Abstract
Background
Metabolic syndrome (MetS) is one of the primary reasons for increased mortality in patients with schizophrenia. The mechanisms involved in its pathogenesis are not well understood.
Objective
To estimate the prevalence of MetS in adult outpatients with schizophrenia according to the presence or absence of negative symptoms.
Materials and methods
A retrospective cohort study using electronic medical records was conducted. The Positive and Negative Syndrome Scale negative-symptom factor (N1–N4, N6, G7, and G16) was used as a framework for characterizing negative symptoms. MetS was defined using the National Cholesterol Education Program Adult Treatment Panel III diagnostic criteria. An analysis of covariance model was used for correction, with significance at P<0.05.
Results
One or more negative symptoms were present in 52.5% of a sample of 1,120 patients (mean age 46.8 years, men 58.4%). Dyslipidemia (48.7%), hypertension (38.2%), and diabetes mellitus (19.3%) were the most frequent comorbidities. The overall prevalence of MetS was 38.6% (95% confidence interval 35.7%–41.5%), and was significantly higher in those patients with negative symptoms (43.9% versus 34.9%, P=0.002). MetS was significantly associated with the presence of negative symptoms, age, and physical comorbidity (odds ratios 1.6, 1.2, and 1.2, respectively; P<0.05).
Conclusion
A sedentary lifestyle and lack of physical exercise due to negative symptomatology may contribute to MetS development. Further studies are necessary to confirm this association and the underlying pathophysiological mechanisms.
Keywords: cardiovascular risk, metabolic abnormalities, comorbidity, physical illness, schizophrenia, negative symptoms
Introduction
Schizophrenia is one of the most severe mental disorders, and is among the twenty leading causes of disability worldwide.1 The mortality rate in patients with schizophrenia is two to three times higher than that expected in the general population, with a reduction in life expectancy of 10–25 years.2 Increased risk of suicide, an unhealthy lifestyle, poor physical health, and side effects of antipsychotic drugs are the four main reasons associated with excess early mortality in schizophrenia.3 Natural causes of death include those deriving from cardiovascular events.4 The high prevalence of cardiovascular risk factors in this population, such as obesity, dyslipidemia, alterations in carbohydrate metabolism, smoking, a sedentary lifestyle, and an unbalanced diet, as well as undesirable effects of antipsychotic drugs, explains the high cardiovascular morbidity and mortality rate in these patients.5
Negative symptoms are a key element of schizophrenia, affecting patients’ ability to cope with daily activities and having a negative impact on their quality of life.6,7 Negative symptoms are relatively common, and account for much of the long-term morbidity and poor functional outcome of patients with schizophrenia.6,8,9 Despite the introduction of atypical antipsychotics in the 1990s, their clinical management continues to be an unmet need.10
Compared with the general population, patients with schizophrenia are twice as likely to suffer from metabolic syndrome (MetS).11 The prevalence of MetS in patients with schizophrenia varies, ranging between 11% and 69% depending on the methodology used and the study population, although some recent meta-analyses have estimated it to be around 32.5%.12–15 Late onset, ethnicity, long duration of the illness, smoking, and exposure to antipsychotic drugs have generally been considered risk factors for developing MetS.11 However, no studies have been conducted to date to assess its prevalence according to the presence of different types of symptomatic domains.
The aim of this study was to analyze the prevalence of MetS and its individual components in a population of outpatients with schizophrenia according to the presence or absence of negative symptoms.
Materials and methods
Data source and patients
This was a noninterventional, retrospective, cohort study using electronic medical records held by the health care provider Badalona Serveis Assistencials (BSA). Key inclusion criteria were: age ≥18 years; a diagnosis of schizophrenia according to Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV-TR criteria; outpatients who required care in 2011; being under antipsychotic treatment; inclusion in the long-term prescription program (with a record of daily dose, time interval, and duration of each treatment administered); and guaranteed regular patient follow-up (presenting two or more health care records in the computer system). Data for these analyses were obtained from an observational study focused on health care-resource utilization and associated costs in patients with schizophrenia from the city of Badalona.16 The study protocol was approved by the investigational review board of the Hospital Universitari Germans Trias i Pujol (Badalona, Spain).
