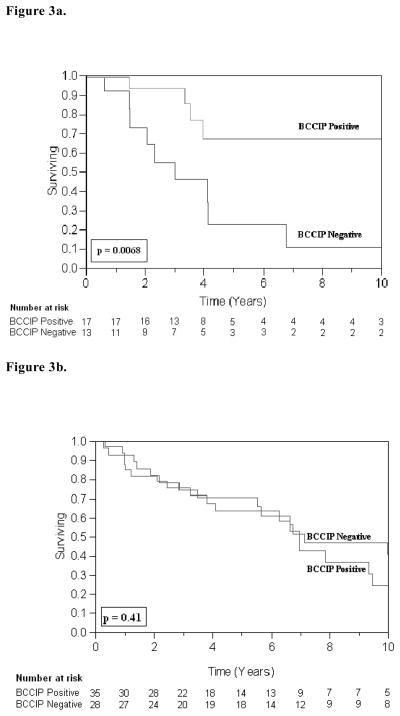
For 5-year poor overall survival, Table 3 illustrates that T2 stage predicted a relative risk of about 1.5 times that of T1 stage for poorer overall survival (RR 1.49; 95% CI 1.10–2.00, p=0.001). Analysis in Table 4 depicts that p53 expression and BCCIP expression were not independently significant predictors for overall survival. However, in the sub-group of patients with intact p53 (i.e. who did not overexpress p53), those who lost BCCIP expression showed over a two fold decrease in overall survival (RR 2.09; 95% CI 1.21–4.00, p=0.008). There was no statistically significant association between patients with overexpression of p53 and BCCIP status. Kaplan-Meier survival curves for 10-year overall survival are presented in Figure 3a and 3b.
Figure 3.
Figure 3a. Overall Survival (OS) in p53 Negative Patients: The 10-year OS was 67% for BCCIP-positive tumors (n = 17) and 11% for BCCIP-negative tumors (n = 13); (p = 0.0068, log-rank test).
Figure 3b. Overall Survival (OS) in p53 Positive Patients: The 10-year OS was 24% for BCCIP-positive tumors (n = 35) and 41% for BCCIP-negative tumors (n = 28); (p = 0.41, log-rank test).
Multivariate Analysis
Multivariate survival estimates were based on the Cox proportional hazards model and assumed no interactions between significant variables in the final model. Multivariate analysis was performed on the subgroup of patients who over-expressed p53 as well as those who did not. In the subgroup of patients with overexpression of p53 there was no statistically significant relationships with respect to tumor subsite, T-stage, or BCCIP status. Analysis of the intact (negative staining) p53 subgroup is presented in Table 5. Although there are no statistically significant correlations with tumor subsite, there are trends to significance in terms of T-stage and BCCIP status. T2 stage was correlated with an increase in local recurrence and poor overall survival within the 90% confidence interval (RR 2.01; 95% CI 0.92–4.38, p=0.07) and (RR 1.72; 95% CI 0.89–3.16, p=0.10), respectively. Loss of BCCIP expression trended toward poor overall survival (RR 1.72; 95% CI 0.94–3.53, p=0.07).
Table 5.
Multivariate Analysis of Prognostic Factors for Local Recurrence and Poor Overall Survival for Subgroup of Patients who Express Wild type p53
| Prognostic Factor | 5-year LR | 5-year Poor OS | ||
|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Tumor Subsite | ||||
| Glottic | 1.00 (Ref.) | 0.77 | 1.00 (Ref.) | 0.81 |
| Supraglottic | 1.14 (0.43–2.39) | 1.08 (0.52–2.00) | ||
| T Stage | ||||
| T1 | 1.00 (Ref.) | 0.07 | 1.00 (Ref.) | 0.10 |
| T2 | 2.01 (0.92–4.38) | 1.72 (0.89–3.16) | ||
| BCCIP Status | ||||
| Positive | 1.00 (Ref.) | 0.23 | 1.00 (Ref.) | 0.07 |
| Negative | 1.61 (0.72–3.79) | 1.72 (0.94–3.53) | ||
RR = Risk ratio; LR = local recurrence; OS = overall survival; CI = confidence intervals; Ref. = reference group;
Statistically significant.
DISCUSSION
Our tissue microarray of 123 patients found that loss of expression of both BCCIP and p53 significantly predicted for 5-year local recurrence and poor overall survival. The magnitude of this benefit was greater than a two-fold increase in local recurrence and poor overall survival (RR 2.04, p=.05; RR 2.09, p=.008), respectively. The study found that neither BCCIP status nor p53 wild type status was independently correlated with local recurrence or overall survival. The implications are that in patients with early stage larynx treated with radiation therapy that have an intact p53 pathway, lack of BCCIP expression identifies a group of patients who are at higher risk of local recurrence and poor overall survival.
