Abstract
In recent years the interest in studying the impact of sex steroids and gender on the regulation of blood pressure and cardiovascular disease has been growing. Women are protected from most cardiovascular events compared to men, until after menopause, and postmenopausal women are at increased risk of cardiovascular complications compared to premenopausal women. The pathophysiological mechanisms have not been elucidated but are not likely as simple as the presence or absence of estrogens, since hormone replacement therapy in elderly women in the Women’s Health Initiative or the HERS Trials did not provide primary or secondary prevention against cardiovascular events. Men are also thought to be at risk for cardiovascular disease at earlier ages than women, and these mechanisms too are not likely to be as simple as the presence of testosterone since androgen levels drop in men with cardiovascular and other chronic diseases. In fact, many investigators now believe that it is the reduction in androgen levels that frequently accompanies chronic disease and may exacerbate cardiovascular disease in men. In this review the roles of sex steroids and gender in mediating or protecting against hypertension and cardiovascular disease will be discussed.
Keywords: estrogens, androgens, menopause, obesity, sympathetic nervous system, renin-angiotensin system, endothelin
The roles played by sex and gender in controlling blood pressure have not been completely elucidated, and studies have shown that these mechanisms are not as easily understand as the presence or absence of sex steroids or the sex chromosomal complement present. As discussed below, both sex steroids and chromosomes likely impact the mechanisms of blood pressure control at multiple levels.
The difference between “Sex” and “Gender”
As defined by the Institute of Medicine in its white paper, entitled “Does Sex Matter?” published in 2010 (1), “sex” differences are defined as those caused by sex organs and subsequent sex steroids, estrogens or androgens, and by sex chromosomes, XX and XY. “Gender” is a sociological term that is “a person’s self representation as male or female, or how that person is responded to by social institutions based on the individual’s gender presentation. Gender is rooted in biology and shaped by environment and experience.” For simplicity, unless stated otherwise, animal studies using males and females are studies into the “sex differences” in a system, since animals do not consciously self-represent as “male” or “female”. In humans, unless a single sex trait is studied, e.g. prostate cancer in men or the effect of hormone replacement therapy in postmenopausal women, differences in traits in men and women connote a “gender difference”.
Sex Steroids vs. Sex Chromosomes
There is a significant amount of evidence that sex steroids contribute to cardiovascular disease progression or protection, and more studies are needed to more fully determine their effects. However, in order to evaluate the role played by sex steroids versus sex chromosomes on physiological parameters, Arnold and colleagues developed the Four Core Genotype mouse models that are either Sry null or have Sry transgene expressed on an autosome providing mice that have XX females, XY males, XY females and XX males (2). When the sex steroids are manipulated by castration or ovariectomy, the contribution of sex steroids to physiology in these animals can be separated from the contribution made by sex chromosomal complement.
To date, to our knowledge, there is no information suggesting that chromosomes alone affect cardiovascular disease including hypertension. In fact, Liu and colleagues used the Four Core Genotype mouse models and reported that angiotensin converting enzyme 2 (ACE2) activity in the kidney is modulated by estradiol and the ovarian milieu, but not by testicular steroids or Y chromosome (3). However, Chen and colleagues recently exploited the four core genotype models and have shown that adiposity is due to the dosage of X chromosomes independent of the gonadal sex or Y chromosome, due to lack of X chromosome inactivation of several genes (4). Mice with 2 X chromosomes, regardless of their type of gonad, had greater adiposity and food intake during daylight hours. They also had greater weight gain on high fat diet with higher lipid and insulin levels. Blood pressure was not measured in these animals, but obesity is known to increase blood pressure (5,6). Thus the contribution of chromosomes to hypertension or cardiovascular disease independent of gonadal steroids remains to be determined.
