Abstract
Before planned surgical treatment of lung cancer, the patient's respiratory system function should be evaluated. According to the current guidelines, the assessment should start with measurements of FEV1 (forced expiratory volume in 1 second) and DLco (carbon monoxide lung diffusion capacity). Pneumonectomy is possible when FEV1 and DLco are > 80% of the predicted value (p.v.). If either of these parameters is < 80%, an exercise test with VO2 max (oxygen consumption during maximal exercise) measurement should be performed. When VO2 max is < 35 % p.v. or < 10 ml/kg/min, resection is associated with high risk. If VO2 max is in the range of 35-75% p.v. or 10-20 ml/kg/min, the postoperative values of FEV1 and DLco (ppoFEV1, ppoDLco) should be determined. The exercise test with VO2 max measurement may be replaced with other tests such as the shuttle walk test and the stair climbing test. The distance covered during the shuttle walk test should be > 400 m. Patients considered for lobectomy should be able to climb 3 flights of stairs (12 m) and for pneumonectomy 5 flights of stairs (22 m).
Keywords: lung cancer, preoperative evaluation, exercise testing
Abstract
Przed planowanym operacyjnym leczeniem raka płuca należy dokonać oceny czynności układu oddechowego. Według aktualnych wytycznych w pierwszej kolejności powinno się dokonać pomiaru natężonej objętości wydechowej pierwszosekundowej (FEV1) i zdolności dyfuzyjnej płuc dla tlenku węgla (DLco). Zabieg pneumonektomii można przeprowadzić, gdy wartość zarówno FEV1, jak i DLco przekracza 80% wartości należnej. Jeśli jeden z powyższych parametrów jest niższy niż 80% wartości należnej, należy na kolejnym etapie wykonać badanie wysiłkowe z pomiarem VO2 max. Gdy VO2 max jest < 35% wartości należnej lub < 10 ml/kg m.c./min, leczenie operacyjne jest obarczone dużym ryzykiem. Jeśli wartość VO2 max mieści się w przedziale 35–75% wartości należnej lub wynosi 10–20 ml/kg m.c./min, należy wyliczyć należne pooperacyjne wartości FEV1, DLco (ppoFEV1, ppoDLco). Badanie wysiłkowe z oceną VO2 max może być zastąpione testem marszowym wahadłowym lub testem wchodzenia po schodach. Pacjent w teście marszowym wahadłowym powinien pokonać dystans > 400 m. Zdolność pokonania 3 kondygnacji schodów w teście wchodzenia po schodach (12 m) kwalifikuje do lobektomii. Chory planowany do pneumonektomii powinien pokonać różnicę wysokości równą 5 kondygnacjom (22 m).
Introduction
Lung cancer is currently responsible for the largest number of “neoplastic” deaths both among women and men [1, 2]. Surgical treatment is one of the methods for treating this disease. The option of surgical treatment is largely determined by the stage of the neoplasm according to the TNM (tumor, node, metastasis) classification [3]. However, in some patients, the surgical options are also limited by concomitant respiratory system diseases, which impair lung function. One such disease, which most often limits the possibility of resection, is chronic obstructive pulmonary disease (COPD). It is, moreover, a risk factor for lung cancer independent of smoking [4]. Establishing the respiratory reserve is an important element of qualifying patients for resection of lung parenchyma. The currently binding recommendations of the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) from 2009 underscore the significance of cooperation within multispecialist teams consisting of pneumonologists, thoracic surgeons, oncologists, and radiation therapists in determining the optimal treatment strategy for individual patients [5]. In the proposed algorithm for conducting the qualification procedure, the subsequent function tests are aimed at increasing both perioperative safety and the percentage of patients qualified for surgical treatment.
The aim of the study was to present the current guidelines concerning the assessment of respiratory system function before qualifying patients for the surgical treatment of lung cancer and to highlight the changes in relation to previous recommendations.
Preliminary assessment – spirometry and exercise capacity testing
According to the current guidelines of ERS and ESTS, both spirometry, aimed at measuring FEV1 (forced expiratory volume in 1 second), and the assessment of the diffusing capacity of the lungs (DLco), performed by measuring gas diffusion within the lungs, are recommended during the first stage of qualifying patients for lung parenchyma resection [5].
