Abstract
Arabidopsis thaliana mutants lacking the SS4 isoform of starch synthase have strongly reduced numbers of starch granules per chloroplast, suggesting that SS4 is necessary for the normal generation of starch granules. To establish whether it plays a direct role in this process, we investigated the circumstances in which granules are formed in ss4 mutants.
Starch granule numbers and distribution and the accumulation of starch synthase substrates and products were investigated during ss4 leaf development, and in ss4 mutants carrying mutations or transgenes that affect starch turnover or chloroplast volume.
We found that immature ss4 leaves have no starch granules, but accumulate high concentrations of the starch synthase substrate ADPglucose. Granule numbers are partially restored by elevating the capacity for glucan synthesis (via expression of bacterial glycogen synthase) or by increasing the volumes of individual chloroplasts (via introduction of arc mutations). However, these granules are abnormal in distribution, size and shape.
SS4 is an essential component of a mechanism that coordinates granule formation with chloroplast division during leaf expansion and determines the abundance and the flattened, discoid shape of leaf starch granules.
Keywords: ADPglucose, Arabidopsis thaliana, chloroplast, leaf expansion, starch granule, starch synthase, starch synthesis
Introduction
The process by which starch granules arise is not known. Suggestions range from largely physico-chemical mechanisms (Doi, 1965; Geddes & Greenwood, 1969; Ziegler et al., 2005) to the existence of specific protein primers analogous to the glycogenins of fungi and animals (e.g. Rothschild & Tandecarz, 1994; Singh et al., 1995; Langeveld et al., 2002; Chatterjee et al., 2005). Recent attention has focussed on the role of one isoform of soluble starch synthase, starch synthase 4 (SS4, At4 g18240). Although SS4 contributes little to total starch synthase activity, ss4 mutants of Arabidopsis have at most one or two starch granules per chloroplast (Roldán et al., 2007) rather than the normal five or six (Crumpton-Taylor et al., 2012). No other starch synthase is individually necessary for normal granule numbers (Roldán et al., 2007), thus SS4 may have a specific function in granule formation. However, other isoforms of starch synthase may partially substitute for this function. The additional loss of SS3 further reduces starch granule numbers in the ss4 mutant background (Szydlowski et al., 2009; Mérida & D′Hulst, 2012).
The importance of SS4 for starch granule formation remains to be established. First, it is not known whether SS4 is required primarily for maintenance of starch granule numbers in mature leaves, or whether it also has a role in immature leaves where new granules arise in concert with chloroplast division (Crumpton-Taylor et al., 2012). Second, it is not clear whether the reduction in starch granule numbers in ss4 mutants is a direct or an indirect consequence of the loss of SS4. Mutants have several additional phenotypes including reduced growth rates, altered starch granule anatomy and morphology and a reduction in the extent of diel starch turnover (Roldán et al., 2007). It remains possible that the reduction in granule numbers in ss4 mutants is an indirect consequence of one of these alterations. Third, a recent study suggests that SS4 may be limiting for starch synthesis in wild-type plants. Its overexpression reportedly results in higher concentrations of starch at the end of the day and accelerated plant growth (Gámez-Arjona et al., 2011). These results have important implications for the control of starch turnover and are of biotechnological interest, but the relationship between starch concentrations and starch granule numbers and sizes in plants with elevated SS4 was not reported.
The aim of our work was to establish whether SS4 has a direct or an indirect role in starch granule formation, and to shed further light on where and when its actions are required for the establishment of normal granule numbers. To this end we examined the phenotype of the ss4 mutant through leaf development, and investigated the impact of loss of SS4 in mutant and transgenic backgrounds in which starch metabolism is altered or chloroplast volumes are abnormally large. Our results indicate that SS4 is directly and specifically required for the establishment of normal numbers and distributions of starch granules during leaf expansion, and that it is also necessary for the normal flattened, discoid shape of leaf starch granules.
Materials and Methods
Plant material
Arabidopsis thaliana plants were grown in compost at 20°C with 12 h light (200 μmol photons m−2 s−1), 12 h dark and were used as mature, nonflowering rosettes unless otherwise stated. For examination of roots, plants were grown on Phytogel with nutrients (Haughn & Somerville, 1986). The ss4-1 mutant and the double mutant ss3ss4 were described in Roldán et al. (2007) and Szydlowski et al. (2009), respectively. The ss4-3 mutant (SALK_096130) was used unless otherwise stated. It carries a T-DNA insertion in intron 4 of the SS4 gene, + 2186 bp from the start codon. The sex1-8 (gwd) mutant (SALK_077211) was described in Ritte et al. (2006). It carries a T-DNA insertion in an intron of GWD, lacks detectable GWD protein, and is phenotypically indistinguishable from the null mutant sex1-3 (Yu et al., 2001). arc mutants were: arc3-2 (SALK_057144), arc5-2 (SAIL_71_D11), arc6-5 (SAIL_693_G04) and arc10-2 (SALK_073878) (Crumpton-Taylor et al., 2012). Double mutants were identified in F2 populations derived from crosses by PCR on genomic DNA, using the oligonucleotide primers listed in Supporting Information Table S1.
Complementation of the ss4 mutant
A full-length SS4 cDNA was introduced into the destination vector pK7FWG2,0 (Karimi et al., 2002) by Gateway® LR clonase™ II (Invitrogen) recombination, under the control of a 35S promoter and upstream of eGPF present in the vector. The resulting binary vector was introduced into Agrobacterium tumefaciens strain GV3101 and used to transform ss4-3 plants. Transformants were selected on media containing kanamycin. Presence of the transgene was confirmed using the oligonucleotide primers shown in Table S1 and homozygous lines were developed.
Generation of lines expressing Agrobacterium glgA
The full-length coding sequence of glycogen synthase glgA was amplified from genomic DNA of A. tumefaciens strain GV3101 and cloned into pDONR 221 via Gateway® BP clonase™ II (Invitrogen) recombination. Using Gateway® LR clonase™ II recombination, glgA was transferred into the binary vector pB7WGY2, modified to contain a chloroplast targeted transit peptide (cTP) sequence (the first 53 amino acids of the Rubisco small subunit; At5 g38430) upstream and in frame with the N-terminal YFP (Fig. S9a). The resulting GS-containing construct (YFP-GS) was transformed into ss3ss4 double mutant plants. Four independent GS-transformed plants (GS-5-3, GS-2-2, GS-2-3 and GS-2-4) were obtained by Basta® resistance screening. Transgene expression was confirmed by YFP fluorescence observation using a Zeiss LSM510 confocal fluorescence microscope.
Generation and analysis of lines with dexamethasone-inducible RNAi
Dexamethasone-inducible constructs were made with the pOpOff2(hyg) destination vector system (Wielopolska et al., 2005). The two targeted regions of the SS4 gene are shown in Fig. S8, and oligonucleotide primers are in Table S1. PCR products SS4A and SS4B were cloned into the GATEWAY-ready pCR8/GW/TOPO TA entry plasmid and transferred into the destination vector to create pOpOff2(hyg)::SS4A and pOpOff2(hyg)::SS4B. These plus an empty vector were separately transformed into plants. Transformants were selected on media containing hygromycin, and single-copy homozygous lines were produced.
Ten-day-old plants were sprayed with 30 μM dexamethasone daily, 10 h into the 12-h light period, for 10 d. For measurements of SS4 transcript and protein, plants were harvested immediately before spraying and 24 h after the final spraying. RNA was extracted using the RNeasyTM Plant Mini kit (Qiagen), digested with RQ1-DNAse (Promega), and used for cDNA synthesis. Semi-quantitative PCR was carried out with oligonucleotide primers listed in Table S1, using the TUBULIN gene as a control. For starch analysis, samples were taken at the end of the night and the day following the final spraying (14 h and 26 h afterwards, respectively).
Microscopy
Sample preparation for electron and light microscopy was as described by Crumpton-Taylor et al. (2012) and Delatte et al. (2005).
Starch analysis
Analysis of chain lengths was as described by Streb et al. (2008). Starch samples (100 mg) were boiled for 10 min in water, then debranched by incubation with isoamylase and pullulanase at pH 4.8 and 37°C for 2 h. Neutral glucans were separated by passage through sequential Dowex 50 and Dowex 1 minicolumns, lyophilized, redissolved, then subjected to HPAEC-PAD analysis on a Dionex PA-200 column. The relative proportions of each chain length in the total population (from d.p. 3 to d.p. 50) are expressed as a percentage of the total number of chains.
Metabolite and enzyme assays
Starch, soluble glucans and sugars were extracted and assayed enzymatically (Critchley et al., 2001; Delatte et al., 2005). Other metabolites were measured by high pressure anion exchange chromatography coupled to tandem mass spectrometry (HPAEC-MS/MS: Lunn et al., 2006). Leaves were frozen as rapidly as possible then extracted in chloroform/methanol. Where young and older leaves were harvested separately, rosettes were placed immediately after excision on a metal plate cooled to −80°C and the centre of the rosette was excised with a 1-cm diameter cork borer.
