Abstract
Objectives
Topical ocular administration is the most convenient route of administration of drugs for the treatment of eye diseases. However, the bioavailability of drugs following eye instillations of eye drops is very low. Over the past 20 years, extensive efforts have been put into research to improve drug bioavailability without compromising treatment compliance and patients' quality of life.
Key findings
One of the most efficient ways to improve drug bioavailability is to increase the precorneal residence time of the eye drop formulations. As a result, new eye drops, with bioadhesive properties, have been developed based on the cationic oil-in-water (o/w) nanoemulsion technology. These low viscosity eye drop nanoemulsions have improved precorneal residence time through the electrostatic interactions between the positively charged oil nanodroplets and the negatively charged ocular surface epithelium.
Summary
This review is the first to present the benefits of this new strategy used to improve ocular drug bioavailability. The roles of the cationic agent in the stabilization of a safe cationic o/w nanoemulsion have been discussed, as well as the unexpected benefits of the cationic o/w nanoemulsion for the protection and restoration of a healthy tear film and corneal epithelium.
Keywords: cationic, cetalkonium chloride, drug delivery, oil-in-water emulsion, ocular surface
Introduction
Aqueous eye-drop solutions are still the most common formulations for topical ocular drug delivery, since they are the simplest, easiest and cheapest ocular dosage forms to produce. The main drawbacks of these conventional ocular dosage forms are that they are rapidly eliminated from the ocular surface following instillation, resulting in a low ocular bioavailability (less than 1%) of the drugs, and are limited to water soluble compounds.1 Variants with viscosifying agents, penetration enhancers or spreading surfactants have not fundamentally changed the paradigm. Suspensions, gels and negatively charged oil-in-water (o/w) nanoemulsions were developed to improve ocular bioavailability of lipophilic or poorly water soluble drugs.2–4 Among them, o/w nanoemulsions were demonstrated to be effective ocular drug delivery vehicles.5,6
Anionic o/w nanoemulsions with ciclosporin (cyclosporine A; Restasis, Allergan) were developed to increase tear production in patients whose tear production was presumed to be suppressed due to ocular inflammation (i.e. for patients with dry eye disease), or with difluprednate (Durezol, Alcon) for the treatment of inflammation and pain associated with eye surgery.7 Oil-in-water nanoemulsions were demonstrated to be excellent vehicles for lipophilic drugs, such as ciclosporin or prostaglandin analogues like latanoprost or tafluprost, but also for delivering water unstable drugs.8,9
Cationic o/w nanoemulsions extended one step further the benefits of the o/w nanoemulsions for drug delivery by improving their residence time over that observed with the anionic o/w nanoemulsions. These cationic o/w nanoemulsions take advantage of the negatively charged ocular surface to increase through electrostatic interactions their precorneal residence time, and thus the ocular drug bioavailability.10,11 As a consequence, the first generation of cationic o/w nanoemulsions were developed to optimize penetration of drugs (among them ciclosporin) in ocular tissues.9,11–14 This first generation of cationic o/w nanoemulsions used noncompendial cationic surfactants and were not devoid of ocular toxicity side effects.15–17 Hence, the challenges for the development of cationic o/w nanoemulsions are in the choice of the most appropriate cationic agent used to bring the positive charge to the oil nanodroplets, and in the improvement of the ocular tolerance of these positively charged nanoemulsions.
This review is the first to compile the information present in the literature that describes this new strategy used to improve ocular drug delivery: the use of cationic o/w nanoemulsion vehicles in ocular drug delivery. The main steps involved in the pharmaceutical development will be discussed, particularly the ones that led to the choice of cetalkonium chloride (CKC) as a cationic agent compatible with the ocular surface.