Previous guidelines, both those published by the American College of Chest Physicians (ACCP) in 2007 and those published by the British Thoracic Society (BTS) in 2001, recommended only spirometry with FEV1 measurement as the preliminary examination [6, 7]. The DLco examination was recommended only for patients with lowered FEV1, post-exercise dyspnea disproportionate to FEV1, or suspicion of interstitial lung disease [6, 7]. The latest guidelines include the DLco measurement in the preliminary function examination, as numerous studies have demonstrated that reduced DLco levels constitute an independent risk factor for increased mortality and perioperative complications, even in patients with normal FEV1 [8–13]. In a large study encompassing 872 patients qualified for lung parenchyma resection, Brunelli et al. found reduced DLco levels in 508 patients (63%) with normal FEV1, which resulted in an increased frequency of perioperative complications. This is one of the reasons for the recommendation that DLco assessment be performed routinely in all candidates for pulmonary parenchyma resections [9].
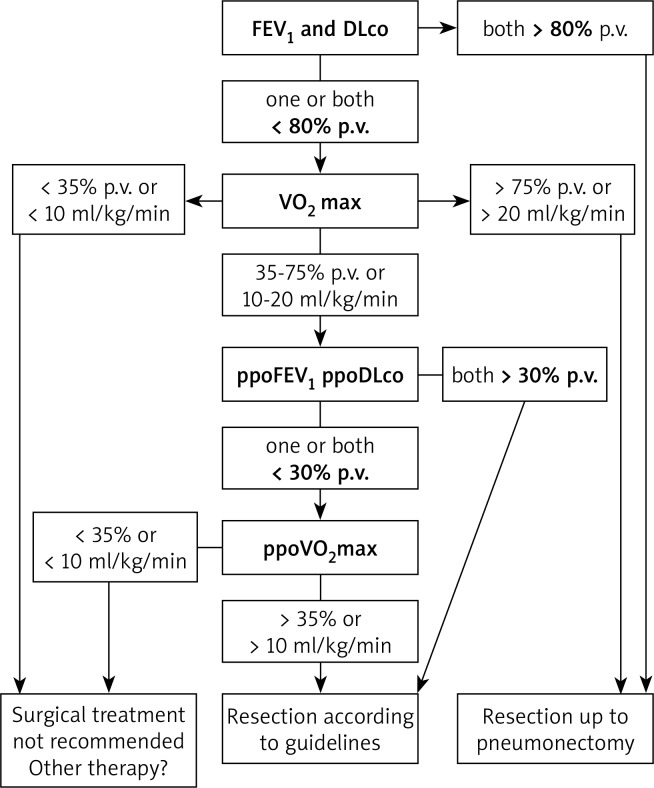
Spirometry should be conducted in accordance with the ERS/ATS standards [14]. The previously published guidelines established independent boundary values of FEV1 for planned lobectomy and pneumonectomy (1.5 l and 2 l, respectively) [6]. Later guidelines added that FEV1 should not be lower than 80% of the predicted value (p.v.), both in the case of planned lobectomy and pneumonectomy [7]. The current values employ only a percentage threshold value, which is 80% of the predicted value for both FEV1 and DLco (Fig. 1) [5]. The preliminary assessment of respiratory function may be concluded if the values of both indices (FEV1 and DLco) exceed 80% p.v. This means that surgical treatment is not burdened with an increased risk of complications and perioperative mortality [5].
Fig. 1.
Algorithm for assessing respiratory system function in lung cancer surgery candidates
When at least one of the parameters (FEV1 or DLco) is lowered (< 80% p.v.), the next stage of the qualification process for lung parenchyma resection should establish the patient's exercise capacity (Fig. 1).
Exercise tests
Exercise tests are widely used in pulmonary diagnostics (including the process of qualifying patients for the surgical treatment of lung cancer) due to their higher prognostic value with regard to FEV1 and DLco measurement. Engaging large groups of muscles, exercise tests significantly burden the circulatory and respiratory systems, enabling the estimation of the physiological functional reserve before the planned surgical procedure [15]. Exercise tests should be performed in patients with FEV1 or DLco lower than 80% p.v. (Fig. 1) [5]. The best test among them is the cardiopulmonary exercise test (CPET), also known as ergospirometry. Cardiopulmonary exercise test may be performed on a treadmill or, as recommended for respiratory system diseases, on a cycloergometer [15]. The measurement of exercise capacity in ergospirometry is peak oxygen uptake expressed by the VO2 max parameter [15].