Variables and measurement instruments
The seven items of the Positive and Negative Syndrome Scale (PANSS) Marder negative-symptom factor were used as a framework for characterizing negative symptoms from the database text fields: blunted affect (N1), emotional withdrawal (N2), poor rapport (N3), passive/apathetic social withdrawal (N4), lack of spontaneity and conversation flow (N6), motor retardation (G7), and active social avoidance (G16).17,18 The number of chronic diseases (diagnoses), the Charlson Comorbidity Index, and the individual Case-Mix Index, obtained from the Adjusted Clinical Groups (a classification system based on the consumption of health care resources), were used to summarize general comorbidity variables for each patient.19,20
The clinical, biochemical and anthropometric parameters analyzed were systolic and diastolic blood pressure (mmHg), body mass index (BMI; kg/m2), basal blood glucose (mg/dL), serum triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol (mg/dL), and serum creatinine (mg/dL). The diagnosis of MetS was established when one same individual met three or more of the following components of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) diagnostic criteria: hypertriglyceridemia (fasting triglyceride concentration ≥150 mg/dL or treatment with triglyceride-lowering agents), dyslipidemia (fasting HDL cholesterol <40 mg/dL in males and <50 mg/dL in females), hypertension (systolic and diastolic blood pressure ≥130/85 mmHg or on antihypertensive medication), hyperglycemia (fasting plasma glucose concentration ≥110 mg/dL or on glucose-lowering drug treatment or a previous diagnosis of diabetes), and abdominal obesity (waist circumference >102 cm in males and >88 cm in females).21 In our study, the waist-circumference measurement was replaced by the BMI. A BMI ≥28.8 kg/m2 is considered to be equivalent to abdominal adiposity by different authors.22
Statistical analysis
Patients were distributed into two groups according to the presence or absence of negative symptoms: group 1 for patients presenting one or more negative symptoms, and group 2 for patients without negative symptoms.
A descriptive univariate statistical analysis was performed with mean values, standard deviation (SD), and 95% confidence intervals (CIs), verifying normal distribution using the Kolmogorov–Smirnov test. The bivariate analysis included analysis of variance, the χ2 test and Pearson’s linear correlation. Logistic regression analysis was performed to obtain the variables associated with the patient profile (negative symptoms) and MetS, with the “Enter” method (Wald statistic). SPSS version 19 was used, establishing statistical significance for P-values <0.05.
Results
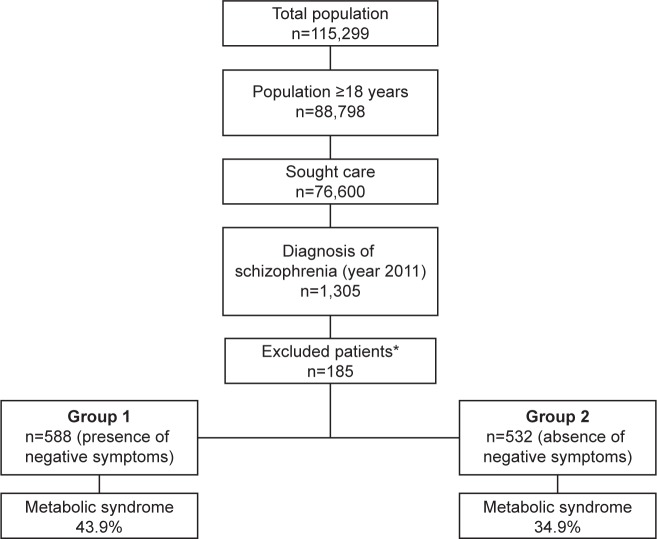
Of the initial selection of 88,798 subjects aged ≥18 years, 1,305 patients presented a diagnosis of schizophrenia and 1,120 were finally analyzed (Figure 1). Major sociodemographic and clinical characteristics of the patients are shown in Table 1. The mean age was 46.8 (SD 13.8) years, and 58.4% were male; 52.5% of the patients presented one or more negative symptoms (n=588, 95% CI 49.6%–55.4%). The prevalence of individual negative symptoms ranged from 26.1% (G16, active social avoidance) to 60.5% (N4, passive/apathetic social withdrawal). Only 27% of the patients simultaneously presented three or more negative symptoms.
