Summary
Background
Necrotizing enterocolitis (NEC) is one of the most serious disorders of gastrointestinal tract during neonatal period. Early diagnosis and adequate treatment are essential in the presence of clinical suspicion of NEC. Plain abdominal radiography is currently the modality of choice for initial evaluation of gastrointestinal tract in neonates. However, when the diagnosis is uncertain, abdominal ultrasound with bowel assessment might be an important complementary examination. The aim of the study was to evaluate usefulness of ultrasound in the diagnosis of NEC and its value for implementation of proper treatment.
Material/Methods
The data of nine neonates diagnosed with NEC, hospitalized at the Provincial Hospital No. 2 in Rzeszow in the period from September 2009 to April 2013 was retrospectively analyzed. Apart from abdominal radiography, abdominal ultrasound with bowel assessment was performed in all nine cases. Imaging findings, epidemiological data, coexisting risk factors and disease course were assessed.
Results
Most children in the group were preterm neonates. Findings in plain abdominal radiography were normal or nonspecific. A wider spectrum of findings was demonstrated in all ultrasound examinations and intestinal pneumatosis, a pathognomonic sign for NEC, was more frequently noted than in plain abdominal x-ray. Most children were treated by surgical intervention with resection of necrotic bowel loops and in more than half of the cases location of changes identified during surgery was concordant with ultrasonographic findings.
Conclusions
Abdominal ultrasound examination might be helpful in the diagnosis of NEC, especially when plain abdominal radiography findings do not correlate with clinical symptoms. However, abdominal radiography is still considered the modality of choice. The range of morphological changes detectable on ultrasound examination is much wider than in plain abdominal radiography. Ultrasound examination allows for more accurate assessment of changes within intestines and adjacent tissues, which aids clinicians in making more accurate therapeutic decisions and implementing proper treatment.
MeSH Keywords: Diagnostic Imaging, Emergency Treatment, Neonatology
Background
Intestinal disorders constitute an important clinical and diagnostic problem in neonatology. Necrotizing enterocolitis (NEC) is one of the most severe gastrointestinal conditions occurring during this developmental period. NEC is an inflammatory condition from a group of the most common gastrointestinal urgencies in neonates, most often affecting those with low birth weight and born prematurely [1–5].
Etiology of NEC is not well recognized. It is related to decreased perfusion and ischemia of intestinal wall (particularly in immature intestines), leading to disruption of intestinal barrier, enabling bacterial passage and activation of inflammatory mediators. Finally, it results in structural and functional changes, including formation of pneumointestine [6–8]. This process begins in mucosa and submucosa. Necrosis encompassing entire thickness of the intestine develops in most severe cases and may lead to perforation [9].
The main factors increasing the risk of this condition include the following: prematurity, perinatal asphyxia, chronic intrauterine hypoxia, early implementation of enteral nutrition in preterm neonates, disorders and congenital diseases of cardiovascular system, umbilical artery catheterization, meconium aspiration syndrome, respiratory distress syndrome or exchange transfusion [6,7,9,10].
Despite significant advances in neonatal care, mortality in NEC remains high (between 20 and 60% in a group of most immature neonates) and is maintained at the same level. Therefore, in cases of clinically suspected NEC quick diagnostics and implementation of appropriate treatment are crucial [4,5,10]. Clinical symptoms suggestive of NEC include abdominal bloating, growing anxiety, retention of intestinal contents, vomiting, ileus, gastrointestinal bleeding [9,11–14]. Abdominal x-ray or, less often contrast radiograms, are diagnostic modalities fundamental for initial assessment of gastrointestinal tract in case of clinically suspected NEC [9]. Due to its ability to visualize intestinal walls, gastrointestinal lumen and neighboring structures ultrasound (US) of gastrointestinal tract and intestines is an accessory method, especially when the x-ray findings are equivocal [9,15–17]. According to a three-stage Bell’s classification and its modifications, symptoms suggestive of NEC in radiograms include: distended intestinal loops, features of intestinal obstruction of various degrees, presence of air bubbles within intestinal wall (intestinal pneumatosis), presence of air bubbles within portal vein, ascites, presence of intraperitoneal free air [16] (Table 1). Changes noted in US include: augmented echogenicity of intestinal wall, changes in intestinal wall thickness (thickening over 2 mm or thinning below 1 mm), intestinal pneumatosis (“circle” or “aurora sign”), air bubbles within portal vein, free air in peritoneal cavity, peritoneal fluid (anechogenic or echogenic), reduced peristalsis. Moreover, Doppler ultrasound examination may reveal disrupted intestinal wall perfusion [15,17]. Among all mentioned diagnostic signs, intestinal pneumatosis is considered pathognomonic for NEC.
Table 1.
Bell’s classification of necrotizing enterocolitis according to radiographic findings.
| Stage | Radiologic signs |
|---|---|
| I |
|
| II |
|
| III |
|
Based on the results of clinical and diagnostic studies suggestive of NEC, conservative treatment consisting of gastrointestinal decompression and administration of antibiotics is implemented in less severe cases, while surgical intervention may be necessary in more severe cases, particularly when signs of peritonitis or perforation are present [4,16,18].
