Abstract
This paper examines the predictive relations between two infant temperamental biases assessed at 4 months and inhibited behavior during the first two years of life in three independent samples from two research laboratories. Although each sample used slightly different criteria for classifying infants, the results across samples were consistent. Infants of both genders who displayed high levels of motor activity and distress to unfamiliar events were more inhibited at 14 months of age. By 24 months there were significant sex differences: boys identified as high reactive were more inhibited than high reactive girls.
Keywords: temperament, behavioral inhibition, infant reactivity
INTRODUCTION
Young infants vary in their patterns of responsiveness to unfamiliar events. Some of this variation has an origin in one or more temperamental biases. Previous work suggested that variation in motor activity and fretting/crying in response to unfamiliar events reflects the two temperamental biases called high and low reactivity, which, in turn, predict inhibited or uninhibited behavior in the second year (Kagan, Reznick, Snidman, Gibbons, & Johnson, 1988; Kagan & Snidman, 1991). Four-month-old infants who display very high levels of limb/trunk movement and distress (called high reactive) are biased to show avoidance of and withdrawal to unfamiliar stimuli or contexts during early childhood, a pattern called behavioral inhibition. Those who show very low levels of motor activity and distress at four months (called low reactive) are biased to become sociable and bold and are called uninhibited (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Kagan and Snidman, 1991).
Although the results from different laboratories find similar biological correlates among the high and low reactive infant groups, one node of disagreement concerns the predictive power of the 4-month classifications of high or low reactivity. One set of studies found that the predictive relations between behavioral inhibition at 1 and 2 years and behavior at later years were significantly higher than the relations between high or low reactivity at 4-months and behavioral inhibition among toddlers (Fox et al., 2001). A second set of studies however, reported that the four-month temperamental biases were better predictors of the behavior and physiology of older children than indexes of behavioral inhibition in the second year. Variations across the samples in recruitment and methodology in selecting the high and low reactive groups could be a source of the differences between labs.
The current paper compares methods and outcomes across the two laboratories and three independent samples. In addition, we examined the possibility of sex differences in the expression of temperament and prediction to behavioral inhibition across the samples. Previous research has been mixed with regard to the role of sex differences in temperament. One set of findings suggests that high reactivity predicted social wariness for boys at 4 years of age, but not for girls (Henderson, Fox, and Rubin, 2001). Similarly, other researchers have shown that the continuity of behavioral inhibition may be stronger in boys than in girls (Crockenberg and Smith, 1982; Fagan, 1990). On the other hand, Kagan (1994) reported that the girls in his sample were, on average, more inhibited at 14 and 21 months. We therefore examined relations in three samples between infant reactivity and behavioral inhibition at 14 and 24 months in both males and females.
METHODS
Participants
Sample 1
Subjects were infants from a large metropolitan area in the Northeast United States who were Caucasian and middle-class. All infants were born within 2 weeks of due date and reported no significant medical difficulties. At 4 month olds, (+/− 1 week) more than 500 children were screened for high or low reactivity using the battery of unfamiliar stimuli designed by Kagan and colleagues (Kagan & Snidman, 1991; Kagan, 1994). High reactive infants were defined as those who displayed high frequencies of both motor movement (arms, trunk, and legs) and fretting or crying. Of these infants, 60% were selected for longitudinal study because they displayed extremely high or low scores for motor activity and/or distress. The group showing high levels of both variables was called high reactive (20% of the total sample) and the group showing low levels on both measures were called low reactive (40% of the total sample). For the analyses presented in this paper, we included only the 128, four-month-old infants who were observed at both 14 and 21 months as they encountered a series of unfamiliar objects or people in unfamiliar laboratory settings (High Reactive Group: n = 55, 28 males; Low Reactive Group: n = 73, 36 males). Refer to Kagan (1994) for reasons for attrition. The frequencies of avoidant behaviors and/or crying were used to construct an index of behavioral inhibition (Kagan & Snidman, 1991).