Figure 1.
Study diagram.
Notes: *Patients were excluded for the following reasons: missing or inconsistent data (n=33; 17.8%), lost to follow-up (n=45: 24.3%), moved to other areas (n=37; 20.0%), and other reasons (n=70; 37.9%).
Table 1.
Baseline characteristics of the study series
| Group 1 n=588 |
Group 2 n=532 |
Total n=1,120 |
P-value | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Mean age, years | 47.3 (12.2) | 46.5 (14.8) | 46.8 (13.8) | 0.351 |
| Range | ||||
| 18–44 years | 40.0% | 47.9% | 44.6% | |
| 45–64 years | 57.0% | 43.2% | 48.8% | |
| 65–74 years | 2.8% | 8.6% | 6.3% | |
| ≥75 years | 0.2% | 0.3% | 0.3% | <0.001 |
| Sex, male | 67.7% | 52.0% | 58.4% | <0.001 |
| Overall comorbidity | ||||
| Mean number of comorbidities | 8.5 (4.7) | 7.0 (4.1) | 7.6 (4.4) | <0.001 |
| Mean Charlson Index | 0.5 (0.8) | 0.4 (0.7) | 0.5 (0.8) | 0.130 |
| Mean RUBs | 3.1 (0.6) | 3.0 (0.7) | 3.0 (0.7) | 0.098 |
| Comorbid diagnoses | ||||
| Hypertension | 44.1% | 34.1% | 38.2% | 0.001 |
| Diabetes mellitus | 22.5% | 17.1% | 19.3% | 0.024 |
| Dyslipidemia | 54.1% | 44.9% | 48.7% | 0.002 |
| Obesity | 28.4% | 18.7% | 22.7% | 0.001 |
| Active smoking | 28.4% | 30.5% | 29.6% | 0.443 |
| Alcoholism | 7.2% | 5.4% | 6.2% | 0.227 |
| Vascular events | 19.0% | 16.3% | 17.4% | 0.245 |
| Bronchial asthma | 8.7% | 5.6% | 6.9% | 0.041 |
| COPD | 5.9% | 3.6% | 4.6% | 0.073 |
| Neuropathies | 3.9% | 3.0% | 3.4% | 0.409 |
| Disease duration, mean, years | 19.9 | 18.2 | 18.9 | 0.093 |
| Time under antipsychotic treatment, mean, years | 16.5 | 14.9 | 15.5 | 0.091 |
| Number of CNS-active drugs, mean | 3.6 | 2.6 | 3.1 | 0.002 |
| Antipsychotic drugs | ||||
| Quetiapine | 34.5% | 30.8% | 32.3% | 0.194 |
| Risperidone | 18.1% | 21.8% | 20.3% | 0.131 |
| Olanzapine | 13.8% | 18.3% | 16.4% | 0.046 |
Notes: Group 1, presence of one or more negative symptoms; group 2, absence of negative symptoms.
Abbreviations: RUBs, resource-utilization bands; COPD, chronic obstructive pulmonary disease; CNS, central nervous system.
Quetiapine (32.3%), risperidone (20.3%), and olanzapine (16.4%) were the most common antipsychotic drugs administered. Time on antipsychotic drugs since diagnosis was 15.5 (SD 15.3) years, slightly longer in patients with negative symptoms (16.5 versus 14.9, P=0.091). Polypharmacy was common. The mean number of drugs per patient was 3.1 (SD 1.9), and was found to be significantly greater in group 1 (3.6 versus 2.6 for group 1 and group 2, respectively; P=0.002).
Dyslipidemia (48.7%), hypertension (38.2%), and diabetes mellitus (19.3%) were the most frequent comorbid conditions. The total mean number of comorbid diagnoses was greater in group 1 than in group 2 (8.5 and 7.0, respectively; P<0.001). Table 2 shows the prevalence of the main cardiovascular risk factors and MetS in the total sample. The overall prevalence of MetS was 38.6% (95% CI 35.7%–41.5%), and was significantly higher in patients with negative symptoms (43.9% versus 34.9%, P=0.002). Three or more components of MetS were found in 44% of the sample. MetS was associated with the presence of negative symptoms (odds ratio [OR] 1.6, 95% CI 1.3–2.0), age (OR 1.2, 95% CI 1.1–1.4), and physical comorbidity (resource-utilization bands; OR 1.2, 95% CI 1.1–1.3; P<0.05).