Definition of a cationic oil-in-water nanoemulsion
By definition a cationic o/w nanoemulsion is a biphasic formulation that comprises positively charged oil nanodroplets (the oil phase) dispersed in water (the continuous phase).18 Table 1 summarizes the physicochemical properties of a cationic o/w nanoemulsion. The positive charge of the oil nanodroplets is brought by a cationic surfactant that localizes itself at the oil interface. Ideally, this cationic agent should be sufficiently lipophilic to be almost exclusively entrapped in the oil with only very low amounts of the cationic agent present in the aqueous phase of the formulation. In addition to the biological effects of the cationic o/w nanoemulsion (discussed below), the positive charge of the oil nanodroplets helps improve the long-term stability of the nanoemulsion by generating a repulsive electrostatic force (measured by the zeta potential) between the positively charged oil nanodroplets, thus preventing their merging and avoiding the coalescence process of the nanoemulsion during shelf life.18–20 Figure 1 presents the sketch of one of the oil nanodroplets present in the cationic o/w nanoemulsion.
Table 1.
Summary of the physicochemical characteristics of a cationic oil-in-water nanoemulsion
| Parameter | Description |
|---|---|
| Aspect | White opaque to slightly translucent |
| pH | 5.0–7.0 |
| Osmolality (mOsmol/kg) | 270 |
| Droplet size (nm)a | < 200 |
| Zeta potential (ζ, mV)a | Positive (+40) |
| Sterility | Sterile |
Droplet size was determined by dynamic light scattering (HPPS, Malvern Instruments), and zeta potential by electrophoretic mobility measurement (Zetasizer 2000, Malvern Instruments).
Figure 1.
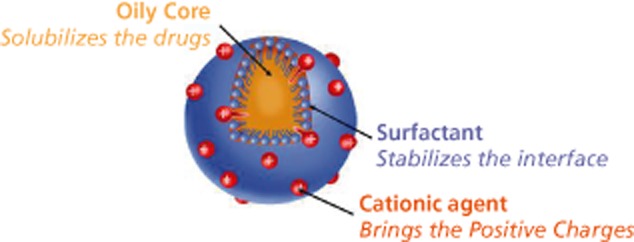
Schematic representation of one of the oil nanodroplets present in the cationic oil-in-water nanoemulsion.
While the oil phase of the nanoemulsion is generally made of inert and stable oils, such as medium chain triglycerides or mineral oil (i.e. non-vegetable liquid paraffin), the choice of the right cationic agent needed to produce a safe and well tolerated cationic o/w nanoemulsion necessitated a thorough examination of the cationic agents at hand.21
Choice of the cationic agent
The positive charge of a cationic o/w nanoemulsion is estimated by measuring the zeta potential. The zeta potential (ζ) is the electrical potential difference (ΔV) between the dispersion medium (i.e. water) and the stationary layer of fluid attached to the dispersed oil nanodroplets.22,23 The zeta potential is a measure of the magnitude of the electrostatic or charge repulsion between the oil nanodroplets, and is one of the fundamental parameters known to affect the stability of dispersed systems (i.e. o/w nanoemulsion); thus the higher the zeta potential, the better (ζ ≥ +40 mV).22 As a consequence, to obtain a high zeta potential for the cationic o/w nanoemulsion, all, to almost all the cationic agent has to be entrapped in the oil nanodroplet, with the positive charge located at the oil–water interface, and no to very low amounts of freely soluble molecules of the cationic agent present in the aqueous phase (i.e. the dispersion medium), where they can contribute to ‘shield’ and reduce the zeta potential of the nanoemulsion. Thus, the cationic agent needs to be lipophilic, i.e. amphiphilic; and the higher the lipophilicity the better.