Sample 2
Infants were recruited in two waves. Wave 1 consisted of infants from a large metropolitan area in the Mideast United States who were predominately Caucasian (95%), and middle- class. All infants were born within 2 weeks of due date and reported no significant medical difficulties. At 4 months (+/− 1 week), 208 children were screened for high or low reactivity using the battery of unfamiliar stimuli designed by Kagan and colleagues (Kagan & Snidman, 1991). The average scores for motor and negative affect for the first 25 percent of the sample were used to determine the criteria for classification. Infants who were above the mean on both motor reactivity and negative affect were classified as high reactive. Infants who were below the mean for both motor reactivity and negative affect were classified as low reactive. Of these infants, 81 were selected for longitudinal study because they displayed extremely high or low scores for motor activity and/or negative affect. The group showing high levels of both variables were called high reactive (representing 14% of the total sample); the group showing low levels on both measures were low reactive (representing approximately 14% of the total sample) (Calkins, Fox, & Marshall, 1996). For the analyses presented in this paper, we included only infants (n = 53) who were observed at both 14 and 24 months of age when measures of behavioral inhibition were collected (High Reactive Group: n = 25, 12 males; Low Reactive Group: n = 28, 12 males) (Fox et al., 2001). Reasons for attrition were unrelated to reactivity grouping, and are further explained in Fox et al., 2001).
A second wave of 225, four-month-old infants from the same metropolitan area in Maryland was observed with the same procedures. The motor activity and negative affect were coded on a Likert scale from 1 to 7 (1 = low reactivity, 7 = extreme reactivity). Subjects whose scores were above 4 on both variables were categorized as high reactive. Subjects whose scores were less than 3 on motor reactivity and negative reactivity were called low reactive. Of these infants, 49 infants were selected for longitudinal study because they displayed extremely high or low scores for motor activity and/or negative affect. The group showing high levels of both variables were called high reactive (representing 12% of the total sample); the group showing low levels on both measures were low reactive (representing approximately 10% of the total sample). For the analyses presented in this paper, we included only infants (n = 42) who were observed at both 14 and 24 months of age when measures of behavioral inhibition were collected (High Reactive Group: n = 23, 10 males; Low Reactive Group: n = 19, 9 males) (Fox et al., 2001). Reasons for attrition were unrelated to reactivity grouping, and are further explained in Fox et al., 2001).
In order for subjects from both Wave 1 and Wave 2 to be coded with the same metric, the 208 subjects from Wave 1 were also coded with the Likert system used to score infants in Wave 2. Correlations between the Likert scales and discrete coded variables were r= .51 for motor reactivity: p < .001 and r = .71 for negative affect: p < .001.
The final make up of Sample 2 analyzed in this paper combined Wave 1 and Wave 2, resulting in 95 infants, 48 (22 males) of which were in the Higher Reactive, and 47 (21 males) of which were Low Reactive.
Sample 3
A third sample was recruited from the same large metropolitan area in the Mideast United States. All infants were born within 2 weeks of due date and reported no significant medical difficulties. At 4 months (+/− 1 week) 779 infants were screened for high or low reactivity with the same battery of unfamiliar stimuli used by Kagan and colleagues (e.g., Kagan & Snidman, 1991). Mean scores for motor activity, negative affect and positive affect of the first 100 infants were used to determine category membership. Subjects whose scores were above the mean for both motor reactivity and negative affect were classified as high reactive (approximately 11% of the total sample). Subjects whose scores were above the mean for both motor reactivity and positive affect were classified as high positive reactive (approximately 9% of the total sample). Infants who did not meet the criteria for either group, and whose scores on both motor and negative reactivity were close to the mean of the entire sample, were selected as a “control” group (representing approximately 7% of the total sample). Of these, 291 subjects were selected for longitudinal study. A total of 187 infants (64.3%) were Caucasian, 41(14.1%) were African American, and 63 (21.6%) were of another ethnicity. (Hane, Fox, Henderson, & Marshall, 2008). For the analyses presented in this paper, we included only infants (n = 140) who were observed at 24 months of age when measures of behavioral inhibition were collected (High Reactive Group: n = 89, 34 males; Low Reactive Group: n = 51, 23 males). Infants in this sample were not seen at 14 months of age (White, McDermott, Degnan, Henderson, & Fox, 2011). Reasons for attrition were unrelated to reactivity grouping, and are further explained in White et al. (2011).
Behavioral Inhibition
The frequency of avoidant behaviors or crying to a series of unfamiliar events defines behavioral inhibition. For Sample 1, crying to any episode or failure to approach a small number of events designated a priori as novel were coded as “fear” responses at 14 and 21 months (See Table 2 for more details). The definition of behavioral inhibition in Sample 1 was the total number of episodes during which the child displayed a fear response (range of 0 to 13; a majority had 2 or fewer fears).