Sex steroids and Receptors
Estrogens are the most important female sex hormones and are synthesized in the granulosa cells of the ovary in females or Sertoli cells in males. Estradiol is produced by the conversion of testosterone and androstenedione by the aromatase enzyme. There are three types of estrogen: estradiol, which is more prevalent in premenopausal women, estrone which is more prevalent during the postmenopausal period, and estriol which is increased during pregnancy primarily due to synthesis in the placenta. There are also, three estrogen receptors (ER), namely estrogen receptor alpha (ERα), estrogen receptor beta (ERβ) (7,8) and the endoplasmic reticulum membrane-bound G-protein-coupled for estrogen receptor (GPER-1) a member of the G-protein-coupled receptor superfamily (9). GPER-1 is located in both intercalated cells and in tubular cells of the kidney (10), and ERβ and ERα are both present in the kidney and the vasculature (8,11,12). In the heart, ERα expression is similar in males and females, but ERβ expression in higher in males (13). The age of the animals/humans studied may also be important in ER localization, since Brandenberger et al., found In the midgestational human fetus that the prevalence of ERβ was greater in the kidney than ERα regardless of gender (14). In the brain, while ERα was located in nuclei of hypothalamic cells in men and premenopausal women, ERα was located in the cytoplasm in postmenopausal women (15). GPER-1 is located in highest concentrations in the hypothalamic-pituitary axis and do not bind either testosterone or progesterone (10). There is some evidence the GPER-1 may act as a mineralocorticoid receptor and bind aldosterone but this hypothesis is controversial (10). In any case, the potential differential expression and cellular and intracellular localization of the ERs and GPER-1 may explain some of the sex differences in the physiological responses to estrogens.
Testosterone is the primary male sex hormone and is synthesized in theca cells in ovaries of females and Leydig cells in testis of males. The androgen receptor (AR) has been found in most tissues. In humans two different forms of AR have been found: A and B. AR-B is predominant in all tissues in which both isoforms were detected. In fact, AR-A is not found in the kidneys of adult humans (16). In the heart, ARs are found in both males and females (13), but the subtype(s) present is not clear.
Both ERs and ARs are transcription factors that bind to estrogen or androgen response elements (EREs or AREs) upstream in the promoter regions of genes that regulate synthesis of proteins whose transcriptional and translational regulation is controlled by the steroids (7,8,16). In addition to genomic effects, sex steroids also have acute, nongenomic effects, especially in the vasculature where both estrogen and androgens cause acute vasodilation (17).
Estrogens and hypertension
Premenopausal women typically have lower blood pressure than do age-matched men (18). The National Health and Nutrition Examination Survey (NHANES) III and IV studies showed that prevalence of hypertension was greater in women, 60 years of age and older than men, regardless of ethnicity (19). The SIMONA epidemiological study (Study on Hypertension Prevalence in Menopause in the Italian population), a large cross-sectional study on 18,326 women ranging in ag from 46–59 years, showed that menopause is associated with a slightly but significantly higher blood pressure, even after adjustment for age and body mass index (BMI), as well as other confounding factors. Similar results were found in other studies that demonstrated natural menopause as a risk factor for higher blood pressure, independent of age and BMI (20).
The results of the Women’s Health Initiative (WHI) and Heart and Estrogen/progestin replacement study (HERS) I and II, are well known now, with hormone replacement therapy (HRT) with conjugated equine estrogen (CEE) and progestin or CEE alone failing to provide prevention against primary or secondary cardiovascular events (21–23). Moreover, these studies showed other deleterious effects of HRT, such as ischemic stroke, pulmonary emboli and other cardiovascular complications.
The effect of HRT on blood pressure is controversial. There are studies that show HRT increases blood pressure, decreases blood pressure, or has no effect (18,24–26). However, the positive or negative potential effect of estradiol on hypertension and cardiovascular disease may be age-related, since observational studies in women with premature or early menopause, estrogen is protective for ischemic stroke before age 50 years, but may become a risk factor after age 50–60 years (27).
Animal models also show age-related effects on blood pressure, some dependent on sex steroids, some not. For example, the Dahl salt sensitive rat exhibits increases in blood pressure when ovariectomized (28). In contrast, we have shown that the blood pressure in the spontaneously hypertensive rat (SHR) is independent of estrogens (29), and even in the postmenopausal period, treatment with estradiol reduces blood pressure only transiently (unpublished data, Yanes and Reckelhoff).
Androgens and hypertension
The role of androgens in mediating hypertension and cardiovascular disease is not clear. Animal studies suggest that androgens may promote cardiovascular-related diseases, including hypertension, since certain diseases are more common in men, such as myocardial infarction at an earlier age when testosterone levels are elevated. However, androgen levels are actually decreased in men with chronic diseases, including hypertension, obesity, heart disease, and chronic kidney disease (30,31). This has lead many investigators to propose that the reduction in testosterone with chronic disease may somehow contribute to the disease progression and is not just a consequence of the disease (30).
