Abstract
7,8-Dihydro-8-oxo-2′-deoxyguanosine (8-oxo-dGuo) is a useful biomarker of oxidative stress. However, its analysis can be challenging because 8-oxo-dGuo must be quantified in the presence of dGuo, without artifactual conversion to 8-oxo-dGuo. Urine is the ideal biological fluid for population studies, since it can be obtained non-invasively and it is less likely that artifactual oxidation of dGuo can occur because of the relatively low amounts that are present when compared with hydrolyzed DNA. Stable isotope dilution liquid chromatography/selected reaction monitoring-mass spectrometry (LC-SRM/MS) with [15N5]-8-oxo-dGuo as internal standard provided the highest possible specificity for 8-oxo-dGuo analysis. Furthermore, artifact formation was determined by addition of [13C1015N5]-dGuo and monitoring its conversion to [13C1015N5]-8-oxo-dGuo during the analytical procedure. 8-Oxo-dGuo concentrations were normalized for inter-individual differences in urine flow by analysis of creatinine using stable isotope dilution LC-SRM/MS. A significant increase in urinary 8-oxo-dGuo was observed in tobacco smokers when compared with non-smokers using either simple urinary concentrations or after normalization for creatinine excretion. The mean levels of 8-oxo-dGuo were 1.65 ng/mL and the levels normalized to creatinine were 1.72 μg/g creatinine. Therefore, stable isotope dilution LC-SRM/MS analysis of urinary 8-oxo-dGuo complements urinary isoprostane (isoP) analysis for assessing tobacco-smoking-induced oxidative stress. This method will be particularly useful for studies that employ polyunsaturated fatty acids, where reduction in arachidonic acid precursor could confound isoP measurements.
Keywords: stable isotope dilution, liquid chromatography-mass spectrometry, 8-oxo-dGuo, urine, oxidative DNA damage
Introduction
Reactive oxygen species (ROS) generated during normal cellular metabolism are detoxified by a suite of antioxidant enzymes including superoxide dismutases, catalases, glutathione peroxidases, and thioredoxins as well as by dietary antioxidants [1–5]. Oxidative stress occurs when ROS overwhelm the endogenous detoxification pathways such as during inflammation [6], viral and bacterial infections [6], metabolism of endogenous molecules such as estrogens [7], metabolism of drugs such as etoposide [8] metabolism of environmental chemicals such as benzo[a]pyrene [9], or tobacco smoking [10]. During oxidative stress, ROS can cause oxidative damage to cellular DNA [1, 11] as well as to the trinucleotide precursors of DNA [12]. 8-Oxo-dGuo is by far the most studied of the DNA-adducts that arise through ROS-mediated oxidative damage to DNA [13, 14].
Previous studies have revealed that significant amounts of dGuo are excreted in the urine [15–20]. This raised the possibility that adventitious oxidation of dGuo to 8-oxo-dGuo could occur during the urine extraction and analysis as we have previously shown for cellular DNA [11, 21]. It is noteworthy that rigorous feeding studies have shown that dietary 8-oxo-dGuo is not excreted in the urine [22, 23] and a number of studies have demonstrated that urinary 8-oxo-dGuo does not arise from cell death [24–26]. However, it is of significant concern that urinary 8-oxo-dGuo measurements could not be validated in the carbon tetrachloride rat model, one of the most widely accepted animal models of oxidative stress [27]. In spite of this potential problem, urinary 8-oxo-dGuo has become widely accepted as a measure of oxidative DNA-base damage [14]. This is because urinary 8-oxo-dGuo is quite stable [19] and urine can be readily acquired through a non-invasive procedure. Furthermore, there are multiple methods available for the analysis of urinary 8-oxo-dGuo including, enzyme-linked immunosorbent assay (ELISA) [18, 24, 28, 29], stable isotope dilution gas chromatography-mass spectrometry (GC-MS) [22, 25, 26, 30], and high performance LC coupled with electrochemical detection (ECD) [15, 18, 19, 31–33]. LC-MS-based methodology has proved to be particularly useful for urinary 8-oxo-dGuo analysis and so the approach described in this critical methods paper is based upon concepts described in these previous studies [16, 17, 20, 28, 34–58].
The clean-up methods employed for the urine before injection into the mass spectrometer have included: offline SPE and immunoaffinity column purification [33], two-steps of off-line clean-up followed by HPLC/ECD [32] or offline HPLC pre-purification followed by GC-MS analysis [30]. Newer methods have used a SPE clean-up step, coupled with LC-SRM/MS analysis [16, 53, 58]. Concentrations determined by LC-MS were correlated with those obtained by ELISA measurements using an assay where the primary antibody incubation was conducted at 4 °C [44]. Interestingly, although the mean amounts determined by LC-MS and ELISA were similar (Table 1), there were substantial inter-individual differences [44]. In a similar study conducted by Garratt et al. [28], there was a much greater difference between the LC-MS and ELISA values at both 4 °C and 37 °C (Table 1). The differences that were observed between LC-MS- and ELISA-based assays can be explained in part by the effect of urea on the antibody-antigen interaction that occurs in the ELISA [29]. As a result, the reported urinary 8-oxo-dGuo concentrations obtained by ELISA-based methodology have questionable validity [14]. This was particularly evident when urine samples are analyzed from individuals with a pathological condition such as cystic fibrosis [28].
Table 1.
Reported values for urinary 8-oxo-dGuo normalized to creatinine concentrations in non-smoking subjects.
| Technique | Non- smoking subjects (n) | Mean (nmol/mmol creatinine) | SD (nmol/mmol creatinine) | Mean (μg/g creatinine) | SD (μg/g creatinine) | Mean (ng/mL) | SD (ng/mL) | Reference |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HPLC-ECD | 60 | 2.70 | 1.88 | 8.26 | 6.11 | [32]*** | ||
| LC-MS | 35 | 4.69 | 1.70 | 5.87 | 2.61 | [39]** | ||
| LC-MS | 20 | 4.65 | 2.09 | [44]* | ||||
| ELISA (4 °C) | 20 | 3.44 | 1.62 | [44]* | ||||
| ELISA (37 °C) | 20 | 7.86 | 3.92 | [44]* | ||||
| HPLC-GC-MS | 115 | 3.86 | NP | 6.01 | 5.22 | [46] | ||
| HPLC-ECD | 115 | 4.20 | NP | 6.52 | 4.59 | [46] | ||
| ELISA (37 °C) | 115 | 18.7 | NP | 29.8 | 31.3 | [46] | ||
| LC-MS | 6 | 2.42 | NP | [16] | ||||
| LC-MS | 33 | 1.27 | 0.93 | [28] | ||||
| ELISA (4 °C) | 33 | 6.88 | 2.33 | [28] | ||||
| ELISA (37 °C) | 33 | 5.92 | 1.95 | [28] | ||||
| LC-MS | 50 | 3.70 | 2.00 | 6.20 | 4.80 | [51] | ||
| LC-MS | 48 | 0.72 | 0.45 | 1.72 | 1.07 | 1.65 | 1.68 | Present study |
NP = not provided;
Converted from pmol/μmol creatinine to nmol/mmol creatinine
Converted from μg/g creatinine to ng/mg creatinine and μg/L to ng/mL.