Table 2.
Descriptions of Scoring Measures
| Sample | 4 month Visit | 14 month Visit | 21 or 24 Month Visit |
|---|---|---|---|
| Sample 1 Thistle | Motor Reactivity: Total # of movements of both arms, both legs, and hyperextension of the arms or legs; (1 pt. each); Bursts: Total # of occurrence of bursts of both arms or legs (two or more movements in quick succession) (2pts each) Negative Reactivity: Crying: Total # of seconds the child cried across the battery Fretting/Fussing: Total # of trials on which these two occurred Other Coded Reactivity: Arching: Total occurrences of arching of the back (2pts each); Fret/Fuss: # of trials on which these two responses occurred. Positive Vocalizations: # of trials on which response occurred; Smiling # of trials on which response occurred. Final Scores: Sum of frequencies for both Motor Reactivity and Negative Reactivity. | Total number of fears (including fretting or crying, or reluctance to approach) to an unfamiliar event or procedure. | Total number of fears (including fretting or crying, or reluctance to approach) to an unfamiliar event or procedure. (Seen at 21 months) |
| Sample 2 Cohort 1: Thistle | Motor Reactivity: Total # of movements of both arms, both legs, and hyperextension of the arms or legs; Can be one or two in succession; Bursts: Total # of occurrence of bursts of both arms or legs (three or more movements in quick succession) (1 pt. each) Arching: Total occurrences of arching of the back (1pts each) Negative Reactivity: Crying: Total # of seconds the child cried across the battery; Fret/Fuss: # of trials on which these two responses occurred. Positive Reactivity: Positive Vocalizations: # of trials on which response occurred; Smiling # of trials on which response occurred. Final Scores: Sum of frequencies for Motor Reactivity, Negative Reactivity, and Positive Reactivity. | Composite score of the following measures: Latency to touch Toy (free play), Latency to vocalize (free play), Time spent in proximity to mother (free play), Latency to vocalize to stranger, Latency to approach stranger, Latency to vocalize to approach robot, Latency to approach robot, Time spent in proximity to mother (robot), Time spent in proximity to mother (truck). | Composite score of the following measures: Time spent in proximity to mother (free play), Time spent in proximity to mother (truck), Time spent in proximity to mother (robot), Time spent in proximity to mother (tunnel), Latency to approach/touch truck, Latency to approach/touch robot, Latency to pass through tunnel. (Seen at 24 months) |
| Cohort 1 Likert | Motor Reactivity: movements of both arms, both legs, and hyperextension of the arms or legs; Can be one or two in succession Bursts: of occurrence of bursts of both arms or legs Arching: Total occurrences of arching of the back (three or more movements in quick succession) (all scored on a Likert Scale from 1 to 7) Negative Reactivity: Crying: seconds the child cried across the battery; Fret/Fuss:(scored on a Likert Scale from 1 to 7). (all scored on a Likert Scale from 1 to 7) Positive Reactivity: Positive Vocalizations; Smiling (all scored on a Likert Scale from 1 to 7). Final Scores: Composite score of Likert scores for Motor Reactivity, Negative Reactivity, and Positive Reactivity. | ||
| Cohort 2 Likert | Motor Reactivity: movements of both arms, both legs, and hyperextension of the arms or legs; Can be one or two in succession Bursts: of occurrence of bursts of both arms or legs Arching: Total occurrences of arching of the back (three or more movements in quick succession) (all scored on a Likert Scale from 1 to 7) Negative Reactivity: Crying: seconds the child cried across the battery; Fret/Fuss:(scored on a Likert Scale from 1 to 7). (all scored on a Likert Scale from 1 to 7) Positive Reactivity: Positive Vocalizations; Smiling (all scored on a Likert Scale from 1 to 7). Final Scores: Composite score of Likert scores for Motor Reactivity, Negative Reactivity, and Positive Reactivity. | Composite score of the following measures: Latency to touch Toy (free play), Latency to vocalize (free play), Time spent in proximity to mother (free play), Latency to vocalize to stranger, Latency to approach stranger, Latency to vocalize to approach robot, Latency to approach robot, Time spent in proximity to mother (robot), Time spent in proximity to mother (truck). | Composite score of the following measures: Time spent in proximity to mother (free play), Time spent in proximity to mother (truck), Time spent in proximity to mother (robot), Time spent in proximity to mother (tunnel), Latency to approach/touch truck, Latency to approach/touch robot, Latency to pass through tunnel. (Seen at 24 months) |