The goal of this work was to evaluate the role of gastrointestinal ultrasound in the diagnostics of NEC, to analyze morphological changes seen in ultrasound examination in the course of the disease and their correlation with radiological findings, as well as to evaluate usefulness of US for planning of further therapeutic management.
Material and Methods
The study included nine children (five girls and four boys) hospitalized at the Clinical Department of Neonatology with Intensive Care Unit, Clinical Department of Intensive Care and Anesthesiology with Toxicology Unit and Department of Pediatric Surgery at the St. Jadwiga the Queen Provincial Hospital No. 2 in Rzeszow between September 2009 and April 2013 with a diagnosis of necrotizing enterocolitis who underwent abdominal US with assessment of gastrointestinal tract and abdominal x-ray examination before implementation of treatment. US examinations were ordered when clinical status and results of additional examinations, including x-ray findings, were equivocal and did not allow for establishing final diagnosis and implementing proper treatment.
We analyzed retrospectively the results of x-rays and subsequent US performed no more than 24 hours later (beside one case where US was the first examination). Radiological signs suggestive of NEC according to Bell’s classification (Table 1) and US features according to classifications available in the literature were taken into consideration [9,15–17] – data acquired from Doppler examination were not subject to analysis, as intramural blood flow analysis is not always possible (most examinations were performed urgently at patient’s bedside). We also evaluated children’s epidemiological data, risk factors for development of NEC and course of treatment.
Ultrasound examinations were performed by a radiology specialist with experience in ultrasound diagnostics of children and gastrointestinal tract, including assessment of the intestines, from the Clinical Department of Radiology and Diagnostic Imaging. If child’s clinical state allowed, US was performed at the Ultrasound Laboratory of the Clinical Department of Radiology and Diagnostic Imaging, while children in severe clinical state had the examinations performed at their bedside. Standard assessment of abdominal organs with a convex probe was supplemented by examination with a linear probe and broadened to include assessment of intestinal structures. Examinations at the Clinical Department of Radiology and Diagnostic Imaging were performed using GE Logiq 7 apparatus with a 2–2.5 MHz convex probe and 7–14 MHz linear probe or GE Logiq E9 apparatus with 2.8–5 MHz convex probe and 7–11 MHz linear probe. Bedside examinations were performed with Esaote MyLab25Gold apparatus with 2.5–6.6 MHz convex probe and 3.5–10 MHz linear probe, Aloka ProSound α7 by Hitachi with 4–10 MHz convex probe and 4–11 MHz linear probe, as well as ATL HDI 3500 apparatus by Philips with a 5–8 MHz Micro-Convex probe and 5–12 MHz linear probe.
Results
Premature children born between 24th and 32nd week of gestation, with birth weight of 540–1960 g, constituted a majority (67%) of neonates included in the study, while the remaining children were born between 38th and 40th week of pregnancy, with birth weight of 2160–2600 g. In the preterm group symptoms of NEC appeared between 1st and 4th week of life, while all term neonates presented with symptoms in the first week of life. Among risk factors for NEC, coexisting respiratory disorders (respiratory distress syndrome or pneumonia) were diagnosed in all premature children, while patent ductus arteriosus (PDA) was found in four (67%) and aortic coarctation was diagnosed in one (16%) neonate.
In a group of term neonates the only identifiable risk factor was low birth weight, which was noted in two children.
Abnormalities were identified in five x-ray studies (56%), while in four children (44%) radiograms showed no changes. In all cases US revealed intestinal changes.
Abnormalities found in x-ray examinations included: signs of obstruction, presence of intramural air bubbles, and paucity of bowel gas. No other signs typical for NEC were noted (Table 2).
Table 2.
Radiographic and ultrasonographic findings identified in the course of NEC in the analyzed group of neonates.
| Radiographic findings | Ultrasonographic findings |
|---|---|
|
|
Abnormalities identified in US are presented in Table 2, including: enhanced echogenicity and increased intestinal wall thickness (2 to 3 mm), intramural air bubbles (hyperechogenic foci within intestinal wall with or without echo enhancement), air bubbles within portal vein (moving echogenic foci identified within portal vein), distended intestinal loops filled with fluid content and reduced or completely absence of peristalsis (signs of obstruction), intestinal wall edema, hyperechogenic, collapsed intestinal wall with reduced or absence of peristalsis suggestive of intestinal immaturity, anechogenic free fluid in peritoneal cavity, echogenic fluid in peritoneal cavity and/or air bubbles within the abdominal cavity (linear or punctate hyperechogenic foci outside the intestine) suggestive of intestinal perforation, or peritoneal calcifications indicating history of peritonitis.