Converted from μmol/mol creatinine to nmol/mmol creatinine and nM to ng/mL.
Principles
Three base excision repair enzymes, human MutY homolog (hMutY) [59], hOGG1 [60], and hOGG2 [61] are involved in the repair of 8-oxo-dGuo-derived lesions in DNA, whereas, the hydrolase enzyme mammalian homologue of E. coli MutT (MTH) 1 removes 8-oxo-dGuo from the trinucleotide pool [40, 62]. It is this latter pathway that is considered to be the major source of urinary 8-oxo-dGuo (Fig. 1) [12]. Stable isotope dilution LC-SRM/MS methods are potentially more specific than ELISA-based methodology for the analysis of 8-oxo-dGuo because they can separate the individual oxidized DNA- and RNA-derived base-adducts. In general, a triple quadrupole (TQ) mass spectrometer operated in the SRM mode is employed for the analysis of urinary 8-oxo-dGuo. In this mode of operation, a precursor ion is pre-selected and resolved in quadrupole (Q) 1 of the TQ, fragmented by collision induced dissociation (CID) in Q2, and the resultant product ion is analyzed in Q3. Under optimal operating conditions, the precursor to product ion “reaction” is monitored many times per second, resulting in extremely reproducible chromatographic peak shape and intensity. In this way, a stable isotope labeled analog internal standard is used to establish the presence of an endogenous analyte using both the LC retention time and MS/MS mass selection of the TQ platform. This level of specificity cannot be attained with any other bioanalytical technique employed for biomarker analysis.
Figure 1.
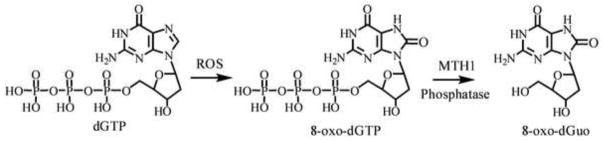
Scheme for the formation of urinary 8-oxo-dGuo.
An authentic stable isotope labeled analog of an analyte has identical physicochemical properties to the endogenous analyte except for its mass. The term stable isotope dilution refers to the use of a stable isotope labeled internal standard spiked into a sample at a known concentration. The response ratio between the analyte and labeled compound can then be interpolated onto a standard curve to calculate the absolute amount of analyte in the unknown sample. Therefore, the stable isotope internal standard offers a means to verify the presence of the analyte and normalize experimental variables such as sample storage and matrix suppression. The use of structural analogs as internal standards, rather than authentic isotope labeled analogs, is undesirable because they will have different retention times and ionization properties compared with the analyte of interest. Therefore, differential ionization can occur between an analyte and a structural analog in the source of the mass spectrometer. This difference arises in part from suppression of ionization by constituents present in the biofluid that is being analyzed and can lead to significant imprecision during quantitative analyses [63]. Unfortunately, suppression effects vary with chromatographic retention time and with biofluid samples from different individuals [64]. It is therefore extremely difficult to standardize the amount of suppression occurring within any particular sample [65].
The ideal control offered by an authentic isotope labeled internal standard is not always possible because for many biomarkers only deuterated and structural analogs are available. Deuterated forms of a compound are not perfect internal standards, since there is a small but significant separation of the deuterium analog internal standards and their corresponding endogenous protium forms during LC analysis [66]. This slight difference in chromatography can result in differential suppression or enhancement of ionization and affect the quality of the analytical data. Fortunately, [15N5]- and [13C1015N5]-dGuo analogs are available so the corresponding labeled 8-oxo-dGuo internal standards can be readily synthesized [11, 34, 53, 67]. Previous reports have described the use of both in-house synthesized stable isotope labeled internal standards as well as commercially available [15N5]-8-oxo-dGuo [14, 58] for the quantification of 8-oxo-dGuo in urine. Typically, [15N]- and [13C]-labeled internal standards have identical LC retention times to the corresponding protium forms [68]. Structural analogs are even less representative of the endogenous compound, since in addition to differences in LC retention time, the structural analog can show different absorptive losses. Selective binding to active sites on glassware or other surfaces can occur during extraction and LC analysis, leading to significant analyte loss. Whereas, a structural analog might not account for this loss, an isotope labeled internal standard has identical physicochemical properties, and is therefore lost at the exact same rate as the endogenous analyte. Due to this feature of stable isotope analogs, they may act as carriers, preventing the loss of trace amounts of analyte during extraction and analyses [69]. Finally, variability introduced during compound isolation can be fully controlled by an authentic isotope labeled standard [68].
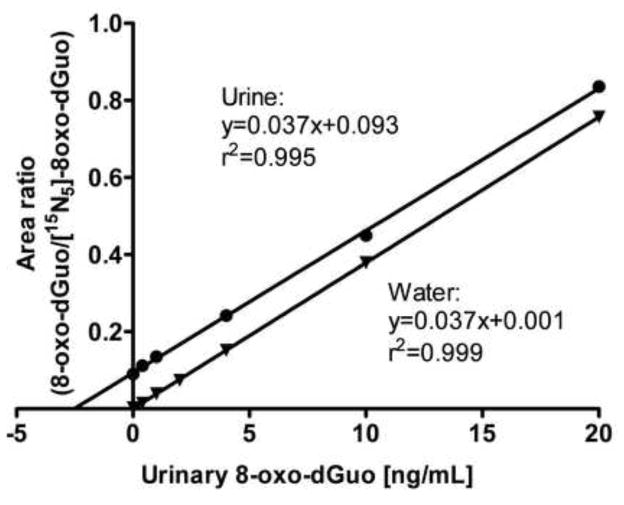
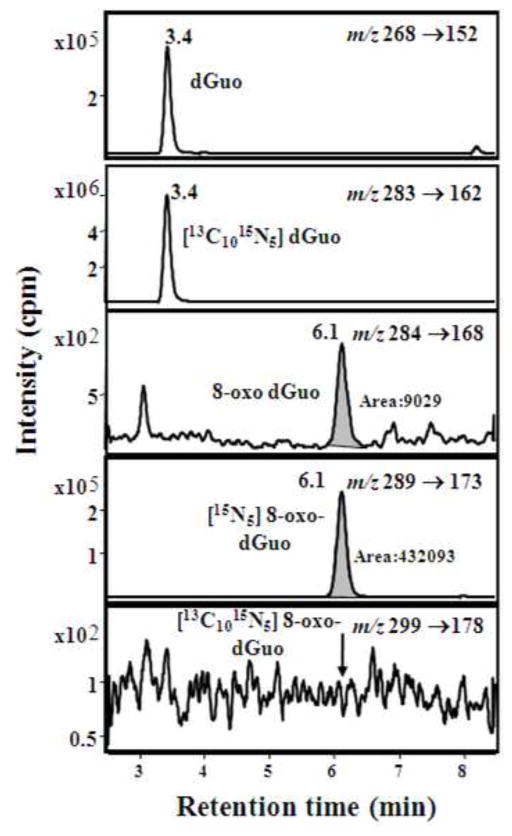
As noted in previous studies (including our own) the specificity of LC-SRM/MS analysis of 8-oxo-dGuo arises from the use of a unique transition from the protonated molecule (MH+) at m/z 284 to a product ion derived from the loss of the protonated ribose moiety (m/z 116) at m/z 168 [11, 34, 53, 58]. Similar specific transitions 5 Da higher in mass were employed for the internal standard [15N5]-8-oxo-dGuo from m/z 289 to m/z 173, and for the marker of artifactual oxidation ([13C1015N5]-8-oxo-dGuo) 15 Da (MH+) and 5 Da (product ion) higher in mass were used from m/z 299 to m/z 178. Thus, three parameters have to be correct in order to satisfy the analytical constraints required for identification of urinary 8-oxo-dGuo. The analyte must have the correct MH+ at m/z 284, the correct product ion at m/z 168 and an identical retention time to the internal standard (Fig. 2). This potentially provides higher specificity than can be obtained with HPLC-ECD because an internal standard with identical physicochemical properties cannot be used with this methodology. Interfering substances present in the urine were removed using SPE columns. A parallel standard curve was obtained in urine compared with a standard curve constructed in water (Fig. 3). Parallelism of the urine and water standard curves, which is important when analyzing endogenous analytes such as 8-oxo-dGuo, provided further validation of the assay specificity [68].