Men typically have higher blood pressure and develop cardiovascular diseases earlier than women. The sexual dimorphism in blood pressure begins at puberty and persists through adult age (32–34). The prevalence of hypertension is also higher in men until after menopause in women when the prevalence of hypertension is higher in women than age-matched men (18). However, as mentioned above, androgen deficiency is associated with cardiovascular disease. For example, the Fourth Tromsø study (1994–1995), in which 1568 randomly-selected, community dwelling men were analyzed for the impact of endogenous testosterone levels on later risk for myocardial infarction and all-cause mortality. Men with free testosterone levels in the lowest quartile had a 24% greater risk for all-cause mortality due to ischemic heart disease (35). These studies did not distinguish whether the men in the lowest testosterone quartile had some undiagnosed disease process and thus had lower testosterone or whether they were healthy and yet had lower testosterone that ultimately contributed to their cardiovascular disease and death. However, in other studies total testosterone levels in older men have been shown to be inversely associated with systolic blood pressure and increased risk of death over the subsequent 20 years, independent of multiple risk factors and several preexisting health conditions (36,37).
Sex Differences in Blood Pressure
We and others have a significant amount of data showing that there are sex differences in blood pressure control in most of the major systems known to be responsible for causing hypertension. For example, we showed many years ago that while renal denervation reduced the blood pressure in both male and female SHR, the reduction was similar in both males and females and that the blood pressure remained significantly elevated (38). These data show that the hypertension in both male and female SHR is mediated by the sympathetic nervous system, but that the sympathetics are not responsible for the sex difference in blood pressure. In addition, the fact that renal denervation failed to reduce the blood pressure to normotensive levels suggests that there are other mechanisms responsible for the hypertension in male and female SHR.
Other mechanisms include the renin-angiotensin system (RAS). We found that blockade of the RAS with enalapril, an angiotensin I converting enzyme inhibitor (ACEI), reduces blood pressure in both males and females and removes the sex difference (39). In addition, we found that treatment with ovariectomized females with testosterone increases the blood pressure in a dose dependent manner (39). In retrospect, enalapril also did not normalize the blood pressure in the SHR, as defined as blood pressure equal to 100 mm Hg, suggesting that other mechanisms contribute to the hypertension. Berecek and colleagues showed that ACE inhibitors given intracerebroventricularly reduced blood pressure to 140 mm Hg in male SHR (40), also showing that other mechanisms than central Ang II contribute to the hypertension in these rats. Since the sympathetic nervous system can stimulate the RAS and vice versa, it would be interesting to see if simultaneous blockade of both systems would normalize the blood pressure in male and female SHR.
In normotensive animals, sex differences in the pressor response to Ang II occur. Xue and colleagues reported that chronic Ang II increased blood pressure to a higher level in in male rats than females (41). Sartori-Valinotti and colleagues performed similar studies in rats chronically treated with enalapril, and found that females had a greater depressor response to ACEI and had a subsequently greater pressor response to chronic Ang II infusion than did the males (42). When placed on a high salt diet, the males responded with a further increase in blood pressure whereas females did not. This is a species effect since male mice exhibited a great pressor response to chronic Ang II regardless of their treatment with chronic ACEI (43).
There are also sex differences in the role that oxidative stress plays in mediating hypertension. In male SHR, inhibiting oxidative stress with superoxide dismutase mimetics or NADPH oxidase inhibitors reduces blood pressure without having an effect on blood pressure in females (26). In addition, increasing oxidative stress with molsidomine in males increases their blood pressure (44). A competent NO system is necessary for antioxidants to reduce blood pressure since blockade of nitric oxide synthase with nitro-L-Arginine methyl ester (L-NAME) prevents antioxidants from reducing the blood pressure in male SHR (45). Whether there are gender differences in the depressor response to antioxidants has not been tested, but in general antioxidants have not been successful in clinical trials in reducing blood pressure. Perhaps the antioxidants reduced blood pressure in men but not women. In addition, if the individuals exhibited considerable endothelial dysfunction perhaps due to chronic, long term hypertension, the antioxidants may not have been able to reduce the blood pressure in the clinical trials.
There are also sex differences in the mechanisms responsible for hypertension in SHR with aging, when the blood pressure in female SHR increases to levels similar to or higher than in males (18). In males, the hypertension is mediated by the RAS since blockade with losartan, the Ang AT1 receptor antagonist, normalizes their blood pressure (46). In aging female SHR, the hypertension is mediated by endothelin (47), the RAS (46), and 20-HETE (48). The combination of losartan (AT1 receptor antagonist), Abt 627 (endothelin ETA receptor antagonist), and 1-ABT (omega-hydroxylase inhibitor) reduced blood pressure to 110 mm Hg in these old females, but still failed to normalize the blood pressure (49). There is a contribution by the sympathetic nervous system as well since α1, β1,2-adrenergic receptor blockade or renal denervation reduce blood pressure but alone do not normalize it (Lima and Reckelhoff, data not published).