| Sample 3 | Motor Reactivity: movements of both arms, both legs, and hyperextension of the arms or legs; Can be one or two in succession Bursts: of occurrence of bursts of both arms or legs Arching: Total occurrences of arching of the back (three or more movements in quick succession) (all scored on a Likert Scale from 1 to 7) Negative Reactivity: Crying: seconds the child cried across the battery; Fret/Fuss:(scored on a Likert Scale from 1 to 7). (all scored on a Likert Scale from 1 to 7) Positive Reactivity: Positive Vocalizations; Smiling (all scored on a Likert Scale from 1 to 7). Final Scores: Composite score of Likert scores for Motor Reactivity, Negative Reactivity, and Positive Reactivity. | Composite z-score of the following measures: Time spent in proximity to mother (free play), Time spent in proximity to mother (truck), Time spent in proximity to mother (robot), Time spent in proximity to mother (tunnel), Latency to approach/touch truck, Latency to approach/touch robot, Latency to pass through tunnel. (Seen at 24 months) |
For sample 2, seen at 14 and 24 months, and sample 3 at 24 months, the index of inhibition was comparable to the one used with Sample 1. The behavioral inhibition scores for samples 2 and 3 included latency to approach an unfamiliar object or person, as well as time spent in close proximity to the caregiver. The final values for behavioral inhibition for Samples 2 and 3 were standardized scores. (Table 2 presents the details of the different coding systems. See also Kagan, 1994; Fox et al. 2001; White et al., 2011).
Although results from each of the three samples have been published, a comparison across samples had not been examined.
RESULTS
4-month Reactivity Groupings
To examine whether motor reactivity1 at 4 months differed between the low and high reactivity groups in samples 1, 2, & 3 and whether observed differences varied by sample and sex, a 3 × 2 × 2 (sample x reactivity group x sex) factorial analysis of variance (ANOVA) was computed. A significant main effect of reactivity group (F1, 351 = 209.210, p < .001, ηp2 = .373) revealed that the high reactive infants (M = .54, SD = .95) were significantly more active and distressed at 4 months than the low reactive infants (M = −.71, SD = .68). There were no main effects of sample (ηp2 = .001), sex (ηp2 = .002), or interactions of reactivity group and sex (ηp2 = .001), reactivity group and sample (ηp2 = .001), sex and sample (ηp2 = .002), or group, sex, and sample (ηp2 = .001).
14 month Behavioral Inhibition
To examine whether behavioral inhibition1 at 14 months differed as a function of reactivity, sample, and gender, a 2 × 2 × 2 (sample x reactivity group x sex) factorial analysis of variance (ANOVA) was computed. Sample 3 was not included in this analysis because 14-month behavioral inhibition data were not collected for this sample. A significant main effect of reactivity group (F1, 206 = 56.253, p < .001, ηp2 = .214) revealed that high reactive infants (M = .50, SD = 1.08) were significantly more inhibited at 14 months than low reactive infants (M = −.41, SD = .68). (See Figure 1). There was no significant main effect of sample (ηp2 = .002), or interaction of sample and reactivity group (ηp2 = .012). However, there were two 2-way interactions involving sex and reactivity (F1, 206 = 4.86, p < .05, ηp2 = .02) and sex and sample (F1, 206 = 6.52, p < .05, ηp2 = .03). Furthermore, these were superseded by a significant three-way interaction of sex x reactivity x sample (F2, 206 = 4.76, p < .05, ηp2 = .02).
Figure 1.
14-Month Behavioral Inhibition Scores
Post hoc analyses using the Bonferronni post hoc criterion to examine significant differences between reactivity groups for each sex within each sample confirmed that, in sample 1, the high reactive infants were significantly more inhibited at 14 months than the low reactive group for both males (F1,206= 25.30 p < .001, ηp2 = .11) and females (F1,206= 25.13 p < .001, ηp2 = .11). In sample 2, the high reactive group was significantly more inhibited at 14 months than the low reactive group for males (F1,206= 19.82 p < .001, ηp2 = .09), but not for females (ηp2 = .002) (See Figure 1).