Starch synthase activity was assayed according to Jenner et al. (1994). To visualise glycogen and starch synthase activities, leaves were extracted with 100 mM MOPS, pH 7.2, 1 mM EDTA, 1 mM DTT, 10% (v/v) glycerol (300 mg leaf ml−1). Extracts (32-μl) were loaded onto nondenaturing polyacrylamide gels containing 0.3% (w/v) glycogen. After incubation for 16 h at 20°C in 100 mM HEPES-NaOH, pH 7.5, 2 mM DTT, 10% (v/v) glycerol, 0.5 mM EDTA, 0.5 M Na-citrate, 2 mM ADPglucose, starch and glycogen synthase activities were revealed by iodine staining.
Immunoblotting
An antiserum was raised commercially in rabbits using the synthetic peptide DIGHDDGKNLDNIT (present in SS4 but not other starch synthase isoforms). Antibodies were affinity-purified using this peptide. Leaf tissue was powdered in liquid nitrogen then extracted in Laemmli sample buffer containing SDS and 1% (v/v) protease inhibitor cocktail (Sigma, www.sigmaaldrich.com). Electrophoresis and immunoblotting were as described by Barratt et al. (2001).
Results
Immature leaves of ss4 plants contain almost no starch
We extended the characterisation of ss4 mutants, using the ss4-1 mutant allele (Roldán et al., 2007) and a further T-DNA insertion line, ss4-3, which lacks SS4 protein (Fig. S1a).
Examination of whole rosettes and mature leaves largely confirmed previous reports of the ss4 phenotype (Roldán et al., 2007). Compared with wild-type plants, ss4 plants had slightly reduced soluble starch synthase activities, 40% less chlorophyll (Table S2), less degradation of starch during the night (measured as end-of-day minus end-of-night starch contents) and higher end-of-night starch contents (Fig. S1b), and slower growth rates under both long and short photoperiods (Fig. S1c).
As reported previously (Roldán et al., 2007), mature ss4 leaves appeared to have only one large, rounded starch granule per chloroplast (Figs 1a,b, S1d,e). Quantification of granule numbers (using Method 2 from Crumpton-Taylor et al., 2012) revealed that mature, nonflowering rosettes of wild-type and ss4-3 plants had 5.54 ± 0.28 and 0.87 ± 0.14 granules per chloroplast, respectively (mean ± SE from eight chloroplast preparations in both cases).
Figure 1.
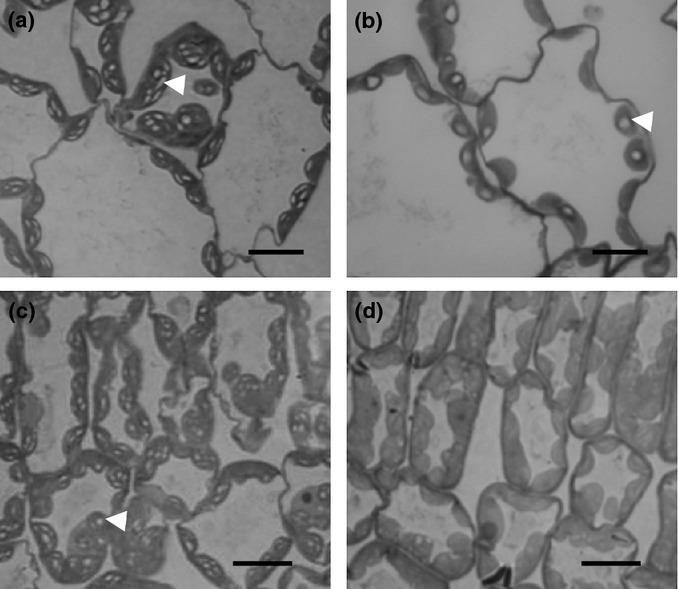
Light micrographs of sections of Arabidopsis leaves stained with toluidine blue. (a) Mature leaf, wild-type plant. (b) Mature leaf, ss4 plant. (c) Immature leaf, wild-type plant. (d) Immature leaf, ss4 plant (see also Fig.4a). (a–c) Arrows indicate starch granules. Bars, 10 μm.
We found a different situation in immature leaves. Whereas immature leaves of wild-type plants have more starch granules per chloroplast than mature leaves (Crumpton-Taylor et al., 2012), no starch granules were visible by light or electron microscopy in immature leaves of ss4 plants (Fig.1c,d). Consistent with this observation, the youngest leaves of ss4 rosettes did not stain with iodine at the end of the day (Fig.2a), and quantitative measurements revealed very low starch contents and little diel starch turnover (Fig.2c,d). Starch content and turnover increased with leaf age, but turnover was limited even in mature leaves. By contrast, in wild-type plants starch content and starch turnover were at their maximum in young leaves (leaves 5–8), and values were similar or somewhat lower in mature leaves (Fig.2b,d). Starch was not replaced with soluble glucan in ss4 leaves. For both mature and immature leaves, ss4 soluble glucan contents were lower than or comparable with wild-type contents (Fig.2e). Thus, loss of SS4 dramatically reduces the rate of acquisition of glucan storage and turnover capacity during leaf development. Mutants fail to form starch granules until late in leaf development, and then only about one granule is formed per chloroplast.
Figure 2.
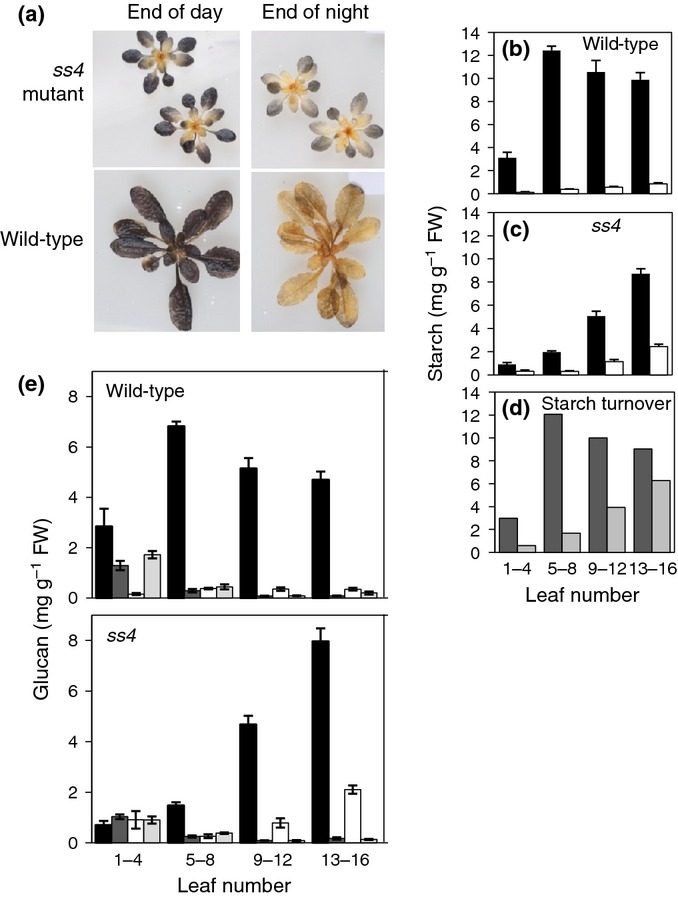
Starch and soluble glucan content and turnover in ss4 leaves. (a) Presence of starch in rosettes of ss4 and wild-type (Col) Arabidopsis plants. Decolourised rosettes were stained with iodine solution. (b) Starch contents of wild-type leaves at the end of the day (black) and the end of the night (white). Leaf 1 is the youngest and leaf 16 the oldest leaf. Values are means of measurements on four plants. Error bars, + SE. (c) Starch contents of ss4 mutant leaves, as for (b). (d) Turnover (end-of-day minus end-of-night starch contents) in wild-type (dark grey) and ss4 mutant (light grey) leaves, calculated from (b) and (c). (e) Starch and soluble glucan contents at the end of the day and the end of the night in leaves of wild-type and ss4 mutant plants. Measurements were made on a separate batch of plants from those shown in (b–d). Leaf 1 is the youngest and leaf 16 the oldest leaf. Starch: black (end of day) and white (end of night). Soluble glucan: dark grey (end of day) and light grey (end of night). Values are means of measurements on at least five rosettes. Error bars, ± SE.
We examined whether loss of SS4 affected starch content in the primary root cap, a region of high starch content in wild-type plants. Starch in the columella cells is essential for the normal gravitropic response of the root (Kiss et al., 1989; Blancaflor et al., 1998; Kiss & Edelmann, 1999). There was wide variation in the amount and location of starch in root caps of ss4 seedlings grown on vertical agar plates. Some ss4 root caps were not distinguishable from those of wild-type plants, but in most cases starch content was reduced with some or all cells having no visible starch. Roots of ss4 seedlings tended to deviate from vertical growth, and the degree of deviation was broadly negatively correlated with starch content (Fig. S2).