Figure 2.
Calibration curve constructed with authentic standards, performed in water and urine.
Figure 3.
LC-SRM/MS chromatograms from a non-smoker’s urine sample.
Validation of the critical methods assay was conducted on 5 separate days with five replicates at the lower limit of quantitation (LLOQ, 0.2 ng/mL), as well as with low quality control (LQC, 0.4 ng/mL), middle quality control (MQC,4 ng/mL) and high quality control (HQC, 20 ng/mL) samples. Precision and accuracy were within the range of ± 15 % and between 85 % and 115 %, respectively. Analysis of study samples was conducted using standard curves covering the range of concentrations found in the urine (Fig. 2) together with two LQC samples, two MQC samples, and two HQC samples. Assays were repeated if the QC values are outside the range of 15 % for precision or 85 % to 115 % for accuracy. Artifact formation was determined by addition of [13C1015N5]-dGuo and monitoring its conversion to [13C1015N5]-8-oxo-dGuo during the analytical procedure (Fig. 2). 8-Oxo-dGuo concentrations were normalized for inter-individual differences in urine flow by analysis of creatinine using a stable isotope dilution LC-SRM/MS assay that was based upon a previously reported procedure [16]. These methods can then be employed to determine whether there is a relationship between urinary 8-oxo-dGuo and tobacco smoking as a biomarker of tobacco smoke-induced oxidative stress.
Materials and methods
Chemicals and supplies
-
1
[15N5]-7,8-Dihydro-8-oxo-2′-deoxyguanosine ([15N5]-8-oxo-dGuo) (Cambridge Isotope Laboratories Inc. Cat. No. NLM-6715).
-
2
[13C1015N5]-dGuo (Cambridge Isotope Laboratories Inc. Cat. No. CNLM-3900).
-
3
8-oxo-dGuo (Sigma Aldrich Cat. No. H5653).
-
4
Desferal (Sigma Aldrich Cat. No. D9533).
-
5
Formic acid (Sigma Aldrich Cat. No. 56302).
-
6
Sodium chloride (Sigma Aldrich Cat. No. S7653).
-
9
Chelex 100 resin (Bio-Rad Cat. No. 143-2832).
-
10
Methanol, acetonitrile, water-all Optima grades were from Fisher Scientific.
-
11
Oasis HLB (30 mg, 1 mL) (Waters Cat. No. 94225).
-
12
Conical glass tubes 10 mL (Kimble Cat. No 73790-10).
Study participants and urine samples
Urine samples were obtained from non-smokers (n=48) or from cigarette smokers (n=85) who had smoked for a minimum of 6 years and a maximum for 60 years (mean = 34 years). Samples, which were provided during a clinic visit, were not collected at pre-determined times after the last cigarette had been smoked. Subjects were healthy individuals participating in an on-going study approved by the University of Pennsylvania Institutional Review Board (Protocol # 800924). Smoking status was assigned based on questionnaires, which requested information on smoking history, packs/day, and use of other tobacco products. All of the smoking subjects were cigarettes smokers except for one individual who also smoked one cigar/day. Urine samples were collected in 20 mL polypropylene tubes fitted with a screw cap. The tubes were capped, labeled and urine samples were stored in −80 °C until analysis.
Sample preparation
Positive Displacement Automated 1 mL pipette (Mettler Toledo, Cat. No. MR-1000).
Hamilton gas tight glass syringe (Fisher Scientific, Cat. No. 13-684-81).
24-port SPE Vacuum manifold (Fisher Scientific, Cat. No. 03-251-253).
Centrifuge (Sorvall Cat. No. 75004377).
Vortex (Fisher Scientific, Cat. No. 02-215-360).
Analytical nitrogen evaporator 24 sample positions (Fisher Scientific, Cat. No. NC9892499).
Liquid-chromatography
Phenomenex Kinetex C18 column (100 × 2.1 mm I.D., 2.6 μm) (Phenomenex, Cat. No. 00D-4462-AN).
Guard column C18 cartridge (0.5u × 0.004 in) (Phenomenex, Cat. No. AF0-8497).
HPLC. An Agilent 1200 series HPLC pump (Agilent Technologies, Santa Clara, CA) was used. It was equipped with an autosampler and thermo controller (set at 4°C). The column heater was set at 30 °C.
The mobile phase A was water with 0.02 % formic acid and mobile phase B was acetonitrile. The linear gradient was as follows: 3 % B at 0 min, 3 % B at 2 min, 20 % B at 8 min, 80 % at 8.1 min, 80 % at 11 min, 3 % B at 11.1 min and 3 % B at 15 min with a flow rate of 0.2 mL/min. Injections of 10 μL were made.
Mass spectrometry
An Agilent Technologies 6460 triple quadrupole mass spectrometer equipped with a JetStream source, was operated in positive mode, but any triple quadruple instrument could be used. The column effluent was diverted to waste for the first 3 min and the last 5 min of the analysis to prevent extraneous material from entering the mass spectrometer. The Agilent 6460 operating conditions were as follows: gas temperature was set at 275°C and the gas flow was set to 8 L/min. Sheath gas temperature was 400 °C and the sheath gas flow was set to 10 L/min. The capillary voltage was set to 3500 V. The nozzle voltage was set to 1000 V. The following transitions were monitored: m/z 284 (MH+) → m/z 168 [MH+-2′-deoxyribose+H] transition for 8-oxo-dGuo and m/z 289 (MH+) → m/z 173 [MH+-2′-deoxyribose+H] transition for [15N5]-8-oxo-dGuo. For dGuo and m/z 268 (MH+) → m/z 152 was monitored and for the labeled [13C1015N5]-dGuo m/z 283 (MH+) → m/z 162. Any labeled [13C1015N5]-8-oxo-dGuo that was formed during samples preparation from the added [13C1015N5]-dGuo was monitored by the transition m/z 299 (MH+) → m/z 178.