How Sex Steroids Could Modulate Blood Pressure
Estradiol is a vasodilator and increases nitric oxide (NO) by upregulating endothelial NO synthase (eNOS) expression chronically (50), but can also acutely increase NO due to its effect to increase intracellular calcium, a cofactor for eNOS activity. Estradiol is also modest antioxidant, although the role of oxidative stress in mediating hypertension in females has not been fully elucidated (see above) (51).
Estrogens can affect the RAS in several ways. Estrogens downregulate ACE, contributing to attenuation of synthesis of Ang II, while increasing synthesis of ACE2, the enzyme mainly responsible for synthesis of the vasodilatory peptide Ang(1-7) (52). Both effects would be anti-hypertensive. Estradiol can also downregulate the AT1 receptor (53), which would also be anti-hypertensive.
The effect of estrogens on sympathetic nervous system activity is not clear. Xue and colleagues reported a sex difference in the pressor response to aldosterone and salt with higher blood pressures developing in male rats than females, ovariectomy exacerbating, but castration having no attenuating effect on the hypertension compared to intact males (54). Interestingly, the sex difference in pressor response took 10–12 days to occur in these groups and females were protected with no significant increase in blood pressure in response to aldosterone. Central administration of estradiol blocked the hypertension in male rats given aldosterone and salt, and this protection was afforded via estrogen receptors since a non-specific estrogen receptor antagonist blocked the depressor response. Estrogen receptor antagonists given centrally also caused a pressor response to aldosterone in intact females, suggesting that the presence of estrogens protected the females from aldosterone-induced hypertension. The authors concluded that estradiol attenuated the sympathetic outflow in response to aldosterone and thus protected the animals from hypertension.
With aging, Esler and colleagues reported that sympathetic activity increases in most tissues except the kidney (55). However, the incidence of obesity increases with aging, and this is accompanied with increases in sympathetic activation (56). Thus studies are needed to fully characterize the effect of sympathetic activation on blood pressure control in pre- and post-menopausal women.
As mentioned previously, studies in animals show that androgens promote hypertension and cardiovascular disease. Studies in children by Weise and colleagues showed that norephinephrine levels increased significantly with puberty and were associated with increases in testosterone in boys, suggesting that puberty (and perhaps testosterone itself) is associated with sympathetic activation (57).
Other mechanisms by which androgens could promote hypertension include their effects to directly stimulate sodium reabsorption via the proximal tubule of the kidney, a mechanism that is blocked by AT1 receptor antagonists (58). Testosterone also increases angiotensinogen synthesis in the kidney (59,60), which could increase renin enzyme activity. Androgens have also been shown to increase endothelin levels in transsexuals taking masculinizing levels of testosterone (61).
Although testosterone supplements have become commonplace, there are few safety studies done on long term androgen treatment. However, in a short term crossover study, testosterone replacement in obese men decreased diastolic blood pressure and improved other components of metabolic syndrome (62). In male Zucker rats that are morbidly obese and have testosterone levels that are reduced by 50% compared to lean controls, testosterone supplements for 10 weeks attenuated body weight, inflammation, dyslipidemia, and insulin resistance, but unlike in the human studies, increased blood pressure (63), suggesting that additional long term studies with chronic testosterone in men with obesity are necessary since androgen supplements may be beneficial for improvement of characteristics of metabolic syndrome but their blood pressures need to be monitored carefully.
Androgen supplements in aging women and cardiovascular diseases
Androgens improve libido in women and thus are given to postmenopausal women to treat sexual dysfunction (64,65). However, there are no long term studies regarding the safety of androgen supplements, cardiovascular disease and hypertension in women. Boxer and colleagues reported data from a double-blind, randomized, placebo-controlled trial of 99 aging, frail women, that short-term therapy with dehydroepiandrosterone was safe with regard to cardiovascular risk factors (66). Nevertheless, other studies suggest that in menopause, androgen therapy could increase the risk factors for cardiovascular diseases, such as obesity, diabetes and hypertension. In a rat model of polycystic ovary syndrome produced by dihydrotestosterone (DHT) supplements, DHT caused increased food intake and body weights, increased adiposity, insulin resistance, and elevated blood pressure (67). In women there is evidence that the sympathetic nervous system is activated and may contribute to the elevated blood pressure in young women with PCOS (68), although the specific role played by androgens in the increase in sympathetic activity and the increase in blood pressure is not clear. In addition, the presence of PCOS with elevated androgens is a risk factor for increased cardiovascular disease after menopause (69). There is also evidence that androgen levels remain elevated after menopause in women who have had PCOS during their reproductive years (70). Thus the role of androgens in mediating hypertension and cardiovascular disease in aging women requires significantly more investigation.