Starch synthesis in immature ss4 leaves is not restored by blocking starch degradation
We considered the possibility that starch granules are formed in immature ss4 leaves, but are immediately degraded. To examine this possibility, we introduced into the ss4 background a mutation that blocks starch degradation in wild-type plants. The sex1 mutation affects glucan, water dikinase (GWD), a starch-phosphorylating enzyme that renders the surface of the starch granule accessible to starch degrading enzymes at night (Stitt & Zeeman, 2012). Mutants lacking GWD have a strongly reduced rate of starch degradation at night, and accumulate very high concentrations of starch in all leaves (Zeeman & ap Rees, 1999; Yu et al., 2001).
The ss4sex1 double mutants grew more slowly than ss4 mutants, at about the same rate as sex1 mutants (Fig. S3). Mature leaves had high starch contents at the end of both the day and the night. However, immature leaves contained very little starch and most chloroplasts appeared to contain no granules (Figs3, S3). Some profiles of chloroplasts in sections of mature ss4sex1 leaves also had no starch granules and others contained few, rounded granules of variable size, whereas sex1 chloroplasts in mature leaves were packed with flattened granules (Fig.3). Thus, introduction of a mutation that drastically reduces starch degradation in a wild-type background had only minor effects on starch granule formation in ss4 leaves.
Figure 3.

Effects of the ss4 mutation in a sex1 background. (a) Presence of starch in rosettes of sex1, and two independently selected ss4sex1 double mutant Arabidopsis lines. Decolourised rosettes were stained with iodine solution. (b) Transmission electron micrographs of (left) a cell in a mature sex1 leaf and (right) part of a cell in a mature ss4sex1 leaf. The arrows indicate starch granules. Note that sex1 starch granules are flattened, whereas ss4sex1 granules are rounded and of very variable sizes. Bars, 4 μm.
Leaves of ss4 mutants accumulate very high concentrations of ADPglucose
The experiments described above show that neither starch granules nor soluble glucans accumulate in immature ss4 leaves. This observation suggests that the actions of starch synthase isoforms other than SS4 may be dependent on SS4. To test this idea, we measured the impact of the loss of SS4 on concentrations of the starch synthase substrate ADPglucose. Concentrations were 50–200 times higher in ss4-1 and ss4-3 rosettes than in wild-type rosettes, and were elevated in both mature and immature leaves (Tables1, S3). This was a highly specific effect. Analysis of mutants lacking any of the other three isoforms of soluble starch synthase (the ss1,ss2 and ss3 mutants) revealed less than two-fold differences between their ADPglucose concentrations and those of wild-type plants (Table 1). In the ss4 mutant there were no large alterations with respect to wild-type plants in concentrations of soluble glucans (as previously shown, Fig.2e) or other intermediates of starch and sucrose synthesis, glycolysis and the Calvin–Benson cycle, and the Krebs cycle (Table 1).
Table 1.
Metabolite contents of Arabidopsis rosettes lacking starch synthase isoforms
| Metabolite | Amount (nmol g−1 FW) | |||||
|---|---|---|---|---|---|---|
| Wild-type Col | Wild-type Ws | ss1 (Ws) | ss2 (Col) | ss3 (Col) | ss4-3 (Col) | |
| ADPglucose | 2.14 ± 0.88 | 2.32 ± 1.10 | 4.02 ± 1.16 | 1.78 ± 0.53 | 2.36 ± 0.40 | 124.2 ± 25.8 |
| UDPglucose | 105 ± 11 | 104 ± 14 | 111 ± 15 | 103 ± 11 | 107 ± 10 | 109 ± 4 |
| Trehalose 6-P | 0.23 ± 0.02 | 0.22 ± 0.03 | 0.27 ± 0.02 | 0.25 ± 0.02 | 0.31 ± 0.04 | 0.38 ± 0.05 |
| Glucose 6-P | 151 ± 43 | 175 ± 19 | 212 ± 17 | 167 ± 15 | 204 ± 12 | 265 ± 21 |
| Glucose 1-P | 51.0 ± 2.7 | 50.2 ± 5.2 | 51.8 ± 4.1 | 51.9 ± 3.8 | 54.0 ± 3.3 | 61.0 ± 3.9 |
| Fructose 6-P | 44 ± 15 | 46 ± 12 | 59 ± 8 | 48 ± 6 | 56 ± 7 | 69 ± 4 |
| Fructose 1,6-bisphosphate | 39 ± 6 | 35 ± 11 | 42 ± 7 | 41 ± 9 | 47 ± 7 | 59 ± 10 |
| Glycerol 3-P | 10.9 ± 1.0 | 9.7 ± 0.6 | 10.7 ± 1.4 | 10.7 ± 1.2 | 10.7 ± 1.3 | 8.5 ± 0.8 |
| 3-Phospho-glycerate | 395 ± 86 | 407 ± 43 | 404 ± 60 | 334 ± 78 | 416 ± 55 | 386 ± 83 |
| Phosphoenol-pyruvate | 29 ± 6 | 27 ± 4 | 33 ± 8 | 26 ± 7 | 31 ± 8 | 34 ± 7 |
| Pyruvate | 101 ± 7 | 111 ± 10 | 122 ± 9 | 112 ± 9 | 92 ± 7 | 75 ± 10 |
| Malate | 1207 ± 671 | 1004 ± 271 | 1684 ± 441 | 1293 ± 234 | 1352 ± 267 | 1015 ± 250 |
| Fumarate | 1734 ± 616 | 1826 ± 357 | 2329 ± 289 | 2000 ± 223 | 1948 ± 365 | 1680 ± 218 |
| Aconitate | 91 ± 13 | 90 ± 8 | 95 ± 8 | 87 ± 9 | 93 ± 11 | 83 ± 6 |
| 2-Oxo-glutarate | 84 ± 30 | 85 ± 13 | 105 ± 14 | 120 ± 10 | 102 ± 6 | 64 ± 6 |
| Citrate | 6103 ± 2438 | 5594 ± 1429 | 6441 ± 503 | 5957 ± 562 | 6299 ± 700 | 5710 ± 323 |
| Succinate | 92 ± 71 | 47 ± 42 | 143 ± 41 | 97 ± 39 | 100 ± 53 | 104 ± 45 |
| Shikimate | 21 ± 2 | 21 ± 3 | 21 ± 1 | 23 ± 2 | 22 ± 1 | 32 ± 3 |
Plants were grown together in identical conditions. Rosettes were excised c. 4 h into the 12 h photoperiod, and very rapidly frozen in liquid nitrogen. Metabolites were extracted in chloroform/methanol and assayed by anion exchange chromatography linked to tandem mass spectrometry. Values are means of measurements on six rosettes ± SD.
As expected of any mutant with a reduced capacity to generate sugars from starch at night (e.g. pgm1, Gibon et al., 2004; mex1, Niittylä et al., 2004; lsf1, Comparot-Moss et al., 2010), sucrose concentrations were elevated in ss4 plants during the day, and sucrose and hexose concentrations were lower in ss4 than in wild-type plants during most of the night (Fig. S1b; see also Roldán et al., 2007).
Starch synthesis in an ss4 background is partially but not fully restored by expression of Agrobacterium glycogen synthase
The results above imply that SS4 directly or indirectly provides a ‘primer’ required for the actions of other starch synthases. To investigate this possibility, we overexpressed glycogen synthase (GS) from A. tumefaciens in an ss4 background. We chose this enzyme because it is reported both to elongate glucan chains and to initiate chains de novo via an autoglucosylation mechanism (Ugalde et al., 2003), using ADPglucose as its substrate. Thus it might replace the function of SS4 by providing glucosylated proteins as primers for other starch synthases, resulting in the formation of starch granules. We introduced the GS into plants lacking both SS4 and SS3 (the ss3ss4 double mutant), because these mutants contain even fewer starch granules than the ss4 mutant (Szydlowski et al., 2009; Mérida & D′Hulst, 2012). Indeed, in our growth conditions, (12 h : 12 h, light : dark), ss3ss4 plants were pale, slow growing and contained very little starch (Figs 4, S4).
Figure 4.