Reduced VO2 max results in an increased risk of post-resection complications [16].
This pertains especially to patients with VO2 max < 65% p.v. (or < 16 ml/kg/min) [17]. Brunelli et al. reported that all deaths after lung resections that were at least as extensive as lobectomy occurred among patients whose VO2 max was < 20 ml/kg/min [18].
Low-cost exercise tests
In Poland, CPET is relatively inaccessible and costly, and requires trained personnel. Other, lower-cost methods of assessing exercise capacity include the 6-minute walk test (6MWT), shuttle walk test (SWT), and stair climbing test.
The 6-minute walk test
The distance covered during 6MWT correlates well with VO2 max in lung transplantation candidates [19]. However, it is not a good prognostic indicator in terms of the probability of complication occurrence after lung resection [20–30].
The shuttle walk test
During the test, the patient walks between 2 points, which are 10 m apart, at an increasing speed set by a test-specific sound signal. The distance covered during this test correlates well with VO2 max [24–26]. Previous guidelines recommended 250 m to be the boundary value for increased complication frequency after lung resection [6].
Later reports demonstrated no significant difference in the distance covered during this test by patients with and without postoperative complications [27, 28]. On the other hand, it was revealed that some patients covered distances shorter than 250 m in spite of the fact that their VO2 max values exceeded 15 ml/kg/min [28]. In the study group, all patients who walked more than 400 m in distance achieved VO2 max values exceeding 15 ml/kg/min [28].
As a result, the shuttle walk test is currently recommended as a screening exercise test for patients qualified for resection due to lung cancer [29]. All patients with results over 400 m should undergo additional CPET in order to establish their VO2 max [29].
Stair climbing
Stair climbing is another examination recommended for qualifying patients for lung cancer surgery [30, 31]. During this simple test, the patient climbs flights of stairs, thus ascending a certain distance and number of floors. As demonstrated by Brunelli et al., patients who climb less than 12 m (3 floors) are twice as likely to suffer from complications, while mortality among them increases 13-fold, and the costs of their treatment rise 2.5-fold in comparison with patients who can climb 22 m (5 floors) [32].
Thus, the stair climbing test may be employed as a screening examination for the identification of patients who are able to ascend 22 m (5 floors) of stairs and can, therefore, undergo lung resection up to pneumonectomy without increased risk [32].
Lately, reports have been published, pointing to the significance of the speed of stair climbing with regard to the frequency of complications after lung resection procedures (the majority of the studied patients were operated on for non-neoplastic reasons) [33, 34]. It was demonstrated that climbing 20 m within 80 s (speed ≥ 15 m/min) correlates well with VO2 max [33]. All patients who achieved a result of less than 80 s had VO2 max above 20 ml/kg/min [33].
Also, Ambrozini et al. demonstrated that climbing 12.16 m in a time exceeding 37.5 s indicates a higher risk of complications after thoracic surgery procedures [34].
Perhaps a larger number of reports assessing the stair climbing speed of patients qualified for resections due to lung cancer will result in the preparation of appropriate expert guidelines.
Predicted postoperative values of FEV1 and DLco (ppoFEV1, ppoDLco)
After exercise tests, the next stage of preoperative function evaluation consists in calculating the predicted postoperative values of FEV1 and DLco [5].
Previous guidelines recommended calculating ppoFEV1 and ppoDLco if FEV1 is lowered (< 1.5 l for lobectomy and < 2 l for pneumonectomy) [6, 7]. The current guidelines place ppoFEV1 and ppoDLco calculations after exercise capacity tests and VO2 max measurements (Fig. 1) [5].
Moreover, the latest guidelines include the calculation of predicted oxygen consumption after resection (VO2 max) [5]. This pertains to patients in whom ppoFEV1 or ppoDLco is lower than 30% p.v. (in previous guidelines, the boundary value of ppoFEV1 and ppoDLco in qualifying patients for resection was 40% p.v.) [6, 7]. Considering the improving standards of perioperative care and the modern, less invasive surgical techniques, the boundary value of ppoFEV1 and ppoDLco was lowered to 30% p.v. [5].