A large number of cationic agents were described in the literature that could have been potential cationic agents for the cationic o/w nanoemulsion, such as: stearylamine, oleylamine, poly(ethylenimine), poly(l-lysine), 1,2-di-(9Z-octadecenoyl)-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-di-(9Z-octadecenoyl)-3-trimethylammonium-propane (DOTAP), and alkyl benzyldimethylammonium compounds of various alkyl chain lengths (IUPAC name: benzyl(dimethyl)azanium; but better known under their common name: benzalkonium chloride (BAK) derivatives). However, they were all hampered either by toxicity, stability or regulatory issues which avoided or limited their use in ophthalmologic products.16,23,24 Consequently, the search for the appropriate cationic agent was limited to the ones already registered, used in ophthalmic products or compliant with either the United States (US) or European (EU) pharmacopoeias.11
The most common cationic agents found in ophthalmic products belong to the family of quaternary ammoniums, such as BAK or polycationic polymers also known as polyquaternium (e.g. Polyquaternium-1 (PQ-1) found in Polyquad). However, these quaternary ammonium derivatives are used as preservative agents in conventional ocular drug products for their bactericidal and microbicidal properties (through a detergent action (see Furrer et al.25)) at relatively low concentrations in aqueous solution: 0.001% PQ-1 in Travatan (Alcon) or 0.02% BAK in Xalatan (Pfizer). Over the past twenty years, a very large collection of evidence has been published demonstrating the deleterious effects of quaternary ammonium preserved eye drop solutions for the ocular surface, especially for BAK-preserved solutions.26–33 The actual trend for conventional ocular drug products– i.e. for eye drop aqueous solutions– is to reduce the concentration, or even remove completely quaternary ammoniums, and especially BAK, from their compositions.34 As a consequence many soft-preserved and preservative-free ophthalmic drug products have reached the market in the last few years.35 Hence, can quaternary ammonium chlorides, and among them BAK derivatives still make good cationic agents for cationic o/w nanoemulsions?
BAK derivatives as cationic agent for cationic oil-in-water nanoemulsion
As mentioned previously, the ideal cationic agent should be lipophilic enough to localize itself exclusively within the oil nanodroplets with no freely soluble cationic agent molecule within the aqueous phase for better zeta potential and shelf life stability, and improved safety profile.22,36
According to the latest (2012) US and EU pharmacopoeias BAK is a mixture of alkyl benzyldimethyl quaternary ammonium chlorides of various alkyl chain lengths (Figure 2). The alkyl chains are ranging from 8 to 18 carbons, with the C12, C14 and C16 alkyl derivatives being the most common in the BAK mixture (Figure 2). Indeed, the pharmacopoeias specify that the BAK mixture content of the C12 homologue should not be less than 40%, and the content of the C14 homologue not less than 20% of the total alkyl benzyldimethylammonium chloride content. In addition the sum of the C12 and C14 alkyl derivatives has to represent at least 70% of the BAK composition. Note that even for pharmacopoeia compliant BAK mixtures, the composition and distribution of the alkyl chain derivatives can vary from one manufacturer to another.37 Thus, is there among these alkyl benzyldimethylammonium of various alkyl chain lengths an alkyl derivative that possesses physicochemical properties that would make it compatible with the ideal cationic agent for an o/w cationic nanoemulsion?
Figure 2.
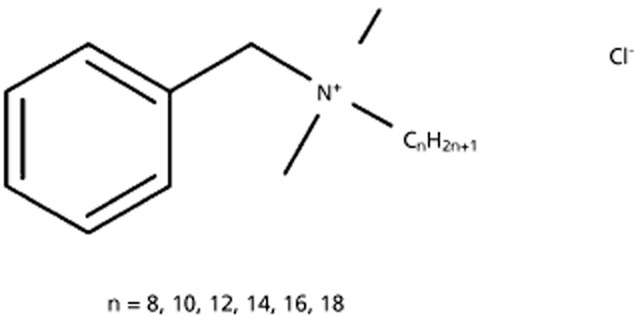
Benzalkonium chloride is a mixture of alkyl benzyldimethylammonium chloride compounds of various chain lengths.