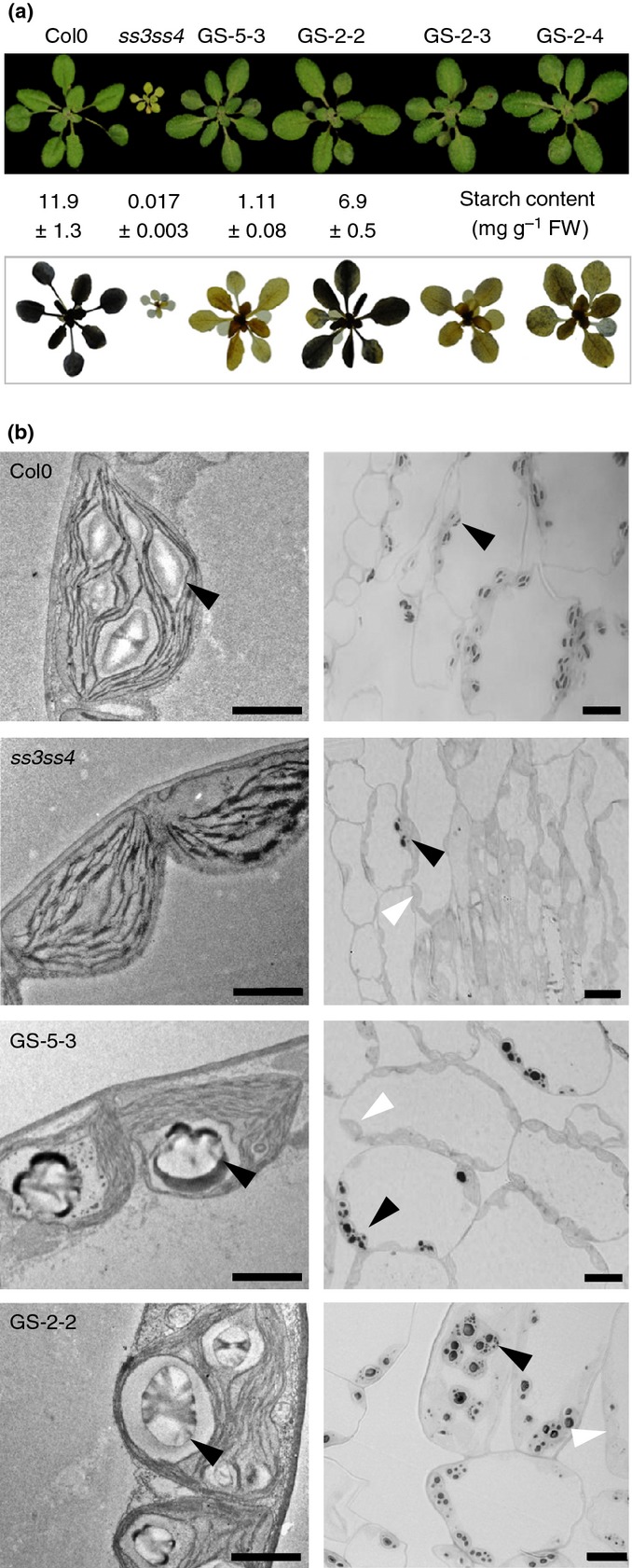
Expression of Agrobacterium tumefaciens glycogen synthase (GS) in the ss3ss4 mutant of Arabidopsis. (a) A Col-0 wild-type plant, a ss3ss4 mutant plant, and the T2 generation of four independent GS transformed ss3ss4 lines. Upper panel, plants grown for 3 wk in 12-h light, 12-h dark conditions. Lower panel, plants harvested at the end of the day, decolourised and stained with iodine solution. Starch contents at the end of the day are given for wild-type, ss3ss4 and two GS-transformed lines. Values are means ± SE of measurements on five 30-d-old plants. (b) Electron (left) and light (right) micrographs of mature leaves from Col-0, ss3ss4 and GS-transformed ss3ss4 plants, 10 h into the light period. Sections for light microscopy were stained with toluidine blue. Bars: 2 μm (for electron micrographs), 10 μm (for light micrographs). Black arrowheads indicate starch granules in plastids. White arrowheads indicate plastids that are devoid of starch.
Agrobacterium GS was introduced into ss3ss4 plants as a fusion protein consisting of an N-terminal Rubisco small subunit chloroplast transit peptide, then a yellow fluorescent protein (YFP), then GS (Fig. S4). Examination of four independent GS-transformed ss3ss4 plants with confocal fluorescence microscopy confirmed that the protein was targeted to chloroplasts. Analysis of crude extracts of leaves by nondenaturing PAGE on gels containing glycogen revealed abundant GS activity in these plants (Fig. S4).
All GS-expressing lines had higher growth rates and more starch than ss3ss4 mutants (Figs4, S4). However, complementation was quantitatively and qualitatively incomplete. Although ADPglucose was reduced from 423 ± 113 nmol g−1 FW in ss3ss4 plants to 40 ± 19 nmol g−1 FW in transformed line G-5-3 (see Figs4, S4), this concentration was still many times greater than that of wild-type plants grown under the same conditions (1.29 ± 0.23 nmol g−1 FW: means ± SD of measurements on three to five plants per line). Importantly, both the distribution and the nature of starch granules in the GS-expressing plants were very different from those of wild-type plants. In leaf sections, starch granules were visible in some chloroplasts but not in others. Furthermore, chloroplasts of a single cell appeared to contain a huge range of granule morphologies and sizes (Fig.4). As expected of amylopectin, polymers from starch granules in GS-expressing lines had a polymodal distribution of chain lengths. However, the pattern of distribution was not the same as that of amylopectin from wild-type leaves (Fig. S4). Thus, either the additional glucan-synthesizing capacity or the autoglucosylation activity provided by Agrobacterium GS can promote starch granule formation in the ss3ss4 background, but neither of these functions of GS can fully restore normal starch synthesis.
Starch synthesis in immature ss4 leaves is partially restored by increased chloroplast volumes
The results above show that formation of new granules occurs much less frequently during leaf development in ss4 leaves than in wild-type leaves. We showed previously that in wild-type plants the number of starch granules present in a chloroplast is a function of its volume, and that the number per unit volume is relatively constant for a given leaf developmental stage (Crumpton-Taylor et al., 2012). Based on these observations, we speculated that the unit volume required per granule formation event might be much larger in ss4 than in wild-type leaves. Average chloroplast volumes double over the course of leaf expansion (Crumpton-Taylor et al., 2012), thus the delayed formation of granules in ss4 leaves might reflect that fact that chloroplast volumes are larger in mature than in immature leaves.
In order to test this idea, we examined the impact on granule formation in the ss4 background of the introduction of mutations that dramatically increase chloroplast volumes. The accumulation and replication of chloroplast (arc) mutants have few, giant chloroplasts per mesophyll cell, but the same number of starch granules per unit chloroplast volume as wild-type plants (Crumpton-Taylor et al., 2012). Chloroplast sizes and numbers in arc3ss4,arc5ss4,arc6ss4 and arc10ss4 double mutants were similar to those in the respective arc parent (between one (arc6) and 25 (arc10) chloroplasts per cell, compared with ∼100 chloroplasts per wild-type cell, not shown). Nondenaturing PAGE analysis revealed no differences in activities of starch synthase isoforms other than SS4 between the parental and double mutant lines (Fig. S5).
The double mutants differed from the ss4 parent in that starch granules were visible in some chloroplasts in sections of immature leaves (Fig. S5) and starch turnover was comparable with that of the arc parent and wild-type plants (Fig. S5 h,i). However, starch synthesis in double mutants was different in several respects from that of arc and wild-type plants. Although some chloroplasts in immature ss4 leaves contained granules, others apparently contained none (Fig. S5f,g). Granule numbers per unit chloroplast volume appeared to be lower than in wild-type plants throughout leaf expansion. In both immature and mature leaves, the range of granule sizes was larger in double mutants than in ss4 or arc mutants, or wild-type plants (Figs5c, S5). The granules of double mutants were rounded rather than flattened, similar to those of ss4 mutants (Fig S5). Thus, although granule formation in the absence of SS4 started earlier during leaf expansion development in arc mutant backgrounds than in a wild-type background, it was highly sporadic and qualitatively different from that in wild-type plants.
Figure 5.