The formulae enabling the calculation of predicted postoperative values are similar for FEV1, DLco, and VO2 max [35].
When lobectomy is planned, the values of ppoFEV1, ppoDLco, and ppoVO2 max are calculated with consideration to the number of all resected segments (Formula 1) or obstructed segments whose number can be assessed using CT or bronchoscopy (Formula 2) [35]. In the case of pneumonectomy, scintigraphy and an assessment of perfusion within the resected lung are required [5–7].
Calculating predicted postoperative values (ppo)
A. Lobectomy
| 1 |
Right lung: Left lung:
upper lobe – 3 upper lobe – 3
middle lobe – 3 lingula – 2
lower lobe – 5 lower lobe – 4
| 2 |
a – number of excised patent segments
b – total number of patent segments
B. Pneumonectomy
ppo = preoperative value × (1 – perfusion percentage within the resected lung)
New indicators in the preoperative assessment of lung cancer patients
The assessment of physical activity by means of simple tests evaluating daily energy expenditure correlates with perioperative mortality among adults [36].
Measuring motor activity with a pedometer facilitates the determination of the degree of exercise capacity impairment resulting from COPD or other diseases [37, 38]. Its usefulness in predicting negative effects of surgery, including thoracic surgery, requires further study.
The slope of the VE/VCO2 curve obtained during CPET is an independent indicator of mortality in patients with moderate and severe COPD undergoing resection due to non-small-cell lung carcinoma [39]. The slope of VE/VCO2 is a better predictive factor than VO2 max, with regard to both mortality and perioperative complications in patients undergoing lung resection [40].
The increasing number of reports concerning the usefulness of these indicators in evaluating the risk of complications after lung cancer surgery procedures may well result in their routine use in preoperative evaluation in the future.
Special circumstances in the preoperative assessment of lung cancer patients
It should be remembered that surgical treatment of lung cancer is also possible in patients with significantly reduced lung function parameters (as long as FEV1 and DLco are not lower than 20% p.v.) if the tumor is located in the upper lobes affected by emphysematous changes [7, 41]. In such cases, concurrent resection of emphysematous bullae serves the role of lung volume reduction surgery in COPD and improves lung ventilation. Another exception occurs if the exercise tests cannot be performed by a patient with orthopedic ailments, e.g. advanced degenerative changes of the hip or knee joints, in which case only the calculation of predicted postoperative lung function indicator values is required.
Conclusions
The qualification of lung cancer patients for resection is a multistage process. Qualifying for the procedure requires not only meeting histopathological and radiological criteria, but also having normal, good lung ventilation and diffusion capacity. In doubtful cases, it must be verified by assessing the patient's exercise capacity. Proper qualification is aimed at both increasing perioperative safety and raising the percentage of patients qualified for lung resection due to cancer.
Biography

Disclosure
The authors report no conflict of interest.