Protocol
Preparation of standards and calibration curves solutions
Individual primary stock solutions of 8-oxo-dGuo and [15N5]-8-oxo-dGuo (1 μg/mL) were prepared in methanol and stored at −80°C. For [13C1015N5]-dGuo a stock of 10 μg/mL was prepared in methanol as well. Working solutions were prepared by serial dilutions with methanol. One large urine sample (500 mL) was obtained from a never-smoker and used for the preparation of quality control (QC) samples. Calibration curves were prepared by spiking 8-oxo-dGuo in 250 μL of urine from a never-smoker who had not been exposed to second-hand smoke with 250 μL of 1 M NaCl with 100 μM desferal in Chelex-treated water, followed by the addition of 20 μl of internal standard solution (500 ng/mL). 8-oxo-dGuo was analyzed in the range 0.4 to 20 ng/mL. Daily eight point calibration samples (0, 0.2, 0.4, 1, 2, 4, 10 and 20 ng/mL) were prepared and analyzed together with two each of low, medium and high QC samples (LQC 1, MQC 4 and HQC 20 ng/mL, respectively). Concentrations are expressed as means ± standard deviation.
Sample preparation
The urine samples were stored at −80 °C until the night before analysis. The samples were thawed at 4 °C overnight and a 250 μL aliquot was taken from each tube after centrifuged for 3 min (10,000 × g) to remove any precipitates. With a set up containing two vacuum manifolds it is best to do at one time 34 urine samples, 8 calibration point samples and 6 QC samples.
Label one set of conical glass tubes with calibration, QC and urine sample numbers.
Add with the glass syringe 20 μL of internal standard solution (500 ng/mL) and 20 μL of [13C1015N5]-dGuo 10 μg/mL to all of the tubes.
Add with the glass syringe 10 μL of corresponding standard solutions to calibration and QC labeled tubes.
Add 250 μL of 1 M NaCl with 100 μM desferal in Chelex-treated water to all tubes with the automated 1 mL pipette.
Add 250 μL of water with 100 μM desferal in Chelex-treated water to all tubes that were used for calibration and QC samples.
Add 250 μL from each thawed urine sample to the tube labeled with the corresponding number with the automated pipette.
Vortex each tube for 5 sec.
SPE preparation
Label Oasis HLB cartridges exactly as the labeled tubes for samples.
Insert them in the vacuum manifold.
Pre-conditioned with 1 mL of acetonitrile added with the automated 1 mL pipette without vacuum.
Pre-conditioned with 1 mL of water added with the automated 1 mL pipette without vacuum.
Load the samples without vacuum. Change the pipette tip for every sample!
Wash with 1 mL Chelex treated water added with the automated 1 mL pipette without vacuum.
Wash with 1 mL 5 % methanol in Chelex treated water added with the automated 1 mL pipette without vacuum.
With the vacuum attached, the cartridges are dried under vacuum for 5 min.
Insert a labeled set of clean glass tubes to collect the samples.
Add to the SPE tubes 0.7 mL of 50% acetonitrile with the automated pipette to elute the analytes. It might be necessary to apply the vacuum for few seconds to get the cartridges wet, but the elution should be done without vacuum.
Remove the tubes from the manifold and dry the samples with the nitrogen evaporator.
HPLC sample preparation
Add 100 μL of water/acetonitrile (97/3) to the tubes containing the dried-down samples using an automated pipette.
Vortex for 10 sec.
Label the HPLC vials.
Move the re-suspended samples into the labeled HPLC with the pipette. Change the tip for every sample!
Calculations and Expected Results
Usually each analytical instrument has software that would do the calibration and QC samples automatically, after which would calculate the amount of 8-oxo-dGuo in all the analyzed samples. The instrument software package is used to calculate the peak areas based on the correct retention time. The peak areas for 8-oxo-dGuo and [15N5]-8-oxo-dGuo are shadowed in Fig 2. To get the calibration curve, one would calculate the area ratio for each of the calibration points, and those ratios were plotted against known concentrations of 8-oxo-dGuo (Fig. 3). From the calibration point one would find out the equation of the line in the form: , where y represents the area ratio and x the concentration.
For an unknown sample, once could find the y value by doing the area ratio of the analyte (8-oxo-dGuo) area over the internal standard ([15N5]-8-oxo-dGuo) area. Whit the calculated y, one could back-calculate the concentration
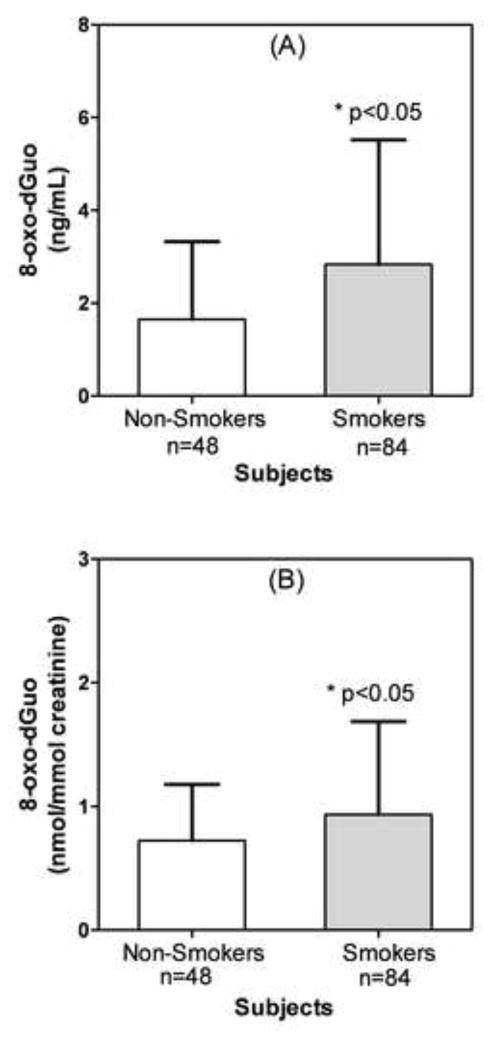
Using this method, urine samples from apparently health smokers (85) and non-smokers (48) were analyzed (Fig. 4). The concentration of 8-oxo-dGuo was found to vary widely, between 0.6 ng/mL to 15.7 ng/mL for the smokers (Fig. 4A). The concentrations in the non-smoker subjects were closer in range, varying between 0.2 ng/mL to 4.1 ng/mL (Fig. 4A). The mean urinary 8-oxo-dGuo concentration for 48 non-smokers was 1.65 ng/mL with a standard deviation (SD) of 1.68 ng/mL and the mean concentration for 85 smokers was 2.83 ng/mL with a SD of 2.67 ng/mL (Fig. 4A). When the values were normalized for creatinine concentrations, there was little effect on the range of values. The mean of the 8-oxo-dGuo concentrations in non-smokers’ urine was 0.72 nmol/mmol creatinine (SD = 0.45 nmol/mmol creatinine) and the mean concentration in the smokers’ urine was significantly higher at 1.07 nmol/mmol creatinine (SD, 1.50 nmol/mmol creatine) (Fig. 4B). These values correspond to a mean of 1.72 μg/mg creatinine (SD = 1.10 μg/mg creatinine) for the non-smokers and a mean of 2.21 μg/mg creatinine (SD = 1.79 μg/mg creatinine) for the smokers. There was no significant difference in the urinary creatinine concentrations between non-smokers and smokers. The mean values for non smokers (n = 48) were 1.22 mg/mL (SD = 1.16 ng/mL) or 10.77 mM (SD = 10.25 mM) and for smokers (n=84) were 1.42 mg/mL (SD = 1.10 ng/mL) or 12.59 mM (SD = 9.81 mM).