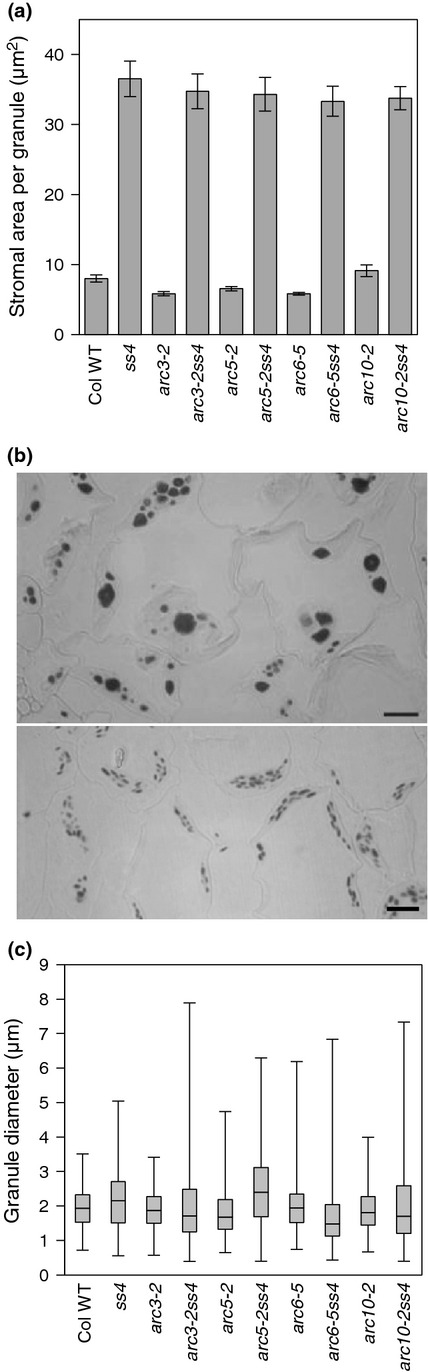
Effects of the ss4 mutation in Arabidopsis arc mutant backgrounds. Values for single mutants are from Crumpton-Taylor et al. (2012). All genotypes were grown together in the same conditions. (a) Stromal area per starch granule for mature leaves of wild-type (WT), ss4,arc and arc x ss4 mutants. Values are chloroplast cross-sectional area divided by numbers of granules in that area. Measurements were on either 40 chloroplasts (WT and single mutants) or 120 chloroplasts (arc × ss4 mutants) in five random sections of two leaves from two different plants, harvested 9 h into the light period. Error bars, ± SE. (b) Sections of mature leaves, stained with iodine. Upper, arc3-2ss4; lower, arc3-2. Bars, 10 μm. (c) Granule diameters in wild-type, ss4,arc and arc x ss4 plants. The solid line in the box is the median (50th percentile). The bottom and top of the box are the 25th and 75th percentiles. Upper and lower bars indicate the range of values. Between 319 and 2270 granules were measured on 11 randomly-selected scanning electron micrographs of starch from 40 25-d-old plants per genotype, at the end of the light period.
Loss of SS4 from established plants affects granule numbers in immature but not mature leaves
Taken together, the observations above suggest that the primary effect of loss of SS4 is a strongly delayed and infrequent formation of new starch granules during leaf development. The alterations in granule number and starch turnover in mature leaves may be secondary consequences of this effect. To distinguish more clearly between primary and secondary effects, we reduced SS4 concentrations in mature rosettes by inducing expression of an RNAi hairpin construct targeted at the SS4 gene, and examined the consequences for granule numbers and starch turnover.
Wild-type plants were transformed with constructs allowing expression of two hairpin RNAs based on different parts of the SS4 gene sequence, under the control of a dexamethasone-inducible promoter (Figs S6, S7). Transgenic plants were treated with dexamethasone daily for 10 d. For each construct, one line showing strong reductions in SS4 protein was selected for further study. Before dexamethasone treatment, SS4 transcript and protein concentrations were the same in transgenic lines and wild-type plants. SS4 protein concentrations were unaffected by dexamethasone treatment in wild-type controls (Fig. S6b and not shown). In transgenic lines, SS4 transcript levels declined to very low values within 24–48 h of the first dexamethasone treatment and remained low. Concentrations of SS4 protein fell slowly to undetectable levels over the first 7 d of dexamethasone treatment (Fig.6a).
Figure 6.
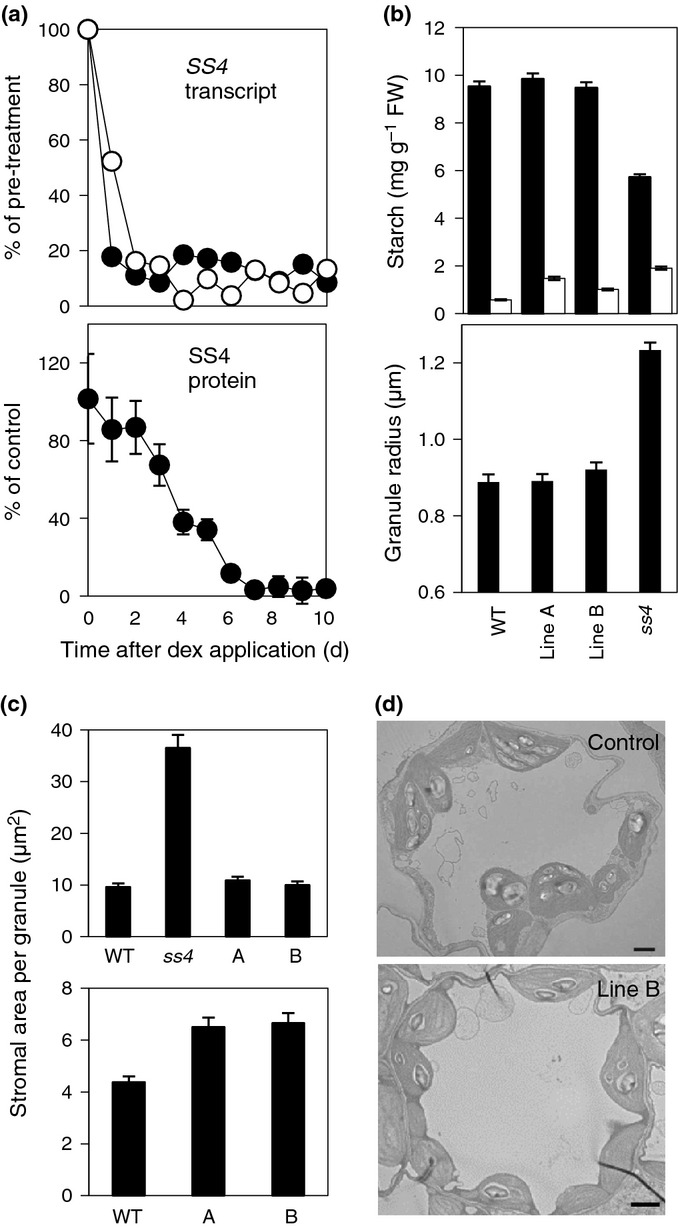
Effects of induction of expression of SS4RNAi in mature Arabidopsis rosettes. (a) Effect on SS4 transcript and protein of daily application of dexamethasone (dex) for 10 d. Harvests were immediately before dex treatment, 10 h into the light period. For each harvest SS4 transcript levels were expressed as a percentage of those of TUBULIN. These values are expressed as a percentage of the day 0 value. Closed circles, RNAi line A; open circles, RNAi line B. SS4 protein concentrations were estimated from immunoblots. Values are percentages of the day 0 value, and are means of measurements from six plants (three from line A, three from line B), all analysed on the same gel. Error bars, ± SE. Further details are in Fig. S6. (b) Starch content (upper panel) at the end of the day (closed bars) and the end of the night (open bars) and granule radii (lower panel) from wild-type, ss4 and RNAi lines after 10 d of dex treatment. Starch values are means of measurements on eight rosettes. Radii of 250 granules per genotype were measured on starch from rosettes harvested at the end of the day. Error bars, ± SE. (c) Stromal area per granule for mature (upper) and immature (lower) leaves of wild-type (WT), ss4 and RNAi line A and B plants after 10 d of dex treatment. Details are as for Fig.5(a) except that 40 chloroplasts were measured in seven sections. Harvest was 10 h into the light period. (d) Transmission electron micrographs of young leaves of wild-type and RNAi line B plants after 10 d of dex treatment. Bar, 2 μm.
After 10 d of dexamethasone treatment, whole rosettes of transgenic lines had higher end-of-night starch contents than control plants (although lower than in ss4 plants), but diel starch turnover was at least 94% of that in wild-type plants and more than twice that in ss4 plants. There was little or no difference between transgenic and control plants in whole-rosette starch content at the end of the day, average granule radius (Fig.6b) and granule size distribution (Fig. S6c). In sections of mature leaves, the chloroplast area per starch granule was the same in transformed and control plants. However, in immature leaves that had emerged during the dexamethasone treatment (leaves 1–4) the chloroplast area per starch granule was c. 50% greater in transgenic than in control plants (Fig.6c,d). In summary, reduced SS4 concentrations strongly reduced starch granule numbers in young leaves, but had little effect in mature leaves.
SS4 concentrations can be substantially reduced without affecting starch granule formation
We investigated whether the amount of SS4 in a wild-type leaf limits starch granule formation. This possibility is suggested by a report that transgenic Arabidopsis plants with elevated concentrations of SS4 protein have increased rates of starch synthesis (Gámez-Arjona et al., 2011). First, we examined starch turnover in leaves of ss4 plants expressing the Arabidopsis SS4 cDNA from the CaMV 35S promoter. Starch content and turnover were restored to near wild-type levels in both immature and mature leaves of several transgenic lines that had much lower concentrations of SS4 protein than wild-type plants (Fig. S8).
Second, we examined growth and starch metabolism of heterozygous (SS4ss4-3) plants. As expected, both mature and immature leaves had approximately half the SS4 protein content of wild-type plants (Figs7, S9). Heterozygous plants were statistically significantly different from ss4 mutant plants but not from wild-type plants with respect to FW, starch granule size distribution, granule volume, starch content and turnover in mature and immature leaves, and mean stromal area per starch granule (measured on light micrographs of leaf sections) (Figs7, S9). Thus, loss of half of the SS4 protein does not affect granule formation.