Table 2.
Prevalence of the main cardiovascular risk factors and metabolic syndrome
| Group 1 n=588 |
Group 2 n=532 |
Total n=1,120 |
P-value | |
|---|---|---|---|---|
| CVRFs, mean (SD) | ||||
| Systolic blood pressure, mmHg | 125.1 (16.5) | 125.0 (17.6) | 125.0 (17.2) | 0.935 |
| Diastolic blood pressure, mmHg | 74.7 (10.8) | 74.1 (11.0) | 74.3 (10.9) | 0.362 |
| Body mass index, kg/m2 | 29.3 (5.6) | 28.6 (5.7) | 28.9 (5.7) | 0.049 |
| Glucose, mg/dL | 102.1 (32.8) | 99.7 (31.8) | 100.7 (32.2) | 0.229 |
| Triglycerides, mg/dL | 134.7 (100.9) | 130.1 (74.0) | 132.0 (86.3) | 0.394 |
| Total cholesterol, mg/dL | 202.3 (44.1) | 194.5 (40.8) | 197.8 (42.3) | 0.003 |
| HDLc, mg/dL | 54.8 (15.4) | 52.4 (16.1) | 53.4 (15.8) | 0.015 |
| LDLc, mg/dL | 121.5 (39.0) | 116.8 (34.3) | 118.8 (36.4) | 0.039 |
| Serum creatinine, mg/dL | 1.1 (0.4) | 1.1 (0.4) | 1.1 (0.4) | 0.851 |
| Metabolic syndrome | ||||
| Prevalence* | 43.9% | 34.9% | 38.6% | 0.002 |
| Components | ||||
| BMI >28.8 kg/m2 | 50.2% | 42.4% | 45.6% | 0.010 |
| BP >130/85 mmHg (or treatment) | 49.1% | 42.0% | 44.9% | 0.018 |
| Triglycerides >150 mg/dL (or treatment) | 27.1% | 24.3% | 25.4% | 0.298 |
| Fasting blood glucose >110 mg/dL | 22.7% | 19.2% | 20.6% | 0.152 |
| HDLc <40 (men) or <50 (women) mg/dL | 19.4% | 20.7% | 20.2% | 0.605 |
| Number of components | ||||
| 1 | 20.7% | 23.9% | 22.6% | |
| 2 | 22.5% | 21.8% | 22.1% | |
| 3 | 21.6% | 19.0% | 20.1% | |
| 4 | 15.9% | 12.4% | 13.8% | |
| 5 | 6.3% | 3.5% | 4.6% | 0.006 |
Notes:
Defined according to the modified National Cholesterol Education Program Adult Treatment Panel III criteria. Group 1, presence of one or more negative symptoms; group 2, absence of negative symptoms.
Abbreviations: CVRFs, cardiovascular risk factors; BMI, body mass index; BP, blood pressure; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; SD, standard deviation.
Discussion
MetS is one of the primary reasons for increased mortality in patients with schizophrenia. In recent years, numerous studies have been published on the prevalence of MetS in different clinical settings and using several methodological approaches, presenting figures ranging from 11% to 69%.11 In five studies previously conducted in Spain, the prevalence of MetS in patients with schizophrenia was 19%–36.8% using the NCEP-ATP III diagnostic criteria.13,14,23–25 Gutiérrez-Rojas et al recently published a new study in which the prevalence was found to be 59.5% in a sample of overweight patients defined by a BMI >25 kg/m2.26
Our study once again confirms the high prevalence of MetS in a population of adult outpatients with schizophrenia (38.6%). However, it also found that the prevalence of MetS was associated with the presence of negative symptoms (OR 1.6, 95% CI 1.3–2.0).
Negative symptoms, such as blunt affect, lack of motivation, asociality, and impoverished speech are better predictors of functioning than positive symptoms, and are often associated with a poor response to available pharmacological treatments.6 In our study, 52% of the patients presented one or more negative symptoms, the most common being passive/apathetic social withdrawal and emotional withdrawal.