Additional post hoc analyses examining sample differences for each sex within each reactivity group revealed that within the high reactivity group, sample 1 males were less inhibited than sample 2 males (F1,206= 4.12 p < .05, ηp2 = .02), while sample 1 females were more inhibited than sample 2 females (F1,206= 6.93 p < .01, ηp2 = .03). There were no sample differences within the low reactivity group for males (ηp2 = .01) or females (ηp2 = .007).
21 or 24-Month Behavioral Inhibition
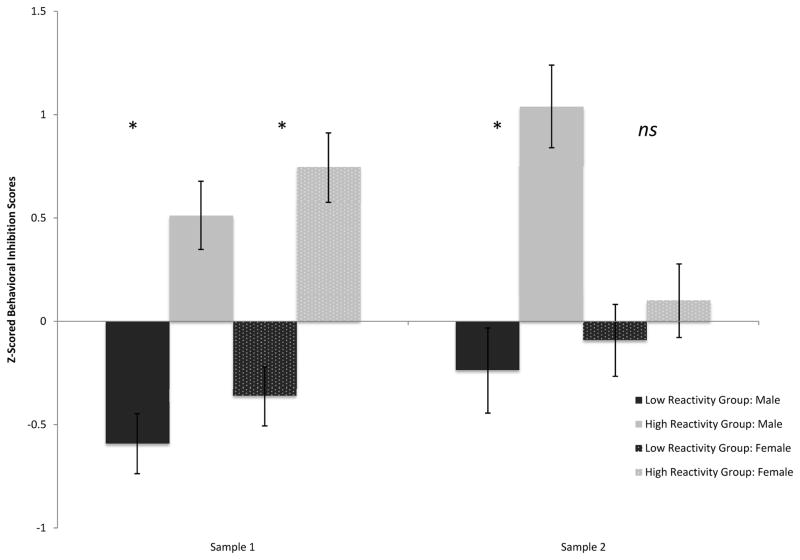
To examine whether behavioral inhibition1 at 21/24 months differed between the low and high reactivity groups in samples 1, 2 & 3, and whether these differences varied by sample and sex, a 3 × 2 × 2 (sample x reactivity group x sex) factorial analysis of variance (ANOVA) was computed. A significant main effect of reactivity group (F1, 335 = 11.845 p < .001, ηp2 = .034), revealed that high reactive infants (M = .17, SD = 1.04) were more behaviorally inhibited at 21/24 months than low reactive infants (M = −.18, SD = .91) (See Figure 2a). There was no significant effect of sample (ηp2 = .001), nor an interaction of sample and reactivity group (ηp2 = .004), or three-way interaction (ηp2 = .004). However, there was a significant two-way interaction of sex and reactivity group (F1, 335 = 4.33, p < .05, ηp2 = .012) and a significant two-way interaction of sex and sample (F2, 335 = 3.19, p < .05, ηp2 = .019).
Figure 2.
Figure 2a: 21/24-Month Behavioral Inhibition Scores
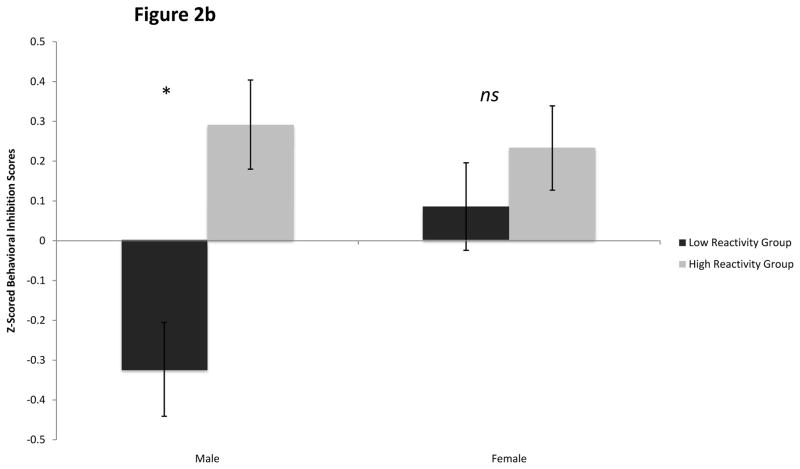
Figure 2b: 24-Month Inhibition by Sex and Reactivity Group
Post hoc analyses using the Bonferronni post hoc criterion to examine significant differences between reactivity groups for each sex confirmed that the high reactive group was significantly more inhibited at 21/24 months than the low reactive group for males (F1,335= 14.33, p < .001, ηp2 = .04), but not for females (ηp2 = .003) (See Figure 2b).