Summary
We hypothesize that hypertension is multifactorial in both animals and humans, and that both sex chromosomes and sex steroids likely modulate the many factors that control blood. As shown in Figure 1, based on the literature, the most important factors that control blood pressure in males and females are activation of the sympathetic nervous system (SNS) and activation of the RAS to increase Ang II, the combination of which would increase sodium reabsorption by the kidney shifting pressure-natriuresis to the left and increasing blood pressure. The SNS can also activate the RAS by increasing renin release, and there is evidence, mainly in rodents, that increases in Ang II can activate the SNS. There is evidence that estrogens (54) can attenuate sympathetic activity (5) and we have unpublished data that androgens can stimulate the SNS, at least in females (Lima and Reckelhoff). Estrogens have been shown to attenuate the RAS by reducing AT1 receptor (11,53) and ACE expression (52) leading to reductions in Ang II, whereas androgens can stimulate synthesis of angiotensinogen (59,60) leading to increases in Ang II. Androgens have also been shown to increase sodium reabsorption in the proximal tubule via both androgen receptors and AT1 receptors (58). Whether estrogens affect sodium reabsorption directly is not clear. Because estrogens stimulate NO production (50), they will attenuate renal vasoconstriction both directly and by attenuating oxidative stress. Acutely androgens cause vasodilation, but whether androgens chronically cause vasoconstriction is not clear. In fact, there is evidence to suggest that the afferent arterioles of male SHR may be vasodilated compared to females since males exhibit renal injury at an earlier age than do females (71). This is supported by data from Schwartzman and colleagues showing that 20-HETE in the renal vasculature is upregulated via androgen-mediated upregulation of cytochrome P450 4A omega-hydroxylases (72). Whether estrogens can attenuate this response has not been studied. Androgens likely stimulate oxidative stress since hypertension in male, but not female, SHR and Dahl salt sensitive rats is mediated in part by oxidative stress (51). Androgens have also been shown to increase (15) and estrogens to attenuate (73) endothelin that would promote or attenuate renal vasoconstriction and sodium reabsorption, respectively.
Figure 1.
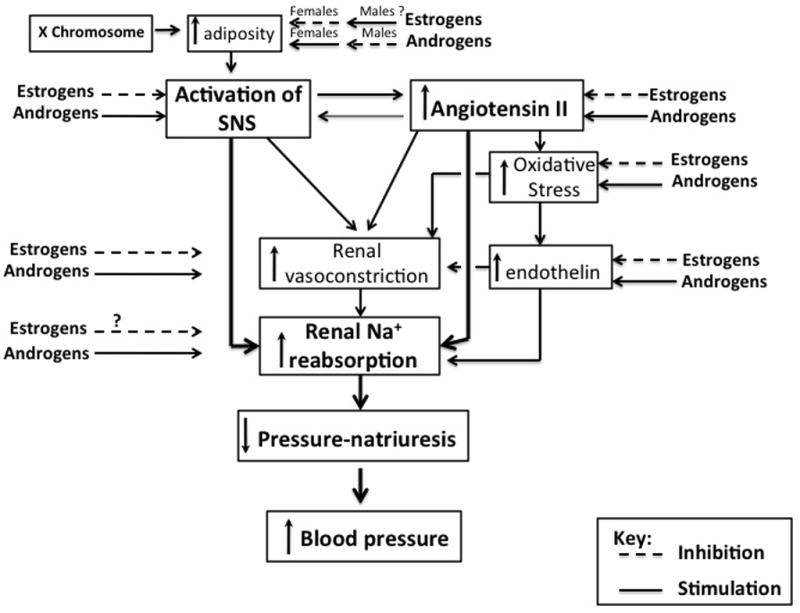
Potential mechanisms by which estrogens and androgens may control blood pressure.