Among signs identifiable in both US and x-ray (Tables 2–4) intestinal pneumatosis typical for NEC was found on ultrasound examination in four patients (44%) and on x-ray in one of those patients (11%) (Figures 1 and 2). Distended intestinal loops were noted in five patients (56%) on US and in three of those patients (33%) on abdominal radiogram (including coexisting obstruction, which was diagnosed in all of those US and two x-ray examinations). Features of intestinal immaturity were noted in four patients (44%) on US, while paucity of bowel gas suggestive of immaturity on x-ray was noted in two patients (22%) only. Other signs identifiable by both modalities were noted on ultrasound only, including portal venous gas in one patient (11%) (Figure 3), intraperitoneal air (Figure 4) in one patient as well (11%), and ascites in six subjects (67%).
Table 4.
Comparison of findings in ultrasound and plain abdominal radiography examinations.
| Signs | Number (%) of children, who presented with certain features in US | Number (%) of children, who presented with certain features in x-ray examination |
|---|---|---|
| Intestinal pneumatosis | 4 (44%) | 1 (11%) |
| Portal venous gas | 1 (11%) | 0 |
| Distended intestinal loops/signs of obstruction | 5/5 (56%) | 3 (33%)/2 (22%) |
| Signs of perforation | 1 (11%) | 0 |
| Features of intestinal immaturity in US (absence/reduced peristalsis)/paucity of bowel gas in x-ray examination | 4 (44%) | 2 (22%) |
| Ascites | 6 (67%) | 0 |
Figure 1.
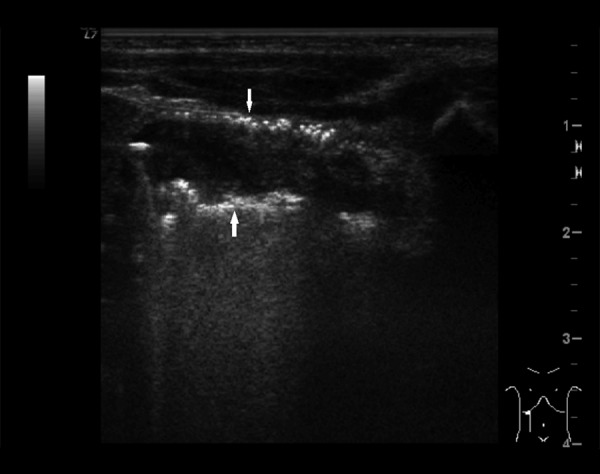
Ultrasound image of intestinal pneumatosis of large intestine (arrows).
Figure 2.
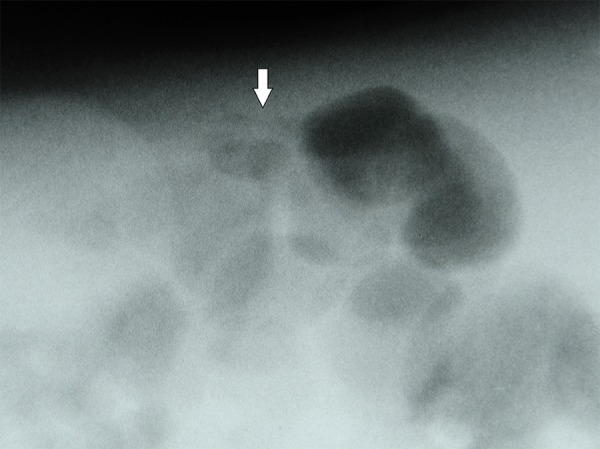
Plain x-ray performed in left lateral decubitus position: tiny air bubbles within intestinal wall (arrow).
Figure 3.
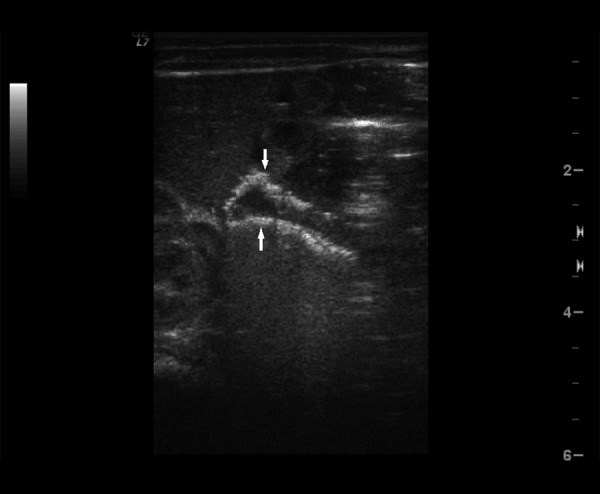
Ultrasound image of portal venous gas (arrows).
Figure 4.