Figure 4.
8-Oxo-dGuo concentrations in urine from apparently healthy non-smokers and smokers. (A) Concentrations in ng/mL urine. B. Concentrations normalized to creatinine (nmol/mmol creatinine). A two-tailed, unpaired t-test with Welch’s correction for unequal variances, and a confidence interval of 95 % was used to determine statistical significance.
Caveats
DNA damage, which occurs during oxidative stress, results in the formation of 8-oxo-dGuo [9, 11, 70]. The 8-oxo-dGuo is excised from DNA by glycosylase-mediated repair, which results in the release of 8-oxo-guanine rather than 8-oxo-dGuo [60, 61]. Therefore, analyses of urinary 8-oxo-guanine cannot distinguish between RNA and DNA-damage. In contrast, oxidative damage to the trinucleotide pool results in the formation of 8-oxo-2′-deoxyguosine triphosphate (8-oxo-dGTP), which is hydrolyzed by MTH1 to release 8-oxo-2′-deoxyguosine monophosphate (8-oxo-dGMP) rather than 8-oxo-guanine [62]. The 8-oxo-dGMP is then converted to 8-oxo-dGuo by cellular phosphatases [71]. Therefore, urinary 8-oxo-dGuo concentrations are thought to reflect oxidative damage to the trinucleotide pool rather than to DNA [12]. Ideally, it would be best to analyze urinary 8-oxo-dGuo in 24 h urine samples so that the possible changes in the glomerular filtration rate (GFR) during that period would have a minimal effect on the concentration of 8-oxo-dGuo. Unfortunately, this is often not possible in biomarker studies as it is difficult to collect urine for an entire 24-h period. Spot urine samples are frequently used as an alternative because they are simple to collect and pose minimal subject inconvenience. However, spot urinary 8-oxo-dGuo concentrations may fluctuate because of many factors (such hydration status) that are unrelated to its rate of formation. This means that changes in urinary 8-oxo-dGuo concentrations from shorter collections times might simply reflect modulation in GFR during a particular collection period.
The concept of creatinine adjustment to normalize for changes in GFR, which was originally proposed by Vought et al. [72], depends upon daily urinary creatine excretion by a healthy individual being constant [73]. Creatinine is formed non-enzymatically from creatine (primarily in the muscle) at an almost steady-state rate of approximately 2 % of the creatine pool per day [74]. Creatine itself can be formed endogenously from glycine and arginine through the transamidinase-mediated intermediate formation of guanidinoacetate, which is then converted into to creatine by N-guanidinoacetate methyltransferase-mediated methylation by S-adenosylmethionine [75]. The rate of creatine synthesis is closely regulated by feedback inhibition of transamidinase. Thus, on a creatine-free vegetarian diet, this pathway is fully activated, and adequate guanidinoacetate is synthesized from its amino acid precursors [76]. Conversely, creatine that is ingested from meat, partially or totally represses transamidinase to modulate its endogenous production. Creatinine is formed non-enzymatically from creatine through cyclization and dehydration or by the intermediate formation of phosphocreatine. The resulting creatinine then diffuses into the circulation and appears in the urine after glomerular filtration.
Daily urinary excretion of creatinine derived from muscles occurs at a rate of approximately 1 g/day (1 g/20 kg of muscle mass) [77]. The normal daily urinary excretion of creatinine is relatively stable for an individual, with a daily variation of between 4 % and 8 %; however, there are substantial inter-individual differences, which are dependent upon sex, height, weight, race, age, and other factors [78]. This means that considerable uncertainty could be introduced when using creatinine excretion is used as a normalization factor. Nevertheless, adjustment for creatinine concentration is commonly used for ELISA-, GC-MS-, HPLC-ECD- and LC-MS-based assays of urinary 8-oxo-dGuo (Table 1) [16, 28, 32, 39, 44, 46, 52]. Conversely, total urinary nicotine concentrations, which provide an index of smoking topography, are rarely normalized for creatinine [79]. Another possible confounding factor is the general use of colorimetric assays for the analysis of urinary creatinine. We have found that this underestimates creatinine concentrations by 20 % (data not shown) when compared with LC-MS-based methodology similar to that described by Teichert et al. [16]. Therefore, it is conceivable that additional uncertainties exist in much of the 8-oxo-dGuo data that has been published when the simple colorimetric assay was employed to analyze urinary creatinine.
Alternative approaches have been advocated such as using timed urine collections and then normalizing to the urinary creatinine excretion rate rather than its concentrations. However, there could still be uncertainty in the actual timing of the urine collections unless they are conducted under carefully controlled conditions. A more innovative approach has been proposed by Warrack et al. for use in metabonomic analyses of urinary metabolites. This involves normalization to urine osmolality, which is a direct measure of total endogenous metabolic output [80]. Using this normalization method, it was possible to reduce variation among biological replicates, which was not corrected by the use of creatinine concentrations [80]. There are as yet no reports on the use of either of these approaches for the analysis of urinary 8-oxo-dGuo. Therefore, in future studies, it will be necessary to evaluate the utility of these methods for normalizing urinary 8-oxo-dGuo concentrations in spot urine samples to take account of potential intra- and inter-individual differences in GFR.
Highlights.
7,8-Dihydro-8-oxo-2′-deoxyguanosine (8-oxo-dGuo) an oxidative stress biomarker.
Urine is the ideal biological fluid for analyzing 8-oxo-dGuo in population studies.
High specificity with LC-SRM/MS and [15N5]-8-oxo-dGuo internal standard.
Artifact formation can be determined by addition of [13C1015N5]-dGuo.
A significant increase in urinary 8-oxo-dGuo was observed in tobacco smokers.
Acknowledgments
This work was supported by NIH grants U01 ES016004, R01 CA130961, and P30 ES013508.