Based on the recent studies using the four core chromosomal mice, sex chromosomes may also contribute to hypertension, whether directly or indirectly, as via X chromosomal increases in adiposity (4) that would then activate the SNS. It is also likely that sex steroids modulate the sex chromosomal effects, and that this modulation may be different in the two sexes. For example, while estrogens limit adiposity in females, androgens via their anabolic effects stimulate adiposity in females (67). Androgens decrease adiposity in males (63), and whether estrogens increase adiposity in males is not clear.
Thus more research is needed to determine how sex steroids and sex chromosomes modulate the important physiological factors that control blood pressure, and makes these exciting times for sex and gender differences studies!
Acknowledgments
The authors would like to thank the National Institutes of Health National Heart, Lung and Blood Institute for funding: RO1 HL 66072, RO1 HL69194, PO1 HL51971.
References
- 1.Wizemann TM, Pardue ML. Exploring the Biological Contributions to Human Health: Does Sex Matter? National Acad Press; 2001. Committee on Understanding the Biology of Sex and Gender Differences, Board on Health Sciences Policy Institute of Medicine; pp. 1–288. [PubMed] [Google Scholar]
- 2.Arnold AP, Chen X. What does “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Zheng W, Wu X, Zhu JJ, Arnold AP, Sandberg K. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ. 2010;1:1–6. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, McCluskey R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–6. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res. 2012;35:4–16. doi: 10.1038/hr.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Arch Toxicol. 2012;116:561–570. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deroo BJ, Buensuceso Minireview: Estrogen receptor-beta: Mechanistic insights from recent studies. Mol Endocrinol. 2010;24:1703–1714. doi: 10.1210/me.2009-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vasc Pharmacol. 2011;55:17–25. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinol. 153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R794–9. doi: 10.1152/ajpregu.00424.2006. [DOI] [PubMed] [Google Scholar]
- 12.Lu H, Lei X, Klaassen C. Gender differences in renal nuclear receptors and aryl hydrocarbon receptor in 5/6 nephrectomized rats. Kidney Int. 2006;70:1920–1928. doi: 10.1038/sj.ki.5001880. [DOI] [PubMed] [Google Scholar]
- 13.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol. 2009;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 14.Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab. 1997;82:3509–12. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 15.Hestiantoro A, Swaab DF. Changes in estrogen receptor-alpha and -beta in the infundibular nucleus of human hypothalamus are related to the occurrence of Alzheimer’s disease neuropathy. J Clin Endocrin Metab. 2004;89:1912–1925. doi: 10.1210/jc.2003-030862. [DOI] [PubMed] [Google Scholar]
- 16.Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Molecular and Cellular Endocrinology. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 17.Feldman RD, Gros R. Rapid vascular effects of steroids-a question of balance? Can J Cardiol. 2010;26(Suppl A):22A–26A. doi: 10.1016/s0828-282x(10)71057-6. [DOI] [PubMed] [Google Scholar]
- 18.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens. 2011;24:740–9. doi: 10.1038/ajh.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burl VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 20.Zanchetti A, Facchetti R, Cesana GC, Modena MG, Pirrelli A, Sega R SIMONA participants. Menopause-related blood pressure increase and its relationship to age and body mass index: the SIMONA epidemiological study. J Hypertens. 2005;23:2269–76. doi: 10.1097/01.hjh.0000194118.35098.43. [DOI] [PubMed] [Google Scholar]
- 21.Burry KA. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. Curr Women’s Health Rep. 2002;2:331–332. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 22.Herrington DM. The HERS trial results: paradigms lost? Heart and Estrogen/progestin Replacement Study. Ann Int Med. 1999;131:463–466. doi: 10.7326/0003-4819-131-6-199909210-00012. [DOI] [PubMed] [Google Scholar]
- 23.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Shakir YA, Samsioe G, Nyberg P, Lidfeldt J, Nerbrand C. Cardiovascular risk factors in middle-aged women and the association with use of hormone therapy: results from a population-based study of Swedish women. The Women’s Health in the Lund Area (WHILA) Study. Climacteric. 2004;7(3):274–83. doi: 10.1080/13697130400001372. [DOI] [PubMed] [Google Scholar]