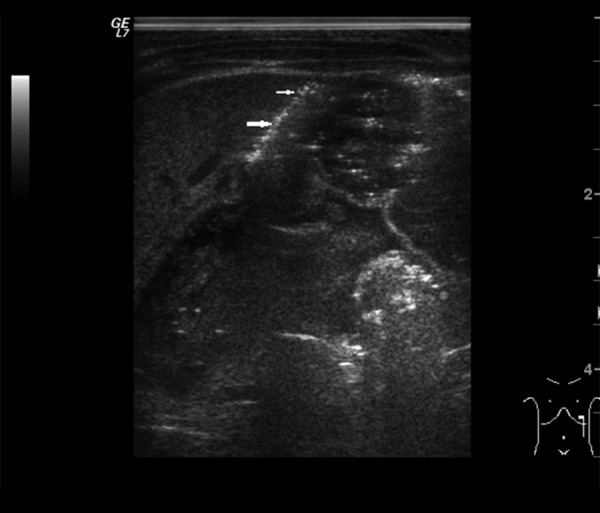
Ultrasound image of subsplenic intraperitoneal free air (arrows).
Among other abnormalities assessable in US (Tables 2–4) thickening/edema of intestinal wall found in 8 patients (89%) was the most common sign (Figure 5). Less common signs included: reduced or absence of peristalsis noted in 2 patients (22%) with obstruction, in 1 patient (11%) with features of intestinal immaturity and in 3 patients (33%) with both of those signs, echogenic fluid (Figure 6) found in 3 patients (33%), increased echogenicity of enteric walls found in 3 patients (33%) and tiny peritoneal calcifications secondary to previous inflammatory process, which were identified in one patient (11%) (Figure 7).
Figure 5.
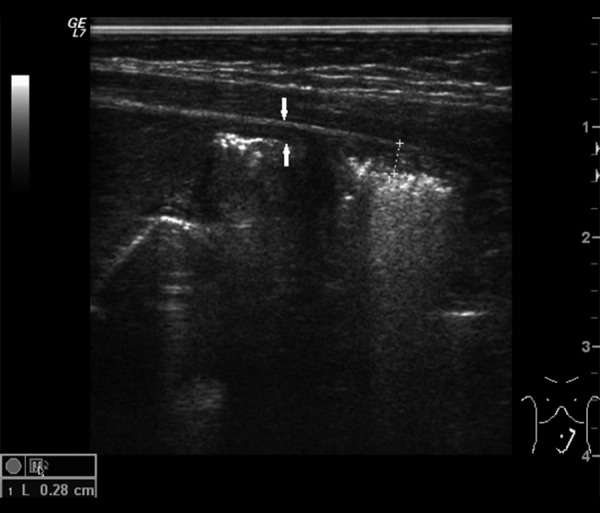
Ultrasound image of thickened, hyperechogenic bowel wall (arrows).
Figure 6.
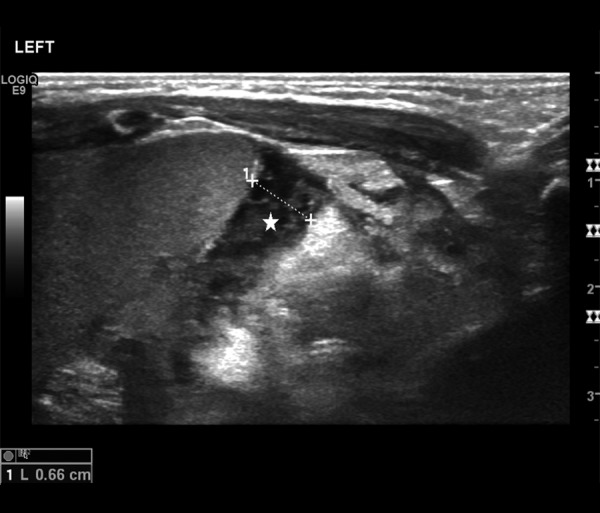
Ultrasound image of echogenic subhepatic intraperitoneal free fluid (star).
Figure 7.
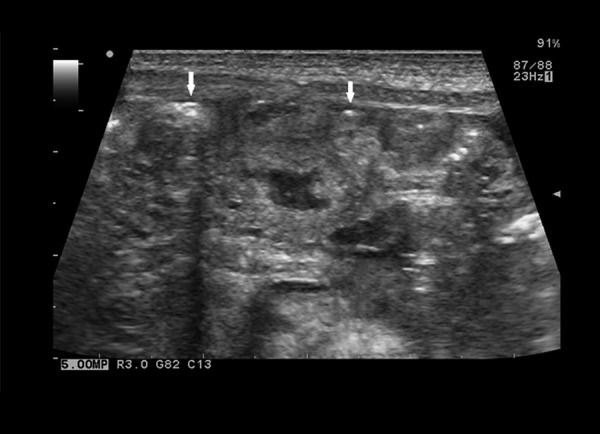
Ultrasound image of tiny intraperitoneal calcifications (arrows).
In the group of preterm children implemented management was more often surgical, involving resection of necrotic intestine and stoma formation – surgery was not performed in only one patient due to severe clinical state and death (Table 5). Mortality was high in this group and five children died (83%). In a group of term neonates in one patient (33%) surgical treatment was not implemented despite radiological and ultrasound features of NEC due to clinical stabilization, resulting in complete recovery (intestinal US performed one month later did not reveal any abnormalities). In another case only exploratory laparotomy was performed, followed by conservative treatment. The third child was treated surgically with resection of necrotic intestine and stoma formation. There were no deaths in the group of term neonates.