Figure 7.
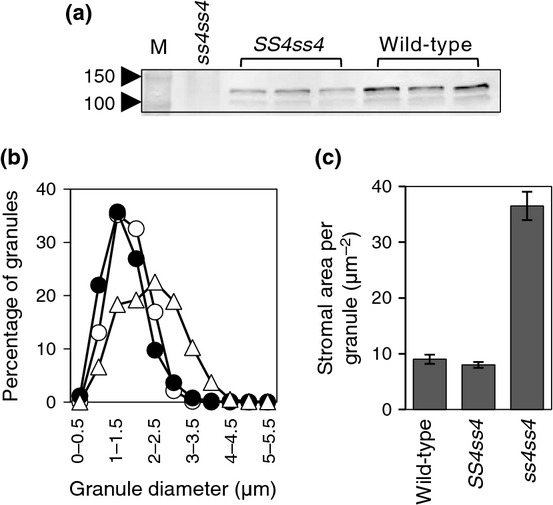
Starch characteristics of heterozygous (SS4ss4) Arabidopsis plants. (a) Immunoblot of extracts of mature wild-type, ss4 mutant and SS4ss4 rosettes, probed with SS4 antibodies (quantification: Fig. S9). M, molecular mass markers (in kDa). Each lane contains 40 μl extract; all extracts contained the same mg tissue per ml. (b) Diameters of granules extracted from wild-type (closed circles), heterozygote (open circles) and ss4 (triangles) mature rosettes at the end of the light period. More than 500 granules were measured for each genotype. Each data point is the percentage of granules falling into the diameter range on the x-axis. (c) Stromal area per starch granule in mature leaves. Details are as for Fig.5(a) except that c. 50 rather than 40 chloroplasts were analysed. Wild-type and heterozygote values are not statistically significantly different.
Discussion
SS4 is necessary for granule formation during leaf expansion
Our results show that SS4 is essential for the coordinated formation of starch granules that occurs during leaf expansion. In wild-type plants, chloroplasts have multiple starch granules from a very early stage of leaf development (Sakamoto et al., 2009; Crumpton-Taylor et al., 2012). Although chloroplasts undergo about three rounds of division as leaf cells expand, resulting in an eight-fold increase in chloroplast numbers (Marrison et al., 1999), the numbers of starch granules per chloroplast remain relatively constant (a 1.5-fold decrease between early expansion and maturity: Crumpton-Taylor et al., 2012). Thus, large numbers of new granules must be produced in association with rounds of chloroplast division. By contrast, no starch granules are visible in chloroplasts of immature leaves of ss4 mutant plants. Granules appear only at a later stage of leaf expansion, and granule numbers per chloroplast are much lower in mature leaves than in wild-type plants.
These observations suggest that the low numbers of starch granules in chloroplasts of mature ss4 leaves are a consequence of the major defect in granule formation during leaf expansion in this mutant. Support for this view comes from our analysis of plants in which concentrations of SS4 protein were progressively reduced by induction of RNAi specifically targeted at the SS4 gene. Loss of SS4 protein was accompanied by reduced granule numbers in newly formed leaves but not in mature leaves, showing that SS4 is required for normal granule formation in immature leaves but not for maintenance of granule numbers in mature leaves. This conclusion is consistent with a specific role for SS4 in granule formation: although new granules arise frequently during leaf expansion this is likely to be a rare event once leaves reach maturity (Crumpton-Taylor et al., 2012).
SS4 also appears to be necessary for a normal frequency of granule formation following cell division in root caps. The root caps of ss4 plants generally contained less starch in fewer cells than wild-type plants, and there was a much higher degree of plant-to-plant variation in root cap starch content.
SS4 plays a direct role in the formation of new granules
Loss of SS4 has several major effects on the Arabidopsis plant in addition to reduced starch granule numbers. These include reduced growth rates, altered patterns of starch turnover and concentrations of sugars, altered starch granule morphology, and reduced starch content and gravitropic response in roots. It is conceivable that failure of starch granule formation during leaf expansion is an indirect consequence of one or a combination of these effects. However, taking our results as a whole, we propose that SS4 is involved directly in the formation of granules, and that the other effects of its loss are secondary consequences of reduced granule numbers. The basis for this conclusion is as follows.
First, the absence of starch granules from immature leaves of SS4 mutants is likely to be caused directly by failure of granule formation rather than indirectly by alterations in other aspects of sugar or glucan metabolism. Immature ss4 leaves did not accumulate abnormal concentrations of soluble glucans, but contained exceptionally high concentrations of ADPglucose, suggesting that in the absence of SS4 the remaining soluble starch synthases are largely inactive. The fact that introduction of the sex1 mutation failed to restore either starch or glucan accumulation is also consistent with the idea that no starch is made. These observations point to a direct role for SS4 in a mechanism that generates a ‘primer’ that is acted on by other starch synthases, resulting in the formation of new granules.
Second, introduction of additional capacity for self-priming glucan synthesis in ss4 leaves complemented some of the effects of loss of SS4 but did not restore normal starch synthesis. Expression of glycogen synthase in the highly compromised ss4ss3 mutant reduced ADPglucose concentrations, increased starch concentrations, and restored wild-type appearance and growth rates. However, although starch granules were formed earlier in leaf expansion in plants expressing glycogen synthase, new granules arose sporadically and there were highly variable numbers per chloroplast. We conclude that glycogen synthase could catalyse the synthesis of glucans from which granules could be formed, but that it could not replace a specific and direct requirement for SS4 in a mechanism that controls the timing of granule formation and its coordination with chloroplast volume.
Third, in ss4 mutants with increased chloroplast volume (arc × ss4 mutants) some granules were present in immature leaves and starch turnover was comparable with that of wild-type plants, but granule distribution, shape and size remained highly variable. Thus, large chloroplast volumes permit more frequent formation of granules and higher starch turnover in the absence of SS4, but do not restore the coordinated production of typical transitory starch granules seen in wild-type leaves.
Fourth, comparison of the ss4 phenotype with those of mutants lacking other components of the pathways of starch synthesis and degradation suggests that SS4 has an exclusive and direct role in granule formation. Several features of the ss4 phenotype are also seen in other starch mutants. For example the starch synthesis mutant pgm1 and the starch degradation mutant sex1 – lacking plastidial phosphoglucomutase and glucan, water dikinase, respectively – have reduced growth rates, elevated daytime concentrations of sugars (Caspar et al., 1985, 1991) and defective gravitropism (Caspar & Pickard, 1989; Vitha et al., 2007). Other starch degradation mutants with reduced growth rates, reduced starch turnover and elevated daytime concentrations of sugars include mex1,pwd1 and dpe1, lacking the chloroplast maltose transporter, phosphoglucan, water dikinase and the plastidial disproportionating enzyme, respectively (Critchley et al., 2001; Niittylä et al., 2004; Baunsgaard et al., 2005; Kötting et al., 2005). However, none of these mutants is reported to have reduced numbers of starch granules per chloroplast, or starch in mature but not immature leaves. Similarly, mutants lacking combinations of isoforms of starch synthase other than SS4 also exhibit reduced starch and elevated sugar contents and reduced growth rates, but are not reported to have reduced numbers of starch granules per chloroplast (ss2ss3 mutants, Zhang et al., 2008; ss1ss2ss3 mutants, Szydlowski et al., 2009). The impact of the ss4 mutation on granule formation is thus likely to reflect a primary role for SS4 in this process, rather than a general secondary effect of perturbations in growth, starch and sugar metabolism, or gravitropism.
We suggest that most of the effects of loss of SS4 may be consequences of a primary failure of granule formation in immature leaves. As discussed above, the exceptionally high concentrations of ADPglucose may result from the absence of a primer required by other starch synthases. The amount of ADPglucose in ss4 leaves is approximately the same as the total amount of ATP + ADP in wild-type leaves (c. 120 nmol g−1 FW: Strand et al., 2000). The sequestration of most of the normal adenine nucleotide pool into ADPglucose probably compromises ATP regeneration during photosynthesis, and hence the operation of the Calvin–Benson cycle. The slow growth of ss4 mutant plants may thus be due largely to reduced photosynthetic CO2 assimilation. Elevated daytime concentrations of sugars in ss4 leaves are probably a secondary consequence of reduced starch turnover. The same phenomenon is seen in other mutants defective in starch turnover (as already described). The loss of gravitropism in roots of ss4 mutants is highly likely to be a consequence of reduced formation of granules in the root cap. We observed a negative correlation between root cap starch content and deviation of the root from vertical growth. Reduced gravitropic responses have been observed previously in roots of Arabidopsis mutants with reduced starch contents (Caspar & Pickard, 1989).