It has been suggested that advanced-age patients with long-standing disease, patients with certain comorbidities, and those taking atypical antipsychotic drugs like clozapine and olanzapine have a greater risk of suffering from MetS.15,27,28 However, no studies have yet been conducted to assess its prevalence according to the presence of different types of symptom domains.
The underlying pathophysiological mechanisms involved in the pathogenesis of MetS in schizophrenia are not well understood.15 A sedentary lifestyle and lack of physical exercise due to decreased spontaneity and drive, as well as other negative symptoms, may contribute to a higher prevalence of MetS. The presence of negative symptoms may also reduce help-seeking and physical health controls, and has recently been negatively correlated with BMI and positively correlated with HDL cholesterol in a sample of 372 chronic inpatients under antipsychotic treatment for more than 2 years. The authors suggested that patients with negative syndrome may have a different lipid pathophysiology in comparison with those who are less severe in this domain.29 Most evidence has emphasized the importance of extrinsic factors (calorie intake, lifestyle, antipsychotic medication) in its development, but the involvement of genetic factors is also supported.11
Our study has several limitations inherent to research based on population databases. The PANSS is not routinely completed in mental health services. Collecting negative symptoms from open text in the medical records may be associated with an underestimation of the true prevalence of these symptoms, due to the underregistration of them by the clinicians. In addition, the most severe cases were possibly not included in the study because they are usually not seen as outpatients. Furthermore, patients with subclinical or severe forms were not identified, as they were not all seen as outpatients and/or treatment adherence or other variables, such as socioeconomic levels, were not evaluated.
Future research regarding schizophrenic patients with MetS should focus on the clinical impact, longitudinal follow-up to understand the factors that contribute to developing MetS, the pathophysiology of schizophrenia, and its impact on quality of life.15 Despite increased cardiometabolic risk in patients with mental disorders receiving antipsychotic drugs, metabolic monitoring is low in routine clinical practice.30 It is important to identify high-risk patients and educate them about preventive measures.31 A sensible approach when prescribing antipsychotic drugs is also advisable, as well as reviewing the medication regularly in order to maintain a balance between adequate symptomatic control and the development of metabolic abnormalities. Moreover, one of the most important objectives of pharmacological research should be to develop new antipsychotic agents with reduced associated risk, fast action, greater effect on negative, affective, and cognitive symptoms, and improved rate of recurrence, along with reduction or disappearance of cumulative comorbidity.
In conclusion, the prevalence of MetS in patients with schizophrenia is high. MetS was associated with age, comorbidity, and the presence of negative symptoms. Due to the proven impact that negative symptoms have on the subjective well-being, functioning, and quality of life of patients, and probably also on their predisposition to develop metabolic disorders, it is essential for clinicians to detect them early and to decide on the best-possible therapeutic approach. Further studies need to be undertaken in order to replicate the findings of this association, and to gain a better understanding of the underlying mechanisms.
Footnotes
Disclosure
This study was funded by Roche Farma, Spain. ASM is an employee of BSA, the health care provider that owns the database that was the subject of this study. JM and ERB are employees of Roche Farma. RNA has no conflicts of interests to declare.