Table 5.
Treatment (with intraoperative assessment) and survival rate in the group of preterm and full term infants.
| Patient data | Preterm neonates (<37 week of gestation) | Full term neonates (>37 week of gestation) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B.c.I. | B.s.I. | B.s.M. | D.s.A. | D.c.B. | K.c.K. | P.P. | R.W. | S.L. | |
| Treatment and survival | |||||||||
| Conservative | − | + | − | − | − | − | + | − | − |
| Exploratory laparotomy | − | − | − | − | − | − | − | + | − |
| Laparotomy and resection of necrotic intestinal fragment | + | − | + | + | + | + | − | − | + |
| Death (D)/survival (S) | D | D | S | D | D | D | S | S | S |
Comparison of US with intraoperative macroscopic assessment revealed concordance between location of lesions with results of four the US studies (57% of operated cases), in two cases (28%) location of lesions identified during surgery was different than that in US, while in one case documentation of intraoperative localization was imprecise, which made data comparison impossible. Suspected intestinal perforation diagnosed in US was confirmed intraoperatively for 66% of ultrasound examinations where intraperitoneal echogenic fluid suggestive of perforation was found (analysis did not include patient with intraperitoneal air bubbles identified in US only – no surgical procedure was performed and intraoperative validation of suspected lesions was not possible). NEC was confirmed macroscopically in all operated cases. Presence of intestinal pneumatosis, pathognomonic for NEC, among operated children was found in 44% of US. Moreover, in one child (11%) treated conservatively, suspicion of NEC was put forward based on ultrasound examination.
Discussion
NEC is one of the most serious pathologies diagnosed in neonates, occurring most often in preterm children [1–4], which was confirmed in the studied group of children with NEC diagnosed intraoperatively (78% of children) or clinically (22% of children).
The most often encountered risk factors for NEC include prematurity and low birth weight, while the others are less common [4,19,20]. Symptoms occur most often between 2nd and 3rd week of life [21]. In our study group NEC was more common in the preterm group, their birth weight was most often below 1000 g (67%), all of them had risk factors for NEC (most commonly respiratory disorders and cardiovascular diseases) with clinical symptoms occurring between 1st and 2nd week of life. In two neonates born full term, of all risk factors, we found low birth weight; one had no risk factors for NEC and all of them presented with clinical symptoms in the first week of life (between 2nd and 6th day).
NEC is associated with high mortality – 20–60% according to various sources, thus early diagnosis is crucial [4,5,9]. According to available NEC diagnostic algorithms x-ray is the examination of choice, while US is considered mostly an accessory modality [9,15–17].
Based on x-ray findings, taking into consideration clinical and laboratory studies, one may establish the degree of disease progression on a scale I–III according to Bell’s classification and its modification proposed by Walsh and Kliegman and subsequently implement appropriate conservative (stages I–II) or surgical (stage III) management [16]. Radiologic signs included in Bell’s criteria may also be identified in US. Moreover, compared to x-ray ultrasound examination enables more precise morphological assessment of intestines and neighboring structures, differentiation between anechogenic and echogenic fluid as well as evaluation of intestinal function (peristalsis, intestinal perfusion) [9,15–17]. In our study group abnormal radiological findings were present in 56% of patients, including signs characteristic for NEC apparent in 33% of x-ray examinations, while intestinal pneumatosis, which is pathognomonic for NEC, was found in one patient only (11%) (Table 3). US changes were found in all examinations and they were characterized by broader spectrum.
Table 3.
Comparison of findings typical of NEC in ultrasound examinations and plain abdominal radiography in a group of preterm and full term infants.