Abbreviations
- 8-oxo-dGuo
7,8-Dihydro-8-oxo-2′-deoxyguanosine
- CID
collision-induced-dissociation
- dGuo
2′-deoxyguanosine
- dGMP
2′-deoxyguanosine-monophosphate
- dGTP
2′-deoxyguanosine-triphosphate
- ECD
electrochemical detection
- ELISA
enzyme-linked immunosorbent assay
- GC-MS
gas chromatography-mass spectrometry
- hMutY
human MutY homolog
- hOGG
human 8-oxo-guanine glycosylase
- isoP
isoprostane
- HPLC
high performance LC
- HQC
high quality control
- LC-SRM/MS
liquid chromatography/selected reaction monitoring-mass spectrometry
- LLOQ
lower limit of quantitation
- LQC
lowest quality control
- MQC
middle quality control
- MTH
mammalian homologue of E. coli MutT
- Q
quadrupole
- SPE
solid phase extraction
- TQ
triple quadrupole
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee SH, Blair IA. Oxidative DNA damage and cardiovascular disease. Trends Cardiovasc Med. 2001;11:148–155. doi: 10.1016/s1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 2.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro L, Freeman BA. Reactive oxygen species in human health and disease. Nutrition. 2001;17:161, 163–161, 165. doi: 10.1016/s0899-9007(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 4.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 8.Zheng N, Felix CA, Pang S, Boston R, Moate P, Scavuzzo J, Blair IA. Plasma etoposide catechol increases in pediatric patients undergoing multiple-day chemotherapy with etoposide. Clin Cancer Res. 2004;10:2977–2985. doi: 10.1158/1078-0432.ccr-03-0221. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Mangal D, Tacka KA, Quinn AM, Harvey RG, Blair IA, Penning TM. Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proc Natl Acad Sci U S A. 2008;105:6846–6851. doi: 10.1073/pnas.0802776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke A, Fitzgerald GA. Oxidative stress and smoking-induced vascular injury. Prog Cardiovasc Dis. 2003;46:79–90. doi: 10.1016/s0033-0620(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 11.Mangal D, Vudathala D, Park JH, Lee SH, Penning TM, Blair IA. Analysis of 7,8-dihydro-8-oxo-2′-deoxyguanosine in cellular DNA during oxidative stress. Chem Res Toxicol. 2009;22:788–797. doi: 10.1021/tx800343c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke MS, Olinski R, Loft S. Measurement and meaning of oxidatively modified DNA lesions in urine. Cancer Epidemiol Biomarkers Prev. 2008;17:3–14. doi: 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- 13.Olinski R, Rozalski R, Gackowski D, Foksinski M, Siomek A, Cooke MS. Urinary measurement of 8-OxodG, 8-OxoGua, and 5HMUra: a noninvasive assessment of oxidative damage to DNA. Antioxid Redox Signal. 2006;8:1011–1019. doi: 10.1089/ars.2006.8.1011. [DOI] [PubMed] [Google Scholar]
- 14.Evans MD, Olinski R, Loft S, Cooke MS. Toward consensus in the analysis of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine as a noninvasive biomarker of oxidative stress. FASEB J. 2010;24:1249–1260. doi: 10.1096/fj.09-147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loft S, Vistisen K, Ewertz M, Tjonneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 16.Teichert F, Verschoyle RD, Greaves P, Thorpe JF, Mellon JK, Steward WP, Farmer PB, Gescher AJ, Singh R. Determination of 8-oxo-2′-deoxyguanosine and creatinine in murine and human urine by liquid chromatography/tandem mass spectrometry: application to chemoprevention studies. Rapid Commun Mass Spectrom. 2009;23:258–266. doi: 10.1002/rcm.3873. [DOI] [PubMed] [Google Scholar]
- 17.Weimann A, Belling D, Poulsen HE. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucleic Acids Res. 2002;30:E7. doi: 10.1093/nar/30.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida R, Ogawa Y, Kasai H. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine values measured by an ELISA correlated well with measurements by high-performance liquid chromatography with electrochemical detection. Cancer Epidemiol Biomarkers Prev. 2002;11:1076–1081. [PubMed] [Google Scholar]
- 19.Loft S, Svoboda P, Kasai H, Tjonneland A, Vogel U, Moller P, Overvad K. Raaschou-Nielsen O. Prospective study of 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis. 2006;27:1245–1250. doi: 10.1093/carcin/bgi313. [DOI] [PubMed] [Google Scholar]
- 20.Lee KF, Chung WY, Benzie IF. Urine 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), a specific marker of oxidative stress, using direct, isocratic LC-MS/MS: Method evaluation and application in study of biological variation in healthy adults. Clin Chim Acta. 2010;411:416–422. doi: 10.1016/j.cca.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Leanderson P, Tagesson C. Cigarette smoke-induced DNA damage in cultured human lung cells: role of hydroxyl radicals and endonuclease activation. Chem Biol Interact. 1992;81:197–208. doi: 10.1016/0009-2797(92)90034-i. [DOI] [PubMed] [Google Scholar]
- 22.Gackowski D, Rozalski R, Roszkowski K, Jawien A, Foksinski M, Olinski R. 8-Oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanosine levels in human urine do not depend on diet. Free Radic Res. 2001;35:825–832. doi: 10.1080/10715760100301321. [DOI] [PubMed] [Google Scholar]
- 23.Rozalski R, Siomek A, Gackowski D, Foksinski M, Gran C, Klungland A, Olinski R. Diet is not responsible for the presence of several oxidatively damaged DNA lesions in mouse urine. Free Radic Res. 2004;38:1201–1205. doi: 10.1080/10715760400017350. [DOI] [PubMed] [Google Scholar]
- 24.Erhola M, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, Uchida K, Osawa T, Nieminen MM, Alho H, Kellokumpu-Lehtinen P. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2′-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett. 1997;409:287–291. doi: 10.1016/s0014-5793(97)00523-1. [DOI] [PubMed] [Google Scholar]
- 25.Faure H, Mousseau M, Cadet J, Guimier C, Tripier M, Hida H, Favier A. Urine 8-oxo-7,8-dihydro-2-deoxyguanosine vs. 5-(hydroxymethyl) uracil as DNA oxidation marker in adriamycin-treated patients. Free Radic Res. 1998;28:377–382. doi: 10.3109/10715769809070806. [DOI] [PubMed] [Google Scholar]
- 26.Siomek A, Tujakowski J, Gackowski D, Rozalski R, Foksinski M, Dziaman T, Roszkowski K, Olinski R. Severe oxidatively damaged DNA after cisplatin treatment of cancer patients. Int J Cancer. 2006;119:2228–2230. doi: 10.1002/ijc.22088. [DOI] [PubMed] [Google Scholar]
- 27.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Garratt LW, Mistry V, Singh R, Sandhu JK, Sheil B, Cooke MS, Sly PD. Interpretation of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine is adversely affected by methodological inaccuracies when using a commercial ELISA. Free Radic Biol Med. 2010;48:1460–1464. doi: 10.1016/j.freeradbiomed.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Song MF, Li YS, Ootsuyama Y, Kasai H, Kawai K, Ohta M, Eguchi Y, Yamato H, Matsumoto Y, Yoshida R, Ogawa Y. Urea, the most abundant component in urine, cross-reacts with a commercial 8-OH-dG ELISA kit and contributes to overestimation of urinary 8-OH-dG. Free Radic Biol Med. 2009;47:41–46. doi: 10.1016/j.freeradbiomed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Ravanat JL, Guicherd P, Tuce Z, Cadet J. Simultaneous determination of five oxidative DNA lesions in human urine. Chem Res Toxicol. 1999;12:802–808. doi: 10.1021/tx980194k. [DOI] [PubMed] [Google Scholar]