The loss of p53 activity and subsequent uncontrolled cell cycle growth without arrest and repair of damaged DNA has been established in laryngeal neoplasms [20]. Previous reports have shown that BCCIP is an important component in maintaining the tumor suppression function of wild type p53 [14]. Without BCCIP, p53 fails to form tetramers, cannot bind with its target promoter sequences, and is defective in transactivation activity. This study suggests that the identification of the functional p53 pathway may be useful in identifying patients who have increased response to treatment (radiosensitivity) resulting in improvements in local control and overall survival.
T stage was also found to be a predictor of local recurrence and overall survival. As expected, higher T stage (T2) in our study also predicted a relative risk of about 1.5 times for increased local recurrence (p = 0.001) and poorer 5-year overall survival (p < 0.003). Several other studies have demonstrated similar outcomes for higher T stage laryngeal disease [21–23].
BCCIP has also been shown to play a role in homologous recombination DNA repair and chromosomal stability [24–26]. It is likely that lack of BCCIP confers additional genomic instability in the aggressive (T2 staged) laryngeal cancer. In this study, the T stage was also found to be correlated with BCCIP status (Table 2). 85% of BCCIP positive patients are a lower T stage (T1) and 64% of T1 stage express BCCIP. This is in good correlation for the projected role of BCCIP in maintaining genomic stability through multiple pathways.
The role of p53 as a tumor suppressor gene has been extensively studied but trying to assess its functional status through immunohistochemistry is not without its limitations. P53 mutants can be heterogenous in terms of loss of DNA binding activity and transactivation (27). Although hot spot mutants found in human cancer exhibit complete loss of their transactivating properties on all target genes, other mutants retain either a partial activity on all genes or on a subset of genes leading to a wide variety of mutant activity. (28)
The covariance between T stage and BCCIP status is a limitation of our findings, because it may imply a confounding factor in the use of BCCIP expression as a marker for improvements in local control and overall survival. However, BCCIP through its maintenance of a functional p53 pathway may be directly involved in the correlation with lower T stage via tumor suppression activity. Another limitation is that the study did not reveal, in multivariate analysis, an association between BCCIP and p53 expression and local recurrence or overall survival. However, there were trends towards statistical significance for BCCIP positive patients with an approximately 1.75 times improvement in overall survival within 90% confidence interval (p=0.07). Clearly a larger study with more events would be required to validate these findings. Given that BCCIP is a novel marker, we consider these findings to be hypothesis generating, and larger prospective, clinical trials are needed to further evaluate the relationship between primary radiotherapy in the larynx and BCCIP status. If validated in larger studies, these observations could have implications regarding selection of patients for radiation therapy or alternative strategies such as altered fractionation or concurrent therapies with radiation to improve outcomes.
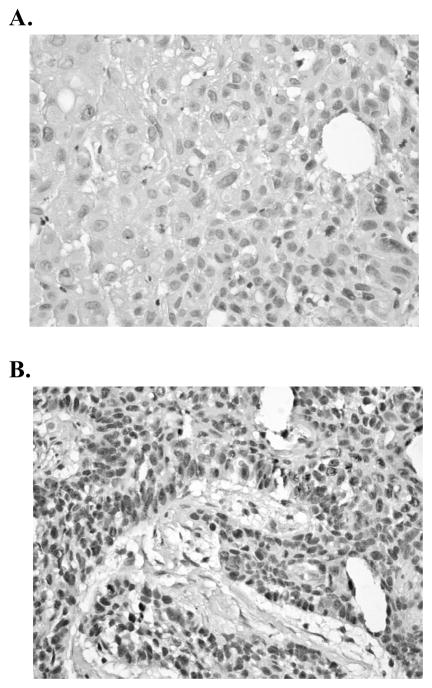
Figure 1.
Staining Characteristics of BCCIP on Tissue Microarrays. Nuclear BCCIP staining levels: 1 (negative) (A); 2 (positive) (B).