Physicochemical properties of the C12, C14 and C16 BAK derivatives
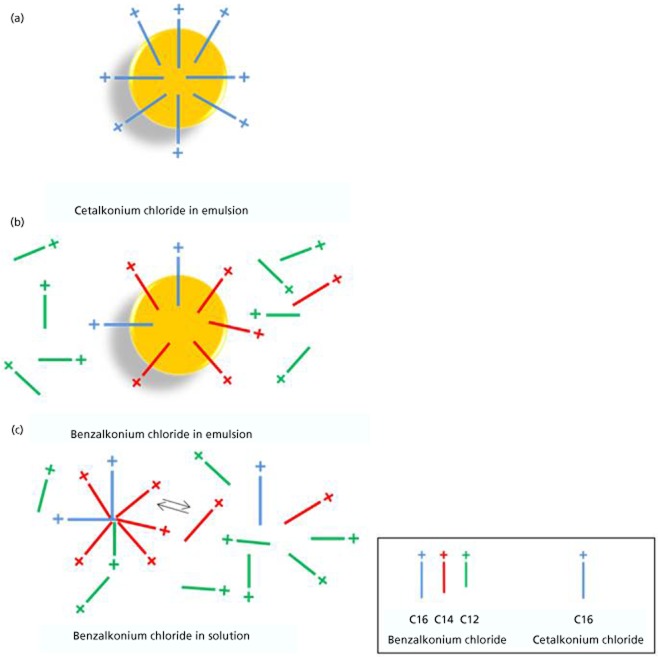
The C12, C14, and C16 alkyl derivatives of BAK are also known as benzododecinium chloride, myristalkonium chloride, and cetalkonium chloride (CKC), respectively. Table 2 summarizes the physicochemical properties of theses alkyl derivatives. It appears that CKC is the most lipophilic and less water soluble among the three major BAK derivatives present in the BAK mixture. Thus, the C16 BAK derivative (i.e. CKC) with a calculated logP of 9.5 is a particularly attractive cationic agent for cationic o/w nanoemulsions. Figure 3 illustrates the phase distribution for the different alkyl derivatives of BAK in o/w nanoemulsions or aqueous solution. Due to their lower lipophilicity, low concentrations of the C12 and C14 BAK derivatives can be found in the aqueous phase of the cationic o/w nanoemulsion, while no C16 BAK derivatives (i.e. CKC) are present in the aqueous phase of the o/w nanoemulsion. This is confirmed by the measure of the zeta potential. For cationic o/w nanoemulsions with BAK (0.02%) as cationic agent the zeta potential is approximately +20 mV, while for a cationic o/w nanoemulsion with CKC (0.005%) as the cationic agent the zeta potential is +40 mV.38,39
Table 2.
Physicochemical properties of C12, C14 and C16 alkyl derivatives of benzalkonium chloride
| BAK derivatives | C12 | C14 | C16 (CKC) |
|---|---|---|---|
| Molecular weight (g/mol) | 340 | 368 | 396 |
| XlogP3 (PubMed compound) | 7.4 | 8.4 | 9.5 |
| logP (calculated) | 3.44 | 4.45 | 5.46 |
| logP (measured)a | −0.17 < logP <−0.07 | 0.24 < logP < 0.44 | 2.4 < logP < 2.6 |
| Critical micellar concentration (CMC)b | 4.5 mm | 0.75 mm | 0.55 mm |
| 1.53 g/l | 0.29 g/l | 0.022 g/l | |
| 0.153% | 0.029% | 0.0022% | |
| Superficial tension at CMC (mN/m) | 38 | 38 | 40 |
| Water solubility (g/l; 25°C) | 1230 | 100 | 8.5 |
BAK, benzalkonium chloride; CKC, cetalkonium chloride.
Maximum solubility ratios in both organic and aqueous phases.
Measured with a Wilhelmy blades tensiometer.
Figure 3.
Illustration of the phase distribution for the different alkyl derivatives of benzalkonium chloride. (a) Cetalkonium chloride (blue) in emulsion; (b) benzalkonium chloride (BAK) mixture in emulsion; and (c) BAK mixture in aqueous solution.