- 31.Shigenaga MK, Gimeno CJ, Ames BN. Urinary 8-hydroxy-2′-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci U S A. 1989;86:9697–9701. doi: 10.1073/pnas.86.24.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Germadnik D, Pilger A, Rudiger HW. Assay for the determination of urinary 8-hydroxy-2′-deoxyguanosine by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl. 1997;689:399–403. doi: 10.1016/s0378-4347(96)00328-3. [DOI] [PubMed] [Google Scholar]
- 33.Park EM, Shigenaga MK, Degan P, Korn TS, Kitzler JW, Wehr CM, Kolachana P, Ames BN. Assay of excised oxidative DNA lesions: isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc Natl Acad Sci U S A. 1992;89:3375–3379. doi: 10.1073/pnas.89.8.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravanat JL, Duretz B, Guiller A, Douki T, Cadet J. Isotope dilution high-performance liquid chromatography-electrospray tandem mass spectrometry assay for the measurement of 8-oxo-7,8-dihydro-2′-deoxyguanosine in biological samples. J Chromatogr B Biomed Sci Appl. 1998;715:349–356. doi: 10.1016/s0378-4347(98)00259-x. [DOI] [PubMed] [Google Scholar]
- 35.Weimann A, Belling D, Poulsen HE. Measurement of 8-oxo-2′-deoxyguanosine and 8-oxo-2′-deoxyadenosine in DNA and human urine by high performance liquid chromatography-electrospray tandem mass spectrometry. Free Radic Biol Med. 2001;30:757–764. doi: 10.1016/s0891-5849(01)00462-2. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, McEwan M, Lamb JH, Santella RM, Farmer PB. An improved liquid chromatography/tandem mass spectrometry method for the determination of 8-oxo-7,8-dihydro-2′-deoxyguanosine in DNA samples using immunoaffinity column purification. Rapid Commun Mass Spectrom. 2003;17:126–134. doi: 10.1002/rcm.883. [DOI] [PubMed] [Google Scholar]
- 37.Hu CW, Wu MT, Chao MR, Pan CH, Wang CJ, Swenberg JA, Wu KY. Comparison of analyses of urinary 8-hydroxy-2′-deoxyguanosine by isotope-dilution liquid chromatography with electrospray tandem mass spectrometry and by enzyme-linked immunosorbent assay. Rapid Commun Mass Spectrom. 2004;18:505–510. doi: 10.1002/rcm.1367. [DOI] [PubMed] [Google Scholar]
- 38.Sabatini L, Barbieri A, Tosi M, Roda A, Violante FS. A method for routine quantitation of urinary 8-hydroxy-2′-deoxyguanosine based on solid-phase extraction and micro-high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:147–152. doi: 10.1002/rcm.1763. [DOI] [PubMed] [Google Scholar]
- 39.Hu CW, Wang CJ, Chang LW, Chao MR. Clinical-scale high-throughput analysis of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine by isotope-dilution liquid chromatography-tandem mass spectrometry with on-line solid-phase extraction. Clin Chem. 2006;52:1381–1388. doi: 10.1373/clinchem.2005.063735. [DOI] [PubMed] [Google Scholar]
- 40.Cooke MS, Singh R, Hall GK, Mistry V, Duarte TL, Farmer PB, Evans MD. Evaluation of enzyme-linked immunosorbent assay and liquid chromatography-tandem mass spectrometry methodology for the analysis of 8-oxo-7,8-dihydro-2′-deoxyguanosine in saliva and urine. Free Radic Biol Med. 2006;41:1829–1836. doi: 10.1016/j.freeradbiomed.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Machowetz A, Poulsen HE, Gruendel S, Weimann A, Fito M, Marrugat J, de la Torre R, Salonen JT, Nyyssonen K, Mursu J, Nascetti S, Gaddi A, Kiesewetter H, Baumler H, Selmi H, Kaikkonen J, Zunft HJ, Covas MI, Koebnick C. Effect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern Europeans. FASEB J. 2007;21:45–52. doi: 10.1096/fj.06-6328com. [DOI] [PubMed] [Google Scholar]
- 42.Malayappan B, Garrett TJ, Segal M, Leeuwenburgh C. Urinary analysis of 8-oxoguanine, 8-oxoguanosine, fapy-guanine and 8-oxo-2′-deoxyguanosine by high-performance liquid chromatography-electrospray tandem mass spectrometry as a measure of oxidative stress. J Chromatogr A. 2007;1167:54–62. doi: 10.1016/j.chroma.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Harri M, Kasai H, Mori T, Tornaeus J, Savela K, Peltonen K. Analysis of 8-hydroxy-2′-deoxyguanosine in urine using high-performance liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:242–246. doi: 10.1016/j.jchromb.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Evans MD, Singh R, Mistry V, Sandhu K, Farmer PB, Cooke MS. Analysis of urinary 8-oxo-7,8-dihydro-purine-2′-deoxyribonucleosides by LC-MS/MS and improved ELISA. Free Radic Res. 2008;42:831–840. doi: 10.1080/10715760802506323. [DOI] [PubMed] [Google Scholar]
- 45.Chao MR, Wang CJ, Wu MT, Pan CH, Kuo CY, Yang HJ, Chang LW, Hu CW. Repeated measurements of urinary methylated/oxidative DNA lesions, acute toxicity, and mutagenicity in coke oven workers. Cancer Epidemiol Biomarkers Prev. 2008;17:3381–3389. doi: 10.1158/1055-9965.EPI-08-0721. [DOI] [PubMed] [Google Scholar]
- 46.Cooke MS, Barregard L, Mistry V, Potdar N, Rozalski R, Gackowski D, Siomek A, Foksinski M, Svoboda P, Kasai H, Konje JC, Sallsten G, Evans MD, Olinski R. Interlaboratory comparison of methodologies for the measurement of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Biomarkers. 2009;14:103–110. doi: 10.1080/13547500802706012. [DOI] [PubMed] [Google Scholar]
- 47.Henriksen T, Hillestrom PR, Poulsen HE, Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2′-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic Biol Med. 2009;47:629–635. doi: 10.1016/j.freeradbiomed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Cooke MS, Henderson PT, Evans MD. Sources of extracellular, oxidatively-modified DNA lesions: implications for their measurement in urine. J Clin Biochem Nutr. 2009;45:255–270. doi: 10.3164/jcbn.SR09-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potdar N, Singh R, Mistry V, Evans MD, Farmer PB, Konje JC, Cooke MS. First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG. 2009;116:637–642. doi: 10.1111/j.1471-0528.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- 50.Manini P, De PG, Andreoli R, Marczynski B, Hanova M, Mozzoni P, Naccarati A, Vodickova L, Hlavac P, Mutti A, Vodicka P. Biomarkers of nucleic acid oxidation, polymorphism in, and expression of, hOGG1 gene in styrene-exposed workers. Toxicol Lett. 2009;190:41–47. doi: 10.1016/j.toxlet.2009.06.862. [DOI] [PubMed] [Google Scholar]