References
- 1.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 3.Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25:83–88. doi: 10.1097/YCO.0b013e32835035ca. [DOI] [PubMed] [Google Scholar]
- 4.Leucht S. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. 2007;116:317–333. doi: 10.1111/j.1600-0447.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 5.De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24:412–424. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Fervaha G, Foussias G, Remington G. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. 2014;29:449–455. doi: 10.1016/j.eurpsy.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645–692. doi: 10.1016/j.euroneuro.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Bobes J, Arango C, Garcia-Garcia M, Rejas J. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry. 2010;71:280–286. doi: 10.4088/JCP.08m04250yel. [DOI] [PubMed] [Google Scholar]
- 9.Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res. 2012;137:147–150. doi: 10.1016/j.schres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Arango C, Garibaldi G, Marder SR. Pharmacological approaches to treating negative symptoms: a review of clinical trials. Schizophr Res. 2013;150:346–352. doi: 10.1016/j.schres.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Papanastasiou E. The prevalence and mechanisms of metabolic syndrome in schizophrenia: a review. Ther Adv Psychopharmacol. 2013;3:33–51. doi: 10.1177/2045125312464385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Bobes J, Arango C, Aranda P, Carmena R, Garcia-Garcia M, Rejas J. Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: results of the CLAMORS study. Schizophr Res. 2007;94:375–376. doi: 10.1016/j.schres.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Sicras-Mainar A, Blanca-Tamayo M, Rejas-Gutiérrez J, Navarro-Artieda R. Metabolic syndrome in outpatients receiving antipsychotic therapy in routine clinical practice: a cross-sectional assessment of a primary health care database. Eur Psychiatry. 2008;23:100–108. doi: 10.1016/j.eurpsy.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull. 2013;39:295–305. doi: 10.1093/schbul/sbs082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sicras-Mainar A, Maurino J, Ruíz-Beato E, Navarro-Artieda R. Impact of negative symptoms on healthcare resource utilization and associated costs in adult outpatients with schizophrenia: a population-based study. BMC Psychiatry. 2014;14:225. doi: 10.1186/s12888-014-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- 18.Edgar CJ, Blaettler T, Bugarski-Kirola D, Le Scouiller S, Garibaldi GM, Marder SR. Reliability, validity and ability to detect change of the PANSS negative symptom factor score in outpatients with schizophrenia on select antipsychotics and with prominent negative and disorganized thought symptoms. Psychiatry Res. 2014;218:219–224. doi: 10.1016/j.psychres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29:452–472. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 21.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment on High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 22.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Araña Moreno T, Touriño-González R, Hernández-Fleta JL, León-Pérez P. Prevalence of the metabolic syndrome among schizophrenic patients hospitalized in the Canary Islands. Actas Esp Psiquiatr. 2007;35:359–367. [PubMed] [Google Scholar]
- 24.Bernardo M, Cañas F, Banegas JR, Casademont J, Riesgo Y, Varela C. Prevalence and awareness of cardiovascular risk factors in patients with schizophrenia: a cross-sectional study in a low cardiovascular disease risk geographical area. Eur Psychiatry. 2009;24:431–441. doi: 10.1016/j.eurpsy.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros-Ferreira L, Obiols JE, Navarro-Pastor JB, Zúñiga-Lagares A. Metabolic syndrome and health-related quality of life in patients with schizophrenia. Actas Esp Psiquiatr. 2013;41:17–26. [PubMed] [Google Scholar]
- 26.Gutiérrez-Rojas L, Azanza JR, Bernardo M, Rojo L, Mesa F, Martínez-Ortega JM. Prevalence of metabolic syndrome in Spanish patients with schizophrenia and overweight. The CRESSOB Study. Actas Esp Psiquiatr. 2014;42:9–17. [PubMed] [Google Scholar]
- 27.Aguilar E, Coronas R, Caixàs A. Metabolic syndrome in patients with schizophrenia and antipsychotic treatment. Med Clin (Barc) 2012;139:542–546. doi: 10.1016/j.medcli.2012.05.028. Spanish. [DOI] [PubMed] [Google Scholar]
- 28.Khalil R. The metabolic syndrome and schizophrenia: a comorbidity or an association? J Pharmacol Pharmacother. 2013;4:174–175. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SF, Huc TM, Lan TH, Chiu HJ, Sheen LY, Loh EW. Severity of psychosis syndrome and change of metabolic abnormality in chronic schizophrenia patients: severe negative syndrome may be related to a distinct lipid pathophysiology. Eur Psychiatry. 2014;29:167–171. doi: 10.1016/j.eurpsy.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42:125–147. doi: 10.1017/S003329171100105X. [DOI] [PubMed] [Google Scholar]
- 31.Steylen PM, van der Heijden FM, Kok HD, Sijben NA, Verhoeven WM. Cardiometabolic comorbidity in antipsychotic treated patients: need for systematic evaluation and treatment. Int J Psychiatry Clin Pract. 2013;17:125–130. doi: 10.3109/13651501.2013.779000. [DOI] [PubMed] [Google Scholar]