However, since these cationic BAK derivatives are surfactants with detergent properties and cellular membrane toxicity, as such, the BAK mixture is very often added to eye drop aqueous solutions for their preservation.40 It was described by Kurup et al.41 that only the free forms of the preservative (i.e. BAK derivatives) present in the aqueous phase of an o/w nanoemulsion were available for antibacterial activity, and therefore exerted ocular surface cell membrane toxicity.42,43 Thus, it is very important to have the lowest concentration of free/micelle BAK derivative molecules in the aqueous phase to avoid or limit as much as possible the side effects induced by these free molecules. Hence, for a better ocular tolerance and safety profile of the cationic o/w nanoemulsion, the higher the lipophilicity of the cationic agent, the better. Consequently, CKC was selected as the cationic agent of choice for the development of unpreserved, well tolerated cationic o/w nanoemulsions as it is the most lipophilic BAK derivative present in the BAK mixture. The following sections will discuss the ocular tolerance and safety profile of the unpreserved CKC-containing cationic o/w nanoemulsions, and the various advantages brought by CKC and its positive charge for the improvement of drug bioavailability and the protection and healing of the ocular surface.
Biological properties of cationic oil-in-water nanoemulsion eye drops
The rationale for developing cationic o/w nanoemulsion eye drops arose from the observation that the ocular mucosa is negatively charged. Both the corneal and conjunctival human cells harbour O-glycosylated transmembrane mucins with only 6% of their glycans not terminated by the negatively charged sialic acid.10 When a cationic o/w nanoemulsion eye drop is instilled onto the ocular surface, the resultant electrostatic attraction between the positively charged oil nanodroplets and the ocular surface manifests itself macroscopically by an improved spreading of the eye drop preparation onto the eye.11 This was evidenced by dynamic contact angle measurements, which were rapidly very low with cationic o/w nanoemulsion (below 3° within the first second) upon instillation, while it remained elevated (above 42°) with either the anionic nanoemulsion or the hyaluronate hydrogel.17 The electrostatic interactions help to increase the residence time of the oil nanodroplets on the ocular surface. The ocular residence time plays a major role for drug absorption, and various strategies were developed to improve drug residence time as a means to improve drug absorption, such as ophthalmic inserts, viscosity enhancers/mucoadhesives, anionic o/w nanoemulsions, and cationic o/w nanoemulsions.4 Classic eye drop solutions are eliminated within minutes following administration, thus greatly reducing drug ocular bioavailability, which seldom exceed 1% of the delivered dose.44 The beneficial role of the cationic o/w nanoemulsion on the ocular bioavailability was demonstrated with ciclosporin.45 Cationic and anionic nanoemulsions of 0.05% ciclosporin absorptions in the conjunctiva and cornea following a single ocular application in the rabbit eye were measured over time (up to 72 h). The pharmacokinetic data demonstrated that ciclosporin's area under the curve (AUC) with the cationic o/w nanoemulsion was approximately twice the one observed with the anionic o/w nanoemulsion (AUC: 26477 vs 14210 ng/g.h, for the cationic vs anionic nanoemulsion, respectively). The maximum ciclosporin concentration (Cmax) in the cornea for the cationic o/w nanoemulsion following instillation was 