- 51.Hu CW, Chao MR, Sie CH. Urinary analysis of 8-oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanosine by isotope-dilution LC-MS/MS with automated solid-phase extraction: Study of 8-oxo-7,8-dihydroguanine stability. Free Radic Biol Med. 2010;48:89–97. doi: 10.1016/j.freeradbiomed.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 52.Hu CW, Huang YJ, Li YJ, Chao MR. Correlation between concentrations of 8-oxo-7,8-dihydro-2′-deoxyguanosine in urine, plasma and saliva measured by on-line solid-phase extraction LC-MS/MS. Clin Chim Acta. 2010;411:1218–1222. doi: 10.1016/j.cca.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 53.Evans MD, Singh R, Mistry V, Farmer PB, Cooke MS. Analysis of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine by liquid chromatography-tandem mass spectrometry. Methods Mol Biol. 2010;610:341–351. doi: 10.1007/978-1-60327-029-8_20. [DOI] [PubMed] [Google Scholar]
- 54.Evans MD, Saparbaev M, Cooke MS. DNA repair and the origins of urinary oxidized 2′-deoxyribonucleosides. Mutagenesis. 2010;25:433–442. doi: 10.1093/mutage/geq031. [DOI] [PubMed] [Google Scholar]
- 55.Boysen G, Collins LB, Liao S, Luke AM, Pachkowski BF, Watters JL, Swenberg JA. Analysis of 8-oxo-7,8-dihydro-2′-deoxyguanosine by ultra high pressure liquid chromatography-heat assisted electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:375–380. doi: 10.1016/j.jchromb.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andreoli R, Manini P, De PG, Alinovi R, Goldoni M, Niessen WM, Mutti A. Quantitative determination of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine, 8-oxo-7,8-dihydroguanine, 8-oxo-7,8-dihydroguanosine, and their non-oxidized forms: daily concentration profile in healthy volunteers. Biomarkers. 2010;15:221–231. doi: 10.3109/13547500903434501. [DOI] [PubMed] [Google Scholar]
- 57.Andreoli R, Mutti A, Goldoni M, Manini P, Apostoli P, De PG. Reference ranges of urinary biomarkers of oxidized guanine in (2′-deoxy)ribonucleotides and nucleic acids. Free Radic Biol Med. 2011;50:254–261. doi: 10.1016/j.freeradbiomed.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Mistry V, Teichert F, Sandhu JK, Singh R, Evans MD, Farmer PB, Cooke MS. Non-invasive assessment of oxidatively damaged DNA: liquid chromatography-tandem mass spectrometry analysis of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Methods Mol Biol. 2011;682:279–289. doi: 10.1007/978-1-60327-409-8_20. [DOI] [PubMed] [Google Scholar]
- 59.Parker AR, Eshleman JR. Human MutY: gene structure, protein functions and interactions, and role in carcinogenesis. Cell Mol Life Sci. 2003;60:2064–2083. doi: 10.1007/s00018-003-3053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss JM, Goode EL, Ladiges WC, Ulrich CM. Polymorphic variation in hOGG1 and risk of cancer: a review of the functional and epidemiologic literature. Mol Carcinog. 2005;42:127–141. doi: 10.1002/mc.20067. [DOI] [PubMed] [Google Scholar]
- 61.Hazra TK, Izumi T, Maidt L, Floyd RA, Mitra S. The presence of two distinct 8-oxoguanine repair enzymes in human cells: their potential complementary roles in preventing mutation. Nucleic Acids Res. 1998;26:5116–5122. doi: 10.1093/nar/26.22.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakabeppu Y, Kajitani K, Sakamoto K, Yamaguchi H, Tsuchimoto D. MTH1, an oxidized purine nucleoside triphosphatase, prevents the cytotoxicity and neurotoxicity of oxidized purine nucleotides. DNA Repair (Amst) 2006;5:761–772. doi: 10.1016/j.dnarep.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Bonfiglio R, King RC, Olah TV, Merkle K. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom. 1999;13:1175–1185. doi: 10.1002/(SICI)1097-0231(19990630)13:12<1175::AID-RCM639>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 64.King R, Bonfiglio R, Fernandez-Metzler C, Miller-Stein C, Olah T. Mechanistic investigation of ionization suppression in electrospray ionization. J Am Soc Mass Spectrom. 2000;11:942–950. doi: 10.1016/S1044-0305(00)00163-X. [DOI] [PubMed] [Google Scholar]
- 65.Matuszewski BK. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC-MS bioanalysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:293–300. doi: 10.1016/j.jchromb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Remane D, Wissenbach DK, Meyer MR, Maurer HH. Systematic investigation of ion suppression and enhancement effects of fourteen stable-isotope-labeled internal standards by their native analogues using atmospheric-pressure chemical ionization and electrospray ionization and the relevance for multi-analyte liquid chromatographic/mass spectrometric procedures. Rapid Commun Mass Spectrom. 2010;24:859–867. doi: 10.1002/rcm.4459. [DOI] [PubMed] [Google Scholar]
- 67.Stadler RH, Staempfli AA, Fay LB, Turesky RJ, Welti DH. Synthesis of multiply-labeled [15N3,13C1]-8-oxo-substituted purine bases and their corresponding 2′-deoxynucleosides. Chem Res Toxicol. 1994;7:784–791. doi: 10.1021/tx00042a011. [DOI] [PubMed] [Google Scholar]
- 68.Ciccimaro E, Blair IA. Stable-isotope dilution LC-MS for quantitative biomarker analysis. Bioanalysis. 2010;2:311–341. doi: 10.4155/bio.09.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oe T, Ackermann BL, Inoue K, Berna MJ, Garner CO, Gelfanova V, Dean RA, Siemers ER, Holtzman DM, Farlow MR, Blair IA. Quantitative analysis of amyloid beta peptides in cerebrospinal fluid of Alzheimer’s disease patients by immunoaffinity purification and stable isotope dilution liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3723–3735. doi: 10.1002/rcm.2787. [DOI] [PubMed] [Google Scholar]
- 70.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakabeppu Y, Tsuchimoto D, Yamaguchi H, Sakumi K. Oxidative damage in nucleic acids and Parkinson’s disease. J Neurosci Res. 2007;85:919–934. doi: 10.1002/jnr.21191. [DOI] [PubMed] [Google Scholar]
- 72.Vought RL, London WT, Lutwak L, Dublin TD. Reliability of estimates of serum inorganic iodine and daily fecal ans urinary iodine from single casual specimens. J Clin Endocrinol Metab. 1963;23:1218–1228. doi: 10.1210/jcem-23-12-1218. [DOI] [PubMed] [Google Scholar]
- 73.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 74.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 75.Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- 76.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 77.Selberg O, Sel S. The adjunctive value of routine biochemistry in nutritional assessment of hospitalized patients. Clin Nutr. 2001;20:477–485. doi: 10.1054/clnu.2001.0427. [DOI] [PubMed] [Google Scholar]
- 78.Greenblatt DJ, Ransil BJ, Harmatz JS, Smith TW, Duhme DW, Koch-Weser J. Variability of 24-hour urinary creatinine excretion by normal subjects. J Clin Pharmacol. 1976;16:321–328. doi: 10.1002/j.1552-4604.1976.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 79.Rangiah K, Hwang WT, Mesaros C, Vachani A, Blair IA. Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LC-MS. Bioanalysis. 2011;3:745–761. doi: 10.4155/bio.11.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warrack BM, Hnatyshyn S, Ott KH, Reily MD, Sanders M, Zhang H, Drexler DM. Normalization strategies for metabonomic analysis of urine samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:547–552. doi: 10.1016/j.jchromb.2009.01.007. [DOI] [PubMed] [Google Scholar]