Acknowledgments
This research was supported by National Institutes of Health grants CA115488 and ES08353 to ZS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.Fremgen AM, et al. Clinical highlights from the National Cancer Data Base, 1999. CA Cancer J Clin. 1999;49(3):145–58. doi: 10.3322/canjclin.49.3.145. [DOI] [PubMed] [Google Scholar]
- 3.Franchin G, et al. Radiation treatment of glottic squamous cell carcinoma, stage I and II: analysis of factors affecting prognosis. Int J Radiat Oncol Biol Phys. 1998;40(3):541–8. doi: 10.1016/s0360-3016(97)00768-2. [DOI] [PubMed] [Google Scholar]
- 4.Rudoltz MS, Benammar A, Mohiuddin M. Prognostic factors for local control and survival in T1 squamous cell carcinoma of the glottis. Int J Radiat Oncol Biol Phys. 1993;26(5):767–72. doi: 10.1016/0360-3016(93)90490-m. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall WM, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100(9):1786–92. doi: 10.1002/cncr.20181. [DOI] [PubMed] [Google Scholar]
- 6.Franchin G, et al. Radiotherapy for patients with early-stage glottic carcinoma: univariate and multivariate analyses in a group of consecutive, unselected patients. Cancer. 2003;98(4):765–72. doi: 10.1002/cncr.11575. [DOI] [PubMed] [Google Scholar]
- 7.Milas L, et al. Chemoradiotherapy emerging treatment improvement strategies. Head Neck. 2003;25:152–167. doi: 10.1002/hed.10232. [DOI] [PubMed] [Google Scholar]
- 8.Hollstein M, et al. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz JL, et al. Functional inactivation of p53 by HPV-E6 transformation is associated witha reduced expression of radiation-induced potentially lethal damage. Int J Radiat Oncol Biol Phys. 1999;75(3):285–91. doi: 10.1080/095530099140465. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez GS, et al. Nat Genet. 2000;26:37–43. doi: 10.1038/79152. [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein B, et al. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 12.Hollstein M, et al. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 13.Jacks T, et al. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 14.Meng X, et al. Abrogation of the transactivation activity of p53 by BCCIP down-regulation. J Biol Chem. 2007;282(3):1570–6. doi: 10.1074/jbc.M607520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimm DL, et al. Amplification of tissue by construction of tissue microarrays. Exp Mol Pathol. 2001;70(3):255–64. doi: 10.1006/exmp.2001.2363. [DOI] [PubMed] [Google Scholar]
- 16.Chen B, et al. Validation of tissue array technology in head and neck squamous cell carcinoma. Head Neck. 2003;25(11):922–30. doi: 10.1002/hed.10308. [DOI] [PubMed] [Google Scholar]
- 17.Jourdan F, et al. Tissue microarray technology: validation in colorectal carcinoma and analysis of p53, hMLH1, and hMSH2 immunohistochemical expression. Virchows Arch. 2003;443(2):115–21. doi: 10.1007/s00428-003-0833-z. [DOI] [PubMed] [Google Scholar]
- 18.Leversha MA, et al. Expression of p53, pRB, and p16 in lung tumours: a validation study on tissue microarrays. J Pathol. 2003;200(5):610–9. doi: 10.1002/path.1374. [DOI] [PubMed] [Google Scholar]
- 19.Liu, et al. Oncogene. 2001;20:336–345. doi: 10.1038/sj.onc.1204098. [DOI] [PubMed] [Google Scholar]
- 20.Maestro R, et al. High frequency of p53 gene alterations associated with protein overexpression in human squamous cell carcinoma of the larynx. ONcogene. 1992;7(6):1159–66. [PubMed] [Google Scholar]
- 21.Medini E, et al. Radiation therapy in early carcinoma of the glottic larynx T1N0M0. Int J Radiat Oncol Biol Phys. 1996;36(5):1211–3. doi: 10.1016/s0360-3016(96)00431-2. [DOI] [PubMed] [Google Scholar]
- 22.Mendenhall WM, et al. T1-T2N0 squamous cell carcinoma of the gottic larynx treated with radiation therapy. J Clin Oncol. 2001;19(20):4029–36. doi: 10.1200/JCO.2001.19.20.4029. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki H, et al. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64(1):77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Meng F, et al. Roles of BCCIP in chromosome stability and cytokinesis. Oncogene. 2007;26(43):6253–60. doi: 10.1038/sj.onc.1210460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu H, et al. BCCIP regulates homologues recombination by distinct domains and suppresses spontaneous DNA damage. Nucleic Acids Res. 2007;35(21):7160–70. doi: 10.1093/nar/gkm732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H, et al. The BRCA2-interacting protein BCCIP functions in RAD51 and BRCA2 focus formation and homologous recombinational repair. Mol Cell Biol. 2005;25(5):1949–57. doi: 10.1128/MCB.25.5.1949-1957.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soussi T, Lozano G. p53 mutation heterogeneity in cancer. Biochem Biophys Res Commun. 2005;331:834–842. doi: 10.1016/j.bbrc.2005.03.190. [DOI] [PubMed] [Google Scholar]
- 28.Soussi T, et al. Reassessment of the TP53 mutation database in human disease by data mining with a library of TP53 missense mutations. Hum Mutat. 2005;25:6–17. doi: 10.1002/humu.20114. [DOI] [PubMed] [Google Scholar]