1371.8 ng/g, while it was only 747.8 ng/g for the anionic o/w nanoemulsion.46 Interestingly, a second peak of ciclosporin absorption was observed in the cornea with the 0.05% ciclosporin cationic o/w nanoemulsion two hours post instillation (and even 11 h after instillation with the 0.1% ciclosporin cationic o/w nanoemulsion), while no such peak was observed with the anionic o/w nanoemulsion.17,46 The presence of this second peak is a strong argument in favour of an extended ocular residence time with cationic o/w nanoemulsions. The extended ocular residence time of the cationic o/w nanoemulsion was suggested following the instillation of a latanoprost-loaded cationic o/w nanoemulsion.47
Safety profile of the cationic oil-in-water nanoemulsions
As indicated previously, the positive charge of the cationic o/w nanoemulsions is brought by CKC, a quaternary ammonium that is structurally closely related to BAK. CKC's alkyl chain contains 16 carbons. This seemingly slight difference in alkyl chain length has a major impact on the physicochemical properties of CKC (Table 2) and as a consequence on the safety profile of the CKC-containing cationic o/w nanoemulsions when compared with BAK-containing cationic o/w nanoemulsion. Liang et al.36 used an in-vivo rabbit model to demonstrate that both BAK and CKC cationic o/w nanoemulsions were much better tolerated by the rabbit ocular surface than their solution counterparts. When BAK is in solution the C12 and C14 alkyl derivatives form micelles that have the possibility to interact with the corneal and conjunctival cell membranes once applied on the ocular surface. Through their detergent properties the C12 and C14 BAK alkyl derivatives alter the epithelial cells integrity, leading to the well-known deleterious effects of BAK solutions. However, when formulated in an o/w nanoemulsion, a significant part of these C12 and C14 alkyl derivatives of BAK is entrapped in the oil (as a consequence of the lipophilicity of the C12 and C14 alkyl chains of BAK). Only a small proportion of the C12 and C14 alkyl derivatives of BAK remain in the aqueous phase of the solution, thus greatly reducing the ocular toxicity of the BAK o/w nanoemulsion (Figure 4). This is emphasized with CKC, the C16 alkyl derivative of BAK. Due to its extreme lipophilicity, when formulated in emulsion almost all of the CKC is entrapped in the oil with no free CKC molecules present in the aqueous phase of the emulsion. As a result, an even better safety profile of the cationic o/w nanoemulsion can be expected with CKC as the cationic agent rather than with BAK (Table 3)48–51. This is confirmed in vivo by the improved ocular tolerance of the CKC cationic nanoemulsion over the BAK cationic nanoemulsion.36 In a rabbit model of acute toxicity, 15 instillations of CKC-containing cationic o/w nanoemulsion had a better Draize test score and a lower in-vivo confocal microscopy (IVCM) score than the BAK-containing cationic o/w nanoemulsion (Figure 4), and were equivalent to the BAK-free saline control. The ciclosporin-containing CKC cationic o/w nanoemulsion was as well tolerated as –if not better than– the BAK-free Restasis.52
Figure 4.
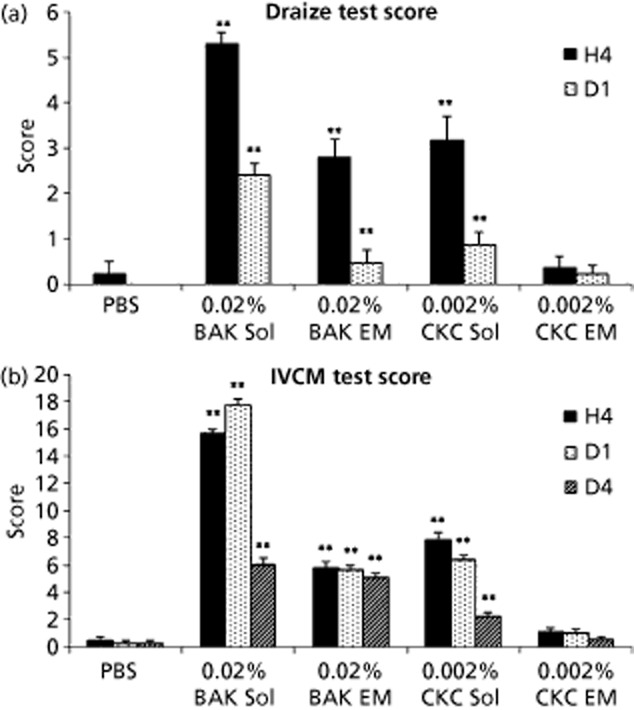
(a) Draize test score and (b) in-vivo confocal microscopy (IVCM) score of cationic oil-in-water nanoemulsion, at four hours (H4), day 1 (D1) and day 4 (D4) after the instillations. **P < 0.01 against phosphate buffered saline (PBS; nonparametric comparisons (Mann-Whitney)). BAK, benzalkonium chloride; CKC, cetalkonium chloride; Sol, solution; EM, nanoemulsion.
Adapted from Liang et al.36.
Table 3.