- 25.Shelley JM, Green A, Smith AM, Dudley E, Dennerstein L, Hopper J, Burger H. Relationship of endogenous sex hormones to lipids and blood pressure in mid-aged women. Ann Epidemiol. 1998;8(1):39–45. doi: 10.1016/s1047-2797(97)00123-3. [DOI] [PubMed] [Google Scholar]
- 26.Masi CM, Hawkley LC, Berry JD, Cacioppo JT. Estrogen metabolites and systolic blood pressure in a population-based sample of postmenopausal women. J Clin Endocrinol Metab. 2006;91(3):1015–20. doi: 10.1210/jc.2005-2339. [DOI] [PubMed] [Google Scholar]
- 27.Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD., Jr Premature menopause or early menopause and risk of ischemic stroke. Menopause. 2012;19(3):272–7. doi: 10.1097/gme.0b013e31822a9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of Dahl hypertension. Hypertension. 2000;35:484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 29.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–9. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 30.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 31.Tamler R. Diabetes, obesity and erectile dysfunction. Gender Med. 2009;6:4–16. doi: 10.1016/j.genm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Himmelmann A, Svensson A, Hansson L. Influence of sex on blood pressure and left ventricular mass in adolescents: the Hypertension in Pregnancy Offspring Study. J Hum Hypertension. 1994;8:485–490. [PubMed] [Google Scholar]
- 33.Harshfield GA, Alpert BS, Pulliam DA, et al. Ambulatory blood pressure recordings in children and adolescents. Pediatrics. 1994;94:180–184. [PubMed] [Google Scholar]
- 34.Stamler J, Stamler R, Riedlinger WF, et al. Hypertension screening of 1 million Americans. Community Hypertension Evaluation Clinic (CHEC) program, 1973 through 1975. JAMA. 1976;235:2299–2306. doi: 10.1001/jama.235.21.2299. [DOI] [PubMed] [Google Scholar]
- 35.Vikan T, Schirmer H, Njølstad I, Svartberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromsø Study. Eur J Endocrinol. 2009;161(3):435–42. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- 36.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 38.Iliescu R, Yanes LL, Bell W, Dwyer T, Batatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol. 2006;290:R341–344. doi: 10.1152/ajpregu.00035.2005. [DOI] [PubMed] [Google Scholar]
- 39.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 40.Berecek K, Kirk KA, Nagahama S, Oparil S. Sympathetic function in spontaneously hypertensive rats after chronic administration of captopril. Am J Physiol. 1987;252:H796–806. doi: 10.1152/ajpheart.1987.252.4.H796. [DOI] [PubMed] [Google Scholar]
- 41.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 42.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension. 2008;51:1170–1176. doi: 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- 43.Venegas-Pont M, Sartori-Valinotti JC, Glover PH, Reckelhoff JF, Ryan MJ. Sexual dimorphism in the blood pressure response to angiotensin II in mice after angiotensin-converting enzyme blockade. Am J Hypertens. 2010;23:92–96. doi: 10.1038/ajh.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortepiani LA, Reckelhoff JF. Increasing oxidative stress with molsidomine increases blood pressure in genetically hypertensive rats but not normotensive controls. Am J Physiol Regul Integr Comp Physiol. 2005;289:R763–770. doi: 10.1152/ajpregu.00526.2004. [DOI] [PubMed] [Google Scholar]
- 45.Yanes L, Romero D, Iliescu R, Cucchiarelli VE, Fortepiani LA, Santacruz F, Bell W, Zhang H, Reckelhoff JF. Systemic arterial pressure response to two weeks of Tempol therapy in SHR: involvement of NO, the RAS, and oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R903–908. doi: 10.1152/ajpregu.00530.2004. [DOI] [PubMed] [Google Scholar]
- 46.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R383–90. doi: 10.1152/ajpregu.00510.2005. [DOI] [PubMed] [Google Scholar]
- 47.Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–33. doi: 10.1152/ajpregu.00697.2003. [DOI] [PubMed] [Google Scholar]
- 48.Yanes LL, Lima R, Moulana M, Romero DG, Yuan K, Ryan MJ, Baker R, Zhang H, Fan F, Davis DD, Roman RJ, Reckelhoff JF. Postmenopausal hypertension: role of 20-HETE. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1543–1548. doi: 10.1152/ajpregu.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lima R, Yanes LL, Davis DD, Reckelhoff JF. Roles played by 20-HETE, angiotensin II and endothelin in mediating the hypertension in aging female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R248–251. doi: 10.1152/ajpregu.00380.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner CP, Lizasain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of Calcium Dependent NO Synthase by Sex Hormones. Proc Natl Acad Sci USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol. 2007;34:938–45. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 52.Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1-7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol. 2008;93:648–57. doi: 10.1113/expphysiol.2007.041392. [DOI] [PubMed] [Google Scholar]