References
- 1.Lung Cancer in European Respiratory Society, the European Lung White Book. 2013. available at: http://www.erswhitebook.org/chapters/lung-cancer/ [Google Scholar]
- 2.Wojciechowska U, Didkowska J, Zatoński W. Nowotwory złośliwe w Polsce w 2008 roku; Warszawa: Krajowy Rejestr Nowotworów; 2010. available at: http://onkologia.org.pl/wp-content/uploads/Nowotwory2008.pdf. [Google Scholar]
- 3.Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, Detterbeck F, American College of Chest Physicians Noninvasive staging of non-small cell lung xancer: ACCP Evidenced-Based Clinical Practice Guidelines (2nd edition) Chest. 2007;132(3 Suppl):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 4.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77:58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, Licker M, Ferguson MK, Faivre-Finn C, Huber RM, Clini EM, Win T, De Ruysscher D, Goldman L. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 6.British Thoracic Society and Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. Guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT, American College of Chest Physicians Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):161S–177S. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 8.Cerfolio RJ, Bryant AS. Different diffusing capacity of the lung for carbon monoxide as predictors of respiratory morbidity. Ann Thorac Surg. 2009;88:405–410. doi: 10.1016/j.athoracsur.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli A, Refai MA, Salati M, Sabbatini A, Morgan-Hughes NJ, Rocco G. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: evidence for systematic measurement before lung resection. Eur J Cardiothorac Surg. 2006;29:567–570. doi: 10.1016/j.ejcts.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Pieretti P, Alifano M, Roche N, Vincenzi M, Forti Parri SN, Zackova M, Boaron M, Zanello M. Predictors of an appropriate admission to an ICU after a major pulmonary resection. Respiration. 2006;73:157–165. doi: 10.1159/000088096. [DOI] [PubMed] [Google Scholar]
- 11.Santini M, Fiorello A, Vicidomini G, Di Crescenzo VG, Laperuta P. Role of diffusing capacity in predicting complications after lung resection for cancer. Thorac Cardiovasc Surg. 2007;55:391–394. doi: 10.1055/s-2007-965326. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg. 2008;85:1158–1164. doi: 10.1016/j.athoracsur.2007.12.071. [DOI] [PubMed] [Google Scholar]
- 13.Bousamra M, 2nd, Presberg KW, Chammas JH, Tweddell JS, Winton BL, Bielefeld MR, Haasler GB. Early and late morbidity in patients undergoing pulmonary resection with low diffusion capacity. Ann Thorac Surg. 1996;62:968–974. doi: 10.1016/0003-4975(96)00476-6. [DOI] [PubMed] [Google Scholar]
- 14.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society; American College of Chest Physicians ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 16.Benzo R, Kelley GA, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: a meta-analysis. Respir Med. 2007;101:1790–1797. doi: 10.1016/j.rmed.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loewen GM, Watson D, Kohman L, Herndon JE, 2nd, Shennib H, Kernstine K, Olak J, Mador MJ, Harpole D, Sugarbaker D, Green M, Cancer and Leukemia Group B Preoperative exercise VO2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol. 2007;2:619–625. doi: 10.1097/JTO.0b013e318074bba7. [DOI] [PubMed] [Google Scholar]
- 18.Brunelli A, Belardinelli R, Refai M, Salati M, Socci L, Pompili C, Sabbatini A. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest. 2009;135:1260–1267. doi: 10.1378/chest.08-2059. [DOI] [PubMed] [Google Scholar]
- 19.Cahalin L, Pappagianopoulos P, Prevost S, Herndon JE, 2nd, Shennib H, Kernstine K, Olak J, Mador MJ, Harpole D, Sugarbaker D, Green M. The relationship of the 6-min walk test to maximal oxygen consumption in transplant candidates with end-stage lung disease. Chest. 1995;108:452–459. doi: 10.1378/chest.108.2.452. [DOI] [PubMed] [Google Scholar]
- 20.Holden DA, Rice TW, Stelmach K, Meeker DP. Exercise testing, 6-min walk, and stair climb in the evaluation of patients at high risk for pulmonary resection. Chest. 1992;102:1774–1779. doi: 10.1378/chest.102.6.1774. [DOI] [PubMed] [Google Scholar]
- 21.Markos J, Mullan BP, Hillman DR, Musk AW, Antico VF, Lovegrove FT, Carter MJ, Finucane KE. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139:902–910. doi: 10.1164/ajrccm/139.4.902. [DOI] [PubMed] [Google Scholar]
- 22.Pierce RJ, Copland JM, Sharpe K, Barter CE. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med. 1994;150:947–955. doi: 10.1164/ajrccm.150.4.7921468. [DOI] [PubMed] [Google Scholar]