Additional post hoc analyses examining sex differences for each sample revealed that in sample 1, males were less inhibited than females (F1,206= 4.12 p < .05, ηp2 = .02). However, there were no differences in inhibition scores between males and females for sample 2 (ηp2 = .001) or sample 3 (ηp2 = .001).
DISCUSSION
The hypothesis that four-month-old infants who displayed high or low levels of motor activity and distress to unfamiliar events would differ in wariness and avoidance in the second year was affirmed for the assessment at 14 months in Samples 1 and 2, and at 21/24 months in Samples 1, 2 and 3. These data suggest that 4-month reactivity reflects early temperamental biases predictive of differences in behavioral inhibition at two years of age.
Although the two laboratories used slightly different coding methods and paradigms, an infant’s classification as high or low reactive predicted behavioral inhibition at 14 months, and at 21 or 24 months for all three samples. These findings support previously published research that high and low reactives retain some features of their temperament through adolescence. (Kagan and Snidman 2004; Kagan Snidman, Kahn & Towsley, 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003; Fox, Henderson, Marshall, Nichols, & Ghera, 2005).
The current data suggest that there is greater preservation of behavioral inhibition in males during the first two years of life, despite a lack of sex differences in reactivity in at four months. One possible post hoc explanation for this observation is that these sex differences are a result of variation in experiences during the first two years of life. Parents may exhibit an intrusive caregiving style with timid sons because they violate the sex role stereotype for males but shyness is less inconsistent with the stereotype for girls (Park, Belsky, Putnam, & Crnic, 1997). Sex differences in rate of maturation of frontal lobe processes might also explain these data. The incentives for an inhibited response were unfamiliar events that were unexpected and none were serious threats to harm or loss of a parent’s comfort. Because frontal lobe functions are able to mute a “fear” response to the unfamiliar, partly through projections to the amygdala, and these projections are likely to be precocious in girls, it is reasonable to suggest that this biological difference could explain the sex difference (Hepper, Shannon, & Dorman 1997).
There is increasing evidence suggesting sex differences in the later outcomes of BI, especially social anxiety. The higher prevalence in males may be due in part to the fact that extreme levels of shyness and timidity are less acceptable in boys than in girls, (Doey, Coplan, & Kingsbury, 2013). For example, stronger associations between toddler shyness and a multitude of less adaptive outcomes, including peer exclusion, depression, anxiety, and low self-esteem, have been reported for boys relative to girls (e.g., Caspi, Elder, & Bem, 1988; Coplan, Closson, & Arbeau, 2007; Spangler & Gazelle, 2009).
However, not all studies report that inhibited boys are at a greater risk for internalizing problems. Schwartz, Snidman, and Kagan (1999) found a stronger association between BI in the second year and social anxiety in adolescent girls. The results in this paper suggest that sex differences begin early. Future work examining caregiving differences in high reactive infants as a function of the child’s sex will illuminate this puzzle.
Table 1.
Description of Testing Procedures
| Sample | 4 month Visit | 14 month Visit | 21 or 24 Month Visit |
|---|---|---|---|
| Sample 1 |
|
Episode 1: Placement of electrodes, Episode 2: Placement of a blood pressure cuff, Episode 3: Facial disapproval from an examiner or the mother, Episode 4: A noisy rotating wheel, Episode 5: Request to taste liquid from a dropper, Episode 6: Invitation to approach an unfamiliar object (a robot) or unfamiliar adults, despite a friendly invitation to do so. | Episode 1: An unfamiliar room containing novel objects Episode 2: Application of heart rate electrodes Episode 3: Blood pressure cuff, Episode 4: Request to accept liquid through an eyedropper Episode 5: An unfamiliar woman, Episode 6: A person dressed as a clown, and Episode 7: A radio-controlled robot. (Seen at 21 months) |
| Sample 2 |
|
Episode 1: 5 minutes of free play Episode 2: Stranger play with truck (1 min quiet play before invite, 2–3 minutes total) Episode 3: Stranger brings in robot (2 minutes) | Episode 1: 5 minutes of free play Episode 2: Stranger play with truck (1 min quiet play before invite, 2–3 minutes total) Episode 3: Stranger brings in robot (2 minutes) Episode 4: Reaction to stranger dressed in clown costume Episode 5: Willingness to climb through inflatable tunnel. (Seen at 24 months) |
| Sample 3 |
|
N/A | Episode 1: 5 minutes of free play Episode 2: Stranger play with truck (1 min quiet play before invite-2–3 minutes total) Episode 3: Stranger brings in robot (2 minutes) Episode 4: Reaction to stranger dressed in clown costume Episode 5: Willingness to climb through inflatable tunnel. (Seen at 24 months) |
Table 3.