Roldán et al. (2007) suggested that the low starch turnover in mature ss4 leaves may result from the very small numbers of starch granules, which provide insufficient surface area for normal rates of synthesis and degradation. Our results provide some support for this idea. First, mature ss4 leaves had elevated concentrations of ADPglucose, consistent with limitation of starch synthesis in these leaves by granule surface area. Second, the arc × ss4 mutants, which had starch from an earlier stage of leaf expansion and a wider range of granule sizes than ss4 mutants, also had higher daily starch turnover than ss4 mutants. However, the possibility that SS4 is directly required for some aspect of starch turnover in mature leaves cannot be ruled out. SS4 can certainly participate in starch synthesis in mature leaves: leaves of triple mutant plants lacking the other isoforms of starch synthase (ss1ss2ss3 mutants) are still capable of starch synthesis and turnover (Roldán et al., 2007).
SS4 is required for coordination of granule formation with chloroplast volume and for determination of granule morphology
ss4 mutants are defective in the relationship between starch granule initiation and chloroplast volume, and in starch granule morphology. In wild-type plants, there is a tight relationship between stromal volume and numbers of starch granules. At a given stage of leaf expansion the number of granules per unit volume of stroma is remarkably constant, regardless of the total volume of the chloroplast. Granule sizes are also relatively uniform within and between chloroplasts (Crumpton-Taylor et al., 2012). These relationships are absent in ss4 mutants, and are not restored by manipulations that increase the frequency of granule initiation. Although immature leaves of ss3ss4 mutant plants expressing glycogen synthase had more starch granules than those of ss4 mutants, these granules were highly variable in distribution and apparent size. Whereas a few chloroplasts appeared to have large numbers of granules, many others had none. Transfer of the ss4 mutation into an arc mutant background, in which chloroplast volumes are greatly increased, had a similar effect. Granule numbers in immature leaves of arc × ss4 mutants were greater than in ss4 mutants. However, granule distribution and size were highly variable, and there was no obvious relationship between starch granule number and chloroplast volume. We conclude that SS4 is an essential component of a mechanism that links granule initiation to chloroplast volume. In its absence, granule initiation appears to be stochastic.
Starch granules in leaves of ss4 mutants are different in shape from those in wild-type plants. Rather than being flattened and discoid, they are rounded with an electron-transparent core (Roldán et al., 2007;Fig. S1d). Manipulations of glucan-synthesizing capacity (by expression of Agrobacterium glucan synthase) and chloroplast volume (by introduction of arc mutations) did not restore normal granule morphology even though they increased the frequency of granule formation in immature leaves. Thus, the mechanism of granule formation in leaves in the presence of SS4 appears to be qualitatively different from that in its absence. It is interesting to note that flattened, discoid granules are found in leaves but not in other plant organs. Starch granules in nonphotosynthetic organs are generally rounded with a distinct core or hilum, resembling the leaf starch granules of ss4 mutants. However, SS4 appears to be necessary for normal granule initiation in root caps as well as leaves, suggesting that it interacts with different granule-initiation mechanisms in the two organs.
What is the function of SS4 in granule initiation? As discussed above, it seems to be an essential part of a mechanism that generates a specific ‘primer’ on which other starch synthases can act, leading to granule formation. The primer might simply be small malto-oligosaccharides, perhaps elaborated by SS4 from ADPglucose alone or from maltose, which is produced de novo during photosynthesis (Linden et al., 1975; Szecowka et al., 2013). This explanation seems unlikely, however, because starch synthases other than SS4 are reported to synthesize malto-oligosaccharides from ADPglucose or maltose. Szydlowski et al. (2009) showed that recombinant SS3 can form glucans from ADPglucose alone. Recombinant SS1, SS2 and SS3 from kidney bean (Phaseolus vulgaris) can elongate both maltose and maltotriose (Senoura et al., 2004, 2007). It is also difficult to explain why SS4 is required for normal granule shape, and why it cannot be fully replaced by Agrobacterium glycogen synthase, if its sole function is to provide small oligosaccharides on which other starch synthases can act. An alternative explanation (Szydlowski et al., 2009; Mérida & D′Hulst, 2012) is that SS4 proteins adopt a quaternary structure – perhaps as part of a complex with other proteins – which serves as a synthetic and/or nucleation centre for specific glucans required for granule formation. This centre could also determine the subsequent direction of granule growth, and hence granule shape. Alone among the starch synthases, SS4 possesses coiled-coil domains in the noncatalytic n-terminal part of the protein. Coiled-coil domains can mediate protein–protein interactions (Mason & Arndt, 2004). Further work is required to establish precisely the nature of the primer made in the presence of SS4, and how it allows initiation of appropriate numbers of uniform granules of defined shape as chloroplasts expand and divide.
SS4 is not limiting for starch granule formation in wild-type leaves
Although SS4 is required for the formation of normal transitory starch granules, it is unlikely that its concentration exerts significant control over the numbers of granules initiated in wild-type plants. First, granule numbers in SS4ss4 heterozygotes with half the wild-type concentrations of SS4 protein were indistinguishable from those of wild-type plants. Second, normal starch metabolism was restored in ss4 mutant plants by transgenic expression of SS4 at concentrations far below those in wild-type plants. These results imply that the tight relationship between stromal volume and numbers of starch granules is not simply a function of numbers of SS4 protein molecules, but is determined by a complex mechanism of which SS4 is an essential but nonlimiting component. In this light, it is interesting that overexpression of SS4 in wild-type Arabidopsis plants results in increased rates of leaf starch synthesis and up to 50% more starch at the end of the day (Gámez-Arjona et al., 2011). Important new information about the role of SS4 may be gained from analysis of the total starch synthase activity and the size, number and distribution of starch granules in these overexpressing plants.
Acknowledgments
This work was supported by grants BB/J004561/1 and BB/C507361/1 from the Biotechnology and Biological Sciences Research Council (UK) and 31003A-131074 from the Swiss National Science Foundation, by the ETH, Zürich, and by the Max Planck Society, Germany. M.C-T. thanks the John Innes Foundation for a PhD studentship; R.F. and J.E.L. thank M. Stitt for helpful advice and discussions. We thank Dr Á. Mérida (University of Seville, Spain) for sharing unpublished data and Dr C. D'Hulst (University of Lille, France) for the gift of ss3ss4 seeds.
Supporting Information
Additional supporting information may be found in the online version of this article.
Characterization of ss4 mutants.
Fig. S2 Growth of ss4 roots.
Fig. S3 Phenotypes of ss4sex1 mutants.
Fig. S4 Expression of Agrobacterium glgA in ss3ss4 mutants.
Fig. S5 Starch granules in arc x ss4 mutants.
Fig. S6 Effects of inducing RNAi targeted at the SS4 gene.
Fig. S7 SS4 coding sequence showing regions targeted by RNAi.
Fig. S8 Transgenic ss4 plants expressing SS4.
Fig. S9 Further characterization of heterozygous (SS4ss4) plants.