| Patient data | Preterm neonates (<37 week of gestation) | Term neonates (>37 week of gestation) | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B.c.M. | B.s.M. | B.s.I | D.s.A. | D.c.B. | K.c.K. | P.P. | R.W | S.L. | ||
| Radiographic findings | +/− | |||||||||
| Distended intestinal loops | − | − | − | − | + | − | − | − | − | 1 (11%) |
| Signs of obstruction of various degrees (fluid levels) | − | − | − | + | − | − | + | − | − | 2 (22%) |
| Presence of air bubbles within intestinal walls | − | − | − | − | − | − | + | − | − | 1 (11%) |
| Presence of air bubbles in portal vein | − | − | − | − | − | − | − | − | − | 0 |
| Ascites | − | − | − | − | − | − | − | − | − | 0 |
| Intraperitoneal free air | − | − | − | − | − | − | − | − | − | 0 |
| Other: paucity of bowel gas | + | + | − | − | − | − | − | − | − | 2 (22%) |
| Grade of disease progression acc. to Bell’s radiological criteria | 0 | 0 | 0 | II | I | 0 | II | 0 | 0 | |
| Ultrasonographic findings | +/− | |||||||||
| Increased intestinal wall echogenicity | + | − | − | + | + | − | − | − | − | 3 (33%) |
| Thickening/edema of intestinal wall | + | + | + | + | + | + | − | + | + | 8 (89%) |
| Thinning of intestinal wall | − | − | − | − | − | − | − | − | − | 0 |
| Presence of air bubbles within intestinal walls | + | − | − | − | + | − | + | − | + | 4 (44%) |
| Presence of air bubbles in portal vein | − | − | − | − | − | − | + | − | − | 1 (11%) |
| Distended intestinal loops filled with fluid, with reduced/absence of peristalsis – sign of obstruction | − | + | + | + | + | − | + | − | − | 5 (56%) |
| Hyperechogenic, collapsed intestinal wall with reduced/absence of peristalsis – sign of intestinal immaturity | + | + | − | + | + | − | − | − | − | 4 (44%) |
| Anechogenic intraperitoneal fluid | − | − | + | + | + | − | − | + | − | 4 (44%) |
| Echogenic intraperitoneal fluid | − | − | − | − | − | + | − | + | + | 3 (33%) |
| Intraperitoneal free air | − | − | − | − | − | − | + | − | − | 1 (11%) |
| Peritoneal calcifications – sign of previous peritonitis | − | − | − | − | − | + | − | − | − | 1 (11%) |
Intestinal pneumatosis is a pathognomonic sign for NEC. Detectability of this sign in US varies between 13% and 100% according to different sources, while in radiograms it ranges 20–95% [16]. In our study group intestinal pneumatosis was diagnosed more often in US (44%), while radiogram showed it in one case only (11%) and was confirmed in US (Table 3). Air bubbles may show within intestinal wall before appearance of clinical symptoms of NEC. One should remember that they might be also sporadically found in the course of other pathologies (e.g. thrombosis or mechanical obstruction), although they rarely occur in neonates [9,15]. This sign is not always present in NEC (19–98% frequency of occurrence) [9] as shown by our study as well, since NEC was confirmed clinically and intraoperatively also in children without signs of intestinal pneumatosis in diagnostic imaging. Presence of intestinal pneumatosis does not always have to be associated with severe clinical course of the disease [9], which was also corroborated in our study group – one patient with intestinal pneumatosis, portal venous gas, obstruction and suspected perforation was treated conservatively due to stabilization of clinical symptoms, resulting in complete regression of symptoms.
Portal venous gas secondary to resorption of air bubbles from the intestine into portal venous system is less common than intestinal pneumatosis. Diagnostic utility of US in detection of this sign is higher than that of x-ray examination [16]. In our study group this sign was detected in one case only (11%) with US. It is not as specific as intestinal pneumatosis and may appear, for example, after umbilical vein catheterization [9].
US allows for detection of both minute and larger amounts of intraperitoneal gas [22]. However, in case of suspected perforation x-ray is the examination of choice [9]. In the analyzed group, x-ray findings did not show signs typical for perforation, while US confirmed presence of air bubbles in one case, although it was not verifiable intraoperatively due to conservative management.
Ability to visualize the amount, location and echogenicity of intraperitoneal fluid is an advantage of US over x-ray, which may visualize large amounts of fluid only [9]. Presence of intraperitoneal fluid is a nonspecific symptom, which may appear in a variety of other pathologies, although visualization of echogenic fluid is an additional factor supporting the diagnosis of perforation, even in the absence of intraperitoneal gas [11]. Echogenic fluid was found in 33% of US. All of those patients underwent laparotomy, which confirmed perforation in 2 cases. X-ray studies did not reveal presence of intraperitoneal fluid.
Intestinal wall thickening/edema (about 2–3 mm) was the most common abnormality diagnosed in US. However, we did not observe intestinal wall thinning related to diffuse necrosis. In x-ray studies assessment of wall thickness is imprecise, while US not only enables wall thickness measurements, but also allows for assessing echogenicity of its individual layers and peristalsis [9]. Thickness of normal intestine in a newborn may vary between 1.1 and 2.6 mm – it is considered thickened when it exceeds 2 mm, while thinning is recognized below 1 mm [22]. One should keep in mind that NEC is mainly diagnosed in preterm children. Radiological changes of the intestine in this group of patients are heterogenic and depand on the stage of development, thus mentioned criteria are not always applicable. Additional symptoms accompanying abnormal wall thickness include increased echogenicity when the wall is thickened and reduced echogenicity with wall thinning [22]. Although quite common, intestinal wall thickening is not specific for NEC. It is also true for abnormal peristalsis. However, when the assessment includes evaluation of perfusion, which shows absence of blood flow in the abnormal intestinal fragment, sensitivity of this examination reaches 100% [22].
Evaluation of intestinal distension and distribution of intestinal gas is an important part of radiographic assessment [9]. Intestinal distension, often secondary to obstruction, is the most common sign visible in 90% of radiograms in neonates with NEC. Low prevalence of this sign (33%) in our study group most likely ensues from inclusion criteria assumed for our study, which required equivocal x-ray findings. US also enables evaluation of the degree of intestinal distension and establishing diagnosis of obstruction. However, in contrast to x-ray examination, ultrasound does not allow for assessment of intestinal gas distribution [9]. In preterm neonates US allows for identification of changes suggestive of intestinal immaturity, which increase the risk of NEC (hyperechogenic, collapsed intestinal wall with reduced peristalsis) [23].