Summary of the physical and biological properties of benzalkonium chloride solutions and cationic oil-in-water nanoemulsions
| Aqueous solutions of BAK (conventional ocular dosage forms) | Cationic oil-in-water nanoemulsions with | ||
|---|---|---|---|
| BAK (C12 +C14) | CKC (C16) | ||
| Solubility in water | Soluble | Soluble | Poorly soluble to insoluble |
| Solubility in oil | Not applicable (aqueous solution) | Soluble | Soluble |
| Zeta potential | / | ∼+20 mV | ∼+40 mV |
| Structural organization | Micelles (10–20 nm) | Emulsion (oil nanodroplet: 150–200 nm) | |
| Stability | Unstable (dynamic equilibrium) | Stable (++) | Stable (+++) |
| Localization in formulation | Water free-flowing molecules in equilibrium with micellar structures | In the oil nanodroplets and a small proportion in the aqueous phase | Bound in the oil nanodroplets |
| Function in formulation | Preservative role (resulting from the free-flowing BAK molecules in the aqueous phase41 | Cationic surfactant role | |
| Help solubilize lipophilic drugs | – Stabilizing the nanoemulsion – Bringing positive charge No preservative role41 |
||
| Effects | Dose-dependent detergent effect with destabilization of biological membranes: – Microbicidal agent – Irritancy of tissues |
No detergent effect (most of the BAK is in the oil droplets)41,43 |
No detergent effect (CKC bound to the oil droplets)41,43 |
| Preservative action | Preserved eye drop from 0.004% to 0.025% depending on formulation (0.005% in Lumigan; 0.02% in Xalatan) | Unpreserved cationic oil-in-water nanoemulsion eye drop | |
| Nonclinical results 26,36 | Toxic for the ocular surface with ocular irritation, inflammation and apoptosis |
Not toxic for the ocular surface No ocular irritation, no inflammation and no apoptosis As safe as saline solution |
|
| Clinical effect 17 |
Tear film instability – Lower tear break-up time with BAK containing eye drops48,49 Ocular surface alterations – Conjunctival epithelial changes50 – Corneal alteration |
Improved tear film stability – Improvement of tear break-up time with both vehicle and Cyclokat after three months of treatment Improved ocular surface – Improvement of corneal staining with both vehicle and Cyclokat after six months of treatment51 |
Improved tear film stability – Improvement of tear break-up time with Cationorm and Cyclokat Improved ocular surface – Improvement of corneal staining with Cationorm and Cyclokat |
BAK, benzalkonium chloride; CKC, cetalkonium chloride.
The CKC cationic o/w nanoemulsion was used as a vehicle for lipophilic drugs such as ciclosporin or latanoprost. Nonclinical studies performed in the rabbit or in the rat demonstrated that these ophthalmic drug products were safe and well tolerated following single and repeated applications, with neither inflammatory cell infiltration nor apoptosis.47,52–55 This good safety profile was confirmed by clinical trials for both drug products, and by the commercialization (since 2008) of the CKC cationic o/w nanoemulsion vehicle (Cationorm) as an improved artificial tear substitute for the relief of mild to moderate dry eye symptoms.17,56–59
Protective properties of the cationic oil-in-water nanoemulsion vehicle
The increased residence time and better spreading properties of the cationic o/w nanoemulsion designed to improve the ocular bioavailability of lipophilic drugs was accompanied by unexpected beneficial effects for the ocular surface.
Applications of the cationic o/w nanoemulsion help restore the integrity of the lacrimal film through the concomitant action of the oil and the slightly hypoosmolar aqueous phase. The oil phase of the cationic o/w nanoemulsion by mixing with the tear film lipid layer contributes to its stability, thus reducing the evaporation of water from the aqueous phase. This is of particular interest for meibomian gland dysfunction (MGD) patients with short tear film break-up times (TFBUTs) due to the lipid deficiency of their tears. The cationic o/w nanoemulsion vehicle Cationorm was able to improve keratitis (corneal fluorescein staining), TFBUT, and significantly reduced the symptoms of dry eye disease (DED) (Figure 5).60 The TFBUT was significantly greater with Cationorm than with Refresh in eyes with MGD. Cationorm was even better in MGD condition than in non-MGD, suggesting a positive correlation, while Refresh showed no better efficacy in eyes with MGD.
Figure 5.