- 53.Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JFM, Daemen MJAP, Vetter H, Bohm M. Estrogen Modulates AT1 Receptor Gene Expression in Vitro and in Vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 54.Xue B, Badaue-Passos D, Jr, Guo F, Gomez-Sanchez CE, HYM, Johnson AK. Sex differences and central protective effect of 17beta-estradiol in the development of aldosterone/NaCl-induced hypertension. Am J Physiol Heart Circ Physiol. 2009;296:H1577–H1585. doi: 10.1152/ajpheart.01255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528(3):407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esler MM, Rumantir G, Wiesner D, Kaye J, Hastings G, Lambert Sympathetic nervous system and insulin resistance. From obesity to diabetes. Am J Hypertension. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- 57.Weise M, Eisenhofer G, Merke DP. Pubertal gender-related changes in the sympathoadrenal system in healthy children. J Clin Endocrinol Metab. 2002;87:5038–5043. doi: 10.1210/jc.2002-020590. [DOI] [PubMed] [Google Scholar]
- 58.Kienitz T, Quickler M. Testosterone and blood pressure regulation. Kidney Blood Press Res. 2008;31:71–79. doi: 10.1159/000119417. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y-F, Naftilan AJ, Oparil S. Androgen-Dependent Angiotensinogen and Renin Messenger RNA Expression in Hypertensive Rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 60.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen Regulation of Rat Renal Angiotensinogen Messenger RNA Expression. J Clin Invest. 1989;83:1941–1943. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Kesteren PJ, Kooistra T, Lansink M, Van Kamp GJ, Asscheman H, Gooren LJ, Emeis JJ, Vischer UM, Stehouwer CD. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb Haemost. 1998;79:1029–1033. [PubMed] [Google Scholar]
- 62.Marin P, Holgans S, Gustafsson C, Jonsson L, Kvist H, Elander A. Androgen treatment of abdominally obese men. Obes Res. 1993;1:245–51. doi: 10.1002/j.1550-8528.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 63.Davis DD, Lopez Ruiz A, Yanes LL, Iliescu R, Yuan K, Moulana M, Reckelhoff JF. Testosterone Supplementation in Male Obese Zucker Rats Reduces Body Weight and Improves Insulin Sensitivity, But Increases Blood Pressure. Hypertension. 2012;59:726–31. doi: 10.1161/HYPERTENSIONAHA.111.180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwenkhagen A. Libido loss after total surgery. Testosterone patch increases sexual desire. MMW Fortschr Med. 2006;148(47):48–9. [PubMed] [Google Scholar]
- 65.Krapf JM, Simon JA. The role of testosterone in the management of hypoactive sexual desire disorder in postmenopausal women. Maturitas. 2009;63:213–9. doi: 10.1016/j.maturitas.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 66.Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA, Kenny AM. Effects of dehydroepiandrosterone (DHEA) on cardiovascular risk factors in older women with frailty characteristics. Age Ageing. 2010;39:451–8. doi: 10.1093/ageing/afq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gender Med. 2011;8:103–15. doi: 10.1016/j.genm.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jedel E, Gustafson D, Waern M, Sverrisdottir YB, Landen M, Janson PO, Labrie F, Ohlsson C, Stener-Victorin E. Sex steroids, insulin sensitivity, and sympathetic nerve activity in relation to affective symptoms in women with polycystic ovary syndrome. Psychoneuroendocrinology. 2011;36:1470–1479. doi: 10.1016/j.psyneuen.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, Blackledge H, Khunti K, Howlett TA. Diabetes and cardiovascular events in women with polycystic ovary syndrome; a 20 years retrospective cohort study. Clin Endocrinol (Oxf) 2013 doi: 10.1111/cen.12068. in press. [DOI] [PubMed] [Google Scholar]
- 70.Krentz AJ, von Mühlen D, Barrett-Connor E. Searching for polycystic ovary syndrome in postmenopausal women: evidence of a dose-effect association with prevalent cardiovascular disease. Menopause. 2007;14:284–292. doi: 10.1097/GME.0b013e31802cc7ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension. 1999;34:920–3. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 72.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lekontseva O, Chakrabarti S, Davidge ST. Endothelin in the female vasculature; a role in aging. Am J Physiol Regul Integr Comp Physiol. 2010;298:R509–516. doi: 10.1152/ajpregu.00656.2009. [DOI] [PubMed] [Google Scholar]