Despite significant progress in neonatal care and improved treatment outcomes, NEC remains an important diagnostic and therapeutic problem, as it is still associated with high mortality, which has remained at the same level for years [4,5]. Implementation of appropriate treatment is crucial for successful outcome, requiring involvement and cooperation of the entire diagnostic-therapeutic team. As previously mentioned, choice of therapeutic method depends on the degree of disease progression. Conservative treatment is implemented in stages I and II, while stage III requires surgical management, as it is associated with a threat of perforation. Literature from recent years regarding utility of US in the diagnostics of intestinal disorders places increasingly more emphasis on the need to include this examination into the diagnostic algorithm [9,15–17,22], particularly when there is a large discrepancy between patient’s clinical state and results of x-ray studies. In an early stage of NEC, despite alarming clinical symptoms, initial radiological findings may be nonspecific. Since disease progression is very rapid, it is crucial to establish the diagnosis as early as possible and US may be helpful in achieving this goal. Signs visualized in US, including intestinal perfusion, may be applied to radiological Bell’s classification in order to establish the degree of disease progression. Distension of intestinal loops with presence of fluid or air, suspicion of intestinal pneumatosis or portal venous gas, as well as increased perfusion in the abnormal portion of the intestine indicate mild disease. Intestinal pneumatosis and portal venous gas, intestinal wall thickening and increased perfusion of diseased intestine indicate moderate severity of the condition. Most advanced stages present with thinning of intestinal wall in the involved region, echogenic intraperitoneal fluid, free intraperitoneal air and absence of blood flow within changed intestinal fragment [22]. In our study group severity of clinical symptoms did not correlate with radiological features, which was the reason for ordering urgent US. Based on the results of US surgical management was implemented in the majority of patients (78% of cases). Other helpful signs identifiable in the US, apart from intestinal pneumatosis, which was diagnosed in 44 % of cases, are intestinal wall thickening and presence of echogenic fluid, and may aid in establishing proper diagnosis.
US also enables initial assessment of extent and location of changes, which is helpful in surgical management – laparotomy with resection of necrotic intestine is indicated in diffuse changes, while limited lesions may be sufficiently treated with less invasive peritoneal drainage, which is associated with improved prognosis in NEC, particularly in neonates with low birth weight [24–28]. In our study group surgical treatment was implemented in 78% of children – one child underwent exploratory laparotomy, while other patients were subjected to laparotomy and removal of necrotic portion of the intestine. Location of changes identified in US was concordant with intraoperative evaluation in 57% of operated cases.
We should point out some limitations of ultrasound examination. Abdominal US may be hindered by large amounts of intestinal gas, although it is rare among small children. Moreover, if the examination is performed by a physician experienced in evaluation of the intestine, with application of gradual pressure, this hindrance may be effectively eliminated [9]. Severe clinical state may also preclude this examination (instability and hypersensitivity to pressure in particular). In such cases it may be advantageous to use large amounts of gel, which enables execution of ultrasound examination without exerting abdominal pressure [9]. In our study group we were able to visualize intestinal structures despite the severe clinical state our patients were in. Among many advantages of US, we should remember about the fact that it does not expose the patient to ionizing radiation, which is very important for preterm as well as term neonates, who are developing rapidly.
Conclusions
Abdominal ultrasound examination with evaluation of the intestine aids in the diagnosis of necrotizing enterocolitis, particularly when results of x-ray do not correlate with severe clinical state of the patient.
Spectrum of morphological changes identifiable in US is much wider than in x-ray examination.
US examination in a course of NEC often enables identification of pathologies before they appear in radiograms.
US enables precise determination of the degree of progression of intestinal changes, which allows clinicians to make proper therapeutic decisions and implement appropriate treatment earlier.