Descriptives
| Time Point | Reactivity Group | Sex | Mean | Std. Error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|
| 4-month Motor Reactivity | Low Reactive | Male | −0.68 | 0.096 | −0.868 | −0.492 |
| Female | −0.757 | 0.088 | −0.93 | −0.583 | ||
| High Reactive | Male | 0.616 | 0.092 | 0.434 | 0.797 | |
| Female | 0.56 | 0.085 | 0.393 | 0.727 | ||
| 14-month Behavioral Inhibition | Low Reactive | Male | −0.494 | 0.125 | −0.74 | −0.248 |
| Female | −0.308 | 0.112 | −0.529 | −0.088 | ||
| High Reactive | Male | 0.684 | 0.128 | 0.431 | 0.937 | |
| Female | 0.34 | 0.121 | 0.101 | 0.579 | ||
| 21/24-month Behavioral Inhibition | Low Reactive | Male | −0.416 | 0.115 | −0.612 | −0.161 |
| Female | 0.015 | 0.107 | −0.194 | 0.225 | ||
| High Reactive | Male | 0.192 | 0.109 | −0.002 | 0.426 | |
| Female | 0.163 | 0.103 | −0.039 | 0.364 |
Acknowledgments
We would like to thank all of the families who participated in this research. Funding for the research from Fox’s lab is from the National Institutes of Health (R37HD17899 and U01 MH093349-04).
Footnotes
Motor Reactivity and Behavioral Inhibition scores of each of the three samples were z-scored in order to account for differences in measurement scales.
References
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Caspi A, Elder GH, Bem DJ. Moving away from the world: Life-course patterns of shy children. Developmental psychology. 1988;24(6):824. [Google Scholar]
- Coplan RJ, Closson L, Arbeau K. Gender differences in the behavioral associates of loneliness and social dissatisfaction in kindergarten. Journal of Child Psychology and Psychiatry. 2007;48:988–995. doi: 10.1111/j.1469-7610.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Crockenberg SB, Smith P. Antecedents of mother-infant interaction and infant irritability in the first three months of life. Infant Behavior and Development. 1982;5(2):105–119. [Google Scholar]
- Doey L, Coplan RJ, Kingsbury M. Bashful Boys and Coy Girls: A Review of Gender Differences in Childhood Shyness. Sex Roles. 2013:1–12. [Google Scholar]
- Fagan J. The interaction between child sex and temperament in predicting behavior problems of preschool age children in day care. Early Child Development and Care. 1990;59(1):1–9. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44(5):1491. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(1):68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Hepper PG, Shannon EA, Dornan JC. Sex differences in fetal mouth movements. Lancet. 1997;350:1820. doi: 10.1016/S0140-6736(05)63635-5. [DOI] [PubMed] [Google Scholar]
- Kagan J. Galen’s Prophecy. New York: Basic Books; 1994. [Google Scholar]
- Kagan J, Reznick JS, Snidman N, Gibbons J, Johnson MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Development. 1988:1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychological Science. 1991;2(1):40–44. [Google Scholar]
- Kagan J, Snidman N. The Long Shadow of Temperament. Belknap Press/Harvard University Press; 2004. [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S, Steinberg L, Fox NA. The preservation of two infant temperaments into adolescence. Monographs of the Society for Research in Child Development. 2007:i–95. doi: 10.1111/j.1540-5834.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Park SY, Belsky J, Putnam S, Crnic K. Infant emotionality, parenting, and 3-year inhibition: Exploring stability and lawful discontinuity in a male sample. Developmental Psychology. 1997;33(2):218. doi: 10.1037//0012-1649.33.2.218. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Spangler T, Gazelle H. Anxious solitude, unsociability, and peer exclusion in middle childhood: A multitrait–multimethod matrix. Social Development. 2009;18(4):833–856. doi: 10.1111/j.1467-9507.2008.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. Journal of abnormal child psychology. 2011;39(5):735–747. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]