Table S1 Oligonucleotide primers used in this study
Table S2 Starch synthase activities and chlorophyll contents of ss4 mutants
Table S3 ADPglucose contents of mature and immature leaves of ss4 mutants
References
- Barratt DHP, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C. Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiology. 2001;127:655–664. [PMC free article] [PubMed] [Google Scholar]
- Baunsgaard L, Lütken H, Mikkelsen R, Glaring MA, Pham TT, Blennow A. A novel isoform of glucan, water dikinase phosphorylates pre-phosphorylated α-glucans and is involved in starch degradation in Arabidopsis. Plant Journal. 2005;41:595–605. doi: 10.1111/j.1365-313X.2004.02322.x. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiology. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiology. 1985;79:11–17. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Lin TP, Kakefuda G, Benbow L, Preiss J, Somerville C. Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiology. 1991;95:1181–1188. doi: 10.1104/pp.95.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Pickard BG. Gravitropism in a starchless mutant of Arabidopsis. Implications for the starch statolith theory of gravity sensing. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- Chatterjee M, Berbezy P, Vyas D, Coates S, Barsby T. Reduced expression of a protein homologous to glycogenin leads to reduction of starch content in Arabidopsis leaves. Plant Science. 2005;168:501–509. [Google Scholar]
- Comparot-Moss S, Kötting O, Stettler M, Edner C, Graf A, Weise SE, Streb S, Lue WL, MacLean D, Mahlow S, et al. A putative phosphatase, LSF1, is required for normal starch turnover in Arabidopsis leaves. Plant Physiology. 2010;152:685–697. doi: 10.1104/pp.109.148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM. A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant Journal. 2001;26:89–100. doi: 10.1046/j.1365-313x.2001.01012.x. [DOI] [PubMed] [Google Scholar]
- Crumpton-Taylor M, Grandison S, Png KMY, Bushby AJ, Smith AM. Control of starch granule numbers in Arabidopsis chloroplasts. Plant Physiology. 2012;158:905–916. doi: 10.1104/pp.111.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte T, Trevisan M, Parker ML, Zeeman SC. Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant Journal. 2005;41:815–830. doi: 10.1111/j.1365-313X.2005.02348.x. [DOI] [PubMed] [Google Scholar]
- Doi K. Formation of amylopectin granules in gelatin gel as a model of starch precipitation in plant plastids. Biochimica et Biophysica Acta. 1965;94:557–565. doi: 10.1016/0926-6585(65)90064-6. [DOI] [PubMed] [Google Scholar]
- Gámez-Arjona FM, Li J, Raynaud S, Baroja-Fernández E, Muñoz FJ, Ovecka M, Ragel P, Bahaji A, Pozueta-Romero J, Mérida Á. Enhancing the expression of starch synthase class IV results in increased levels of both transitory and long-term storage starch. Plant Biotechnology Journal. 2011;9:1049–1060. doi: 10.1111/j.1467-7652.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- Geddes R, Greenwood CT. Observations on the biosynthesis of the starch granule. Starch-Stärke. 1969;6:148–153. [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Hohne M, Gunther M, Stitt M. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant Journal. 2004;39:847–862. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville C. Sulfonylurea-resistant mutants of Arabidopsis thaliana. Molecular and General Genetics. 1986;204:430–434. [Google Scholar]
- Jenner C, Denyer K, Hawker JS. Caution on the use of the generally accepted methanol precipitation technique for the assay of soluble starch synthase in crude extracts of plant tissues. Australian Journal of Plant Physiology. 1994;21:17–22. [Google Scholar]
- Karimi M, Inzé D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Edelmann RE. Spaceflight experiments with Arabidopsis starch deficient mutants support a statolith-based model for graviperception. Advanced Space Research. 1999;24:755–762. doi: 10.1016/s0273-1177(99)00409-3. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- Kötting O, Pusch K, Tiessen A, Geigenberger P, Steup M, Ritte G. Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiology. 2005;137:242–252. doi: 10.1104/pp.104.055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld SMJ, Vennik M, Kottenhagen M, van Wijk R, Buijk A, Kijne JW, de Pater S. Glucosylation activity and complex formation of two classes of reversibly glycosylated polypeptides. Plant Physiology. 2002;129:278–289. doi: 10.1104/pp.010720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden JC, Schilling N, Brackenhofer H, Kandler O. Asymmetric labelling of maltose during photosynthesis in 14CO2. Zeitschrift für Plfanzenphysiologie. 1975;76:176–181. [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochemical Journal. 2006;397:139–148. doi: 10.1042/BJ20060083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrison JL, Rutherford SM, Robertson EJ, Lister C, Dean C, Leech RM. The distinctive roles of five different ARC genes in the chloroplast division process in Arabidopsis. Plant Journal. 1999;18:651–662. doi: 10.1046/j.1365-313x.1999.00500.x. [DOI] [PubMed] [Google Scholar]
- Mason JM, Arndt KM. Coiled coil domains: stability, specificity, and biological implications. ChemBioChem. 2004;5:170–176. doi: 10.1002/cbic.200300781. [DOI] [PubMed] [Google Scholar]
- Mérida A, D′Hulst C. Once upon a prime: inception of the understanding of starch initiation in plants. In: Tetlow IJ, editor. The synthesis and breakdown of starch. SEB essential reviews in experimental biology vol. 5. London, UK: Society for Experimental Biology; 2012. pp. 55–76. [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. A previously unknown maltose transporter essential for starch degradation in leaves. Science. 2004;303:87–89. doi: 10.1126/science.1091811. [DOI] [PubMed] [Google Scholar]
- Ritte G, Heydenreich M, Mahlow S, Haebel S, Kötting O, Steup M. Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Letters. 2006;580:4872–4876. doi: 10.1016/j.febslet.2006.07.085. [DOI] [PubMed] [Google Scholar]
- Roldán I, Wattebled F, Lucas MM, Delvallé D, Planchot V, Jimenez S, Perez R, Ball S, D'Hulst C, Mérida Á. The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant Journal. 2007;49:492–504. doi: 10.1111/j.1365-313X.2006.02968.x. [DOI] [PubMed] [Google Scholar]
- Rothschild A, Tandecarz JS. UDP-glucose-protein transglucosylase in developing maize endosperm. Plant Science. 1994;97:119–127. [Google Scholar]
- Sakamoto W, Uno Y, Zhang Q, Miura E, Kato Y, Sodmergen Arrested differentiation of proplastids into chloroplasts in variegated leaves characterized by plastid ultrastructure and nucleoid morphology. Plant and Cell Physiology. 2009;50:2069–2083. doi: 10.1093/pcp/pcp127. [DOI] [PubMed] [Google Scholar]
- Senoura T, Asao A, Takashima Y, Isono N, Hamada S, Ito H, Matsui H. Enzymatic characterization of starch synthase III from kidney bean (Phaseolus vulgaris L.) FEBS Journal. 2007;274:4550–4560. doi: 10.1111/j.1742-4658.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- Senoura T, Isono N, Yoshikawa M, Asao A, Hamada S, Watanabe K, Ito H, Matsui H. Characterization of starch synthase I and II expressed in early developing seeds of kidney bean (Phaseolus vulgaris L.) Bioscience, Biotechnology and Biochemistry. 2004;68:1949–1960. doi: 10.1271/bbb.68.1949. [DOI] [PubMed] [Google Scholar]
- Singh DG, Lomako J, Lomako WM, Whelan WJ, Meyer HE, Serwe M, Metzger JW. β-Glucosylarginine: a new glucose-protein bond in a self-glycosylating protein from sweet corn. FEBS Letters. 1995;376:61–64. doi: 10.1016/0014-5793(95)01247-6. [DOI] [PubMed] [Google Scholar]
- Stitt M, Zeeman SC. Starch turnover: pathways, regulation and role in growth. Current Opinion in Plant Biology. 2012;15:282–292. doi: 10.1016/j.pbi.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Strand A, Zrenner R, Trevanion S, Stitt M, Gustafsson P, Gardeström P. Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant Journal. 2000;23:759–770. doi: 10.1046/j.1365-313x.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- Streb S, Delatte T, Umhang M, Eicke S, Schorderet M, Reinhardt D, Zeeman SC. Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylase. Plant Cell. 2008;20:3448–3466. doi: 10.1105/tpc.108.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T, Nunes-Nesi A, Vosloh D, Huege J, Feil R, Lunn J, Nikoloski Z, Stitt M, et al. Metabolic fluxes in an illuminated Arabidopsis rosette. Plant Cell. 2013;25:694–714. doi: 10.1105/tpc.112.106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowski N, Ragel P, Raynaud S, Lucas MM, Roldán I, Montero M, Muñoz FJ, Ovecka M, Bahaji A, Planchot V, et al. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell. 2009;21:2443–2457. doi: 10.1105/tpc.109.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde JE, Parodi AJ, Ugalde RA. De novo synthesis of bacterial glycogen: Agrobacterium tumefaciens glycogen synthase is involved in glucan initiation and elongation. Proceedings of the National Academy of Sciences, USA. 2003;100:10 659–10 663. doi: 10.1073/pnas.1534787100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, Ming Y, Sack FD, Kiss JZ. Gravitropism in the starch excess mutant of Arabidopsis thaliana. American Journal of Botany. 2007;94:590–598. doi: 10.3732/ajb.94.4.590. [DOI] [PubMed] [Google Scholar]
- Wielopolska A, Townley H, Moore I, Waterhouse P, Helliwell C. A high throughput inducible RNAi vector for plants. Plant Biotechnology Journal. 2005;3:583–590. doi: 10.1111/j.1467-7652.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Yu TS, Kofler H, Hausler RE, Hille D, Flügge UI, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, et al. The Arabidopsis sex1mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell. 2001;13:1907–1918. doi: 10.1105/TPC.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, ap Rees T. Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant, Cell & Environment. 1999;22:1445–1453. [Google Scholar]
- Zhang X, Szydlowski N, Delvallé D, D'Hulst C, James MG, Myers AM. Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biology. 2008;8:96. doi: 10.1186/1471-2229-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler GR, Creek JA, Runt J. Spherulitic crystallization in starch as a model for starch granule initiation. Biomacromolecules. 2005;6:1547–1554. doi: 10.1021/bm049214p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of ss4 mutants.
Fig. S2 Growth of ss4 roots.
Fig. S3 Phenotypes of ss4sex1 mutants.
Fig. S4 Expression of Agrobacterium glgA in ss3ss4 mutants.
Fig. S5 Starch granules in arc x ss4 mutants.
Fig. S6 Effects of inducing RNAi targeted at the SS4 gene.
Fig. S7 SS4 coding sequence showing regions targeted by RNAi.
Fig. S8 Transgenic ss4 plants expressing SS4.
Fig. S9 Further characterization of heterozygous (SS4ss4) plants.
Table S1 Oligonucleotide primers used in this study
Table S2 Starch synthase activities and chlorophyll contents of ss4 mutants
Table S3 ADPglucose contents of mature and immature leaves of ss4 mutants