- 23.Bagg LR. The 12-min walking distance; its use in the preoperative assessment of patients with bronchial carcinoma before lung resection. Respiration. 1984;46:342–345. doi: 10.1159/000194711. [DOI] [PubMed] [Google Scholar]
- 24.Morgan AD. Simple exercise testing. Respir Med. 1989;83:383–387. doi: 10.1016/s0954-6111(89)80069-1. [DOI] [PubMed] [Google Scholar]
- 25.Singh SJ, Morgan MD, Hardman AE, Rowe C, Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J. 1994;7:2016–2020. [PubMed] [Google Scholar]
- 26.Swinburn CR, Wakefield JM, Jones PW. Performance, ventilation, and oxygen consumption in three different types of exercise test in patients with chronic obstructive lung disease. Thorax. 1985;40:581–586. doi: 10.1136/thx.40.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Win T, Jackson A, Groves AM, Sharples LD, Charman SC, Laroche CM. Comparison of shuttle walk with measured peak oxygen consumption in patients with operable lung cancer. Thorax. 2006;61:57–60. doi: 10.1136/thx.2005.043547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Win T, Jackson A, Groves AM, Wells FC, Ritchie AJ, Munday H, Laroche CM. Relationship of shuttle walk test and lung cancer surgical outcome. Eur J Cardiothorac Surg. 2004;26:1216–1219. doi: 10.1016/j.ejcts.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Brunelli A, Pompoli C, Salati M. Low-technology test in the preoperative evaluation of lung resection candidates. Monaldi Arch Chest Dis. 2010;73:720–728. doi: 10.4081/monaldi.2010.301. [DOI] [PubMed] [Google Scholar]
- 30.Van Nostrand D, Kjelsberg MO, Humphrey EW. Preresectional evaluation of risk from pneumonectomy. Surg Gynecol Obstet. 1968;127:306–312. [PubMed] [Google Scholar]
- 31.Olsen GN, Bolton JW, Weiman DS, Hornung CA. Stair climbing as an exercise test to predict the postoperative complications of lung resection. Two years’ experience. Chest. 1991;99:587–590. doi: 10.1378/chest.99.3.587. [DOI] [PubMed] [Google Scholar]
- 32.Brunelli A, Refai M, Xiume F, Xiumé F, Salati M, Sciarra V, Socci L, Sabbatini A. Performance at symptom limited stair-climbing test is associated with increased cardiopulmonary complications, mortality, and costs after major lung resection. Ann Thorac Surg. 2008;86:240–247. doi: 10.1016/j.athoracsur.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Bernansconi M, Koegelenberg CF, von Groote-Bidlingmaier F, Maree D, Barnard BJ, Diacon AH, Bolliger CT. Speed of ascent during stair climbing identifies operable lung resection candidates. Respiration. 2012;84:117–122. doi: 10.1159/000337258. [DOI] [PubMed] [Google Scholar]
- 34.Ambrozini AR, Cataneo DC, Arruda KA, Cataneo AJ. Time in the star climbing test as a predictor of thoracotomy postoperative complications. J Thorac Cardiovasc Surg. 2013;145:1093–1097. doi: 10.1016/j.jtcvs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Bolliger CT, Guckel C, Engel H, Stöhr S, Wyser CP, Schoetzau A, Habicht J, Solèr M, Tamm M, Perruchoud AP. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration. 2002;69:482–489. doi: 10.1159/000066474. [DOI] [PubMed] [Google Scholar]
- 36.Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 37.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006;27:1040–1055. doi: 10.1183/09031936.06.00064105. [DOI] [PubMed] [Google Scholar]
- 38.Schonhofer B, Ardes P, Geibel M, Köhler D, Jones PW. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997;10:2814–2819. doi: 10.1183/09031936.97.10122814. [DOI] [PubMed] [Google Scholar]
- 39.Torchio R, Guglielmo M, Giardino R, Ardissone F, Ciacco C, Gulotta C, Veljkovic A, Bugiani M. Exercise ventilatory inefficiency and mortality in patients with chronic obstructive pulmonary disease undergoing surgery for non small lung cancer. Eur J Cardiothorac Surg. 2010;38:14–19. doi: 10.1016/j.ejcts.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 40.Brunelli A, Belardinelli R, Pompili C, Xiumé F, Refai M, Salati M, Sabbatini A. Minute ventilation-to-carbon dioxide output (VE/VCO2) slope is the strongest predictor of respiratory complications and death after pulmonary resection. Ann Thorac Surg. 2012;93:1802–1806. doi: 10.1016/j.athoracsur.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Edwards JG, Duthie DJ, Waller DA. Lobar volume reduction surgery: a method of increasing the lung cancer resection rate in patients with emphysema. Thorax. 2001;56:791–795. doi: 10.1136/thorax.56.10.791. [DOI] [PMC free article] [PubMed] [Google Scholar]