References
- 1.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 2.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnabl KL, Van Aerde JE, Thomson AB, et al. Necrotizing enterocolitis: a multifactorial disease with no cure. World J Gastroenterol. 2008;14(14):2142–61. doi: 10.3748/wjg.14.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman RC, Stoll BJ, Curns AT. Necrotising enterocolitis hospitalisations among neonates in the Unites States. Paediatr Perinat Epidemiol. 2006;20:498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 5.Clark RZ, Gordon P, Walker WM, et al. Characteristics of patients who die of necrotizing enterocolitis. J Perinatol. 2012;32:199–204. doi: 10.1038/jp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohosiewicz J. Współczesne poglądy na etiopatogenezę obumierającego zapalenia jelit (NEC). Perinatalne i położnicze czynniki ryzyka. Surg. Child II Sympozjum Chirurgiczno-Neonatologiczne. 2004:56–61. [in Polish] [Google Scholar]
- 7.Kasznia-Brown J, Chilarski A. Współczesne poglądy na etiopatogenezę martwiczego zapalenia jelit. Przegl Pediatr. 2000;30:109–13. [in Polish] [Google Scholar]
- 8.Thompson AM, Bizzarro MJ. Necrotizingenterocolitis in newborns: pathogenesis, prevention and management. Drugs. 2008;68(9):1227–38. doi: 10.2165/00003495-200868090-00004. [DOI] [PubMed] [Google Scholar]
- 9.Epelman M, Daneman A, Navarro OM, et al. Necrotizing enterocolitis: review of state-of-the-art imaging findings with pathologic correlation. Radiographics. 2007;27:285–305. doi: 10.1148/rg.272055098. [DOI] [PubMed] [Google Scholar]
- 10.Luig M, Lui K. Epidemiology of necrotizing enterocolitis – Part II: Risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. 2005;41(4):174–79. doi: 10.1111/j.1440-1754.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- 11.Buonomo C. The radiology of necrotizing enterocolitis. Radiol Clin North Am. 1999;37:1187–98. doi: 10.1016/s0033-8389(05)70256-6. [DOI] [PubMed] [Google Scholar]
- 12.Yost CC. Neonatal necrotizing enterocolitis: diagnosis, management, and pathogenesis. Infus Nurs. 2005;28(2):130–34. doi: 10.1097/00129804-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 13.McElhinney DB, Hedrick HL, Bush DM, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106:1080–87. doi: 10.1542/peds.106.5.1080. [DOI] [PubMed] [Google Scholar]
- 14.Pinheiro JM, Clark DA, Benjamin KG. A critical analysis of the routine testing of newborn stools for occult blood and reducing substanes. Adv Neonatal Care. 2003;3:133–38. doi: 10.1016/s1536-0903(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 15.Silva CT, Danemann A, Navarro OM, et al. Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol. 2007;37:274–82. doi: 10.1007/s00247-006-0393-x. [DOI] [PubMed] [Google Scholar]
- 16.Bohnhorst B. Usefulness of abdominal ultrasound in diagnosing necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2013;98(5):445–50. doi: 10.1136/archdischild-2012-302848. [DOI] [PubMed] [Google Scholar]
- 17.Muchantef K, Epelman M, Darge K, et al. Sonographic and radiographic imaging features of the neonate with necrotizing enterocolitis: correlating findings with outcomes. Pediatr Radiol. 2013;43(11):1444–52. doi: 10.1007/s00247-013-2725-y. [DOI] [PubMed] [Google Scholar]
- 18.Bütter A, Flageole H, Laberge JM. The changing face of surgical indications for necrotizing enterocolitis. J Pediatr Surg. 2002;37:496–99. doi: 10.1053/jpsu.2002.30873. [DOI] [PubMed] [Google Scholar]
- 19.Fanaroff A, Hack M, Walsh M. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003;27:281–87. doi: 10.1016/s0146-0005(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 20.Guner YS, Chokshi N, Petrosyan M, et al. Necrotizing enterocolitis – bench to bedside: novel and emerging strategies. Semin Pediatr Surg. 2008;17:255–65. doi: 10.1053/j.sempedsurg.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Neu J. Neonatal necrotizing enterocolitis: an update. Acta Paediatr. 2005;94(Suppl 449):100–5. doi: 10.1111/j.1651-2227.2005.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 22.Faingold R, Daneman A, Tomlinson G, et al. Necrotizing enterocolitis: assessment of bowel viability with color Doppler US. Radiology. 2005;235:587–94. doi: 10.1148/radiol.2352031718. [DOI] [PubMed] [Google Scholar]
- 23.Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. 2007;85(2):629S–34S. doi: 10.1093/ajcn/85.2.629S. [DOI] [PubMed] [Google Scholar]
- 24.Holland AJ, Shun A, Martin HC, et al. Small bowel perforation in the premature neonate: congenital or acquired. Pediatr Surg Int. 2003;19(6):489–94. doi: 10.1007/s00383-003-0967-8. [DOI] [PubMed] [Google Scholar]
- 25.Calisti A, Perrelli L, Nanni L, et al. Surgical approach to neonatal intestinal perforation. An analysis on 85 cases (1991–2001) Minerva Pediatr. 2004;56(3):335–39. [PubMed] [Google Scholar]
- 26.Cass DL, Brandt ML, Patel DL, et al. Peritoneal drainage as definitive treatment for neonates with isolated intestinal perforation. J Pediatr Surg. 2000;35(11):1531–36. doi: 10.1053/jpsu.2000.18299. [DOI] [PubMed] [Google Scholar]
- 27.Gollin G, Abarbanell A, Baerg JE. Peritoneal drainage as definitive management of intestinal perforation in extremely low-birth-weight infants. J Pediatr Surg. 2003;38(12):1814–17. doi: 10.1016/j.jpedsurg.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Moss RL, Dimmitt RA, Henry MC. A meta-analysis of peritoneal drainage versus laparotomy for perforated necrotizing enterocolitis. J Pediatr Surg. 2001;36(8):1210–13. doi: 10.1053/jpsu.2001.25764. [DOI] [PubMed] [Google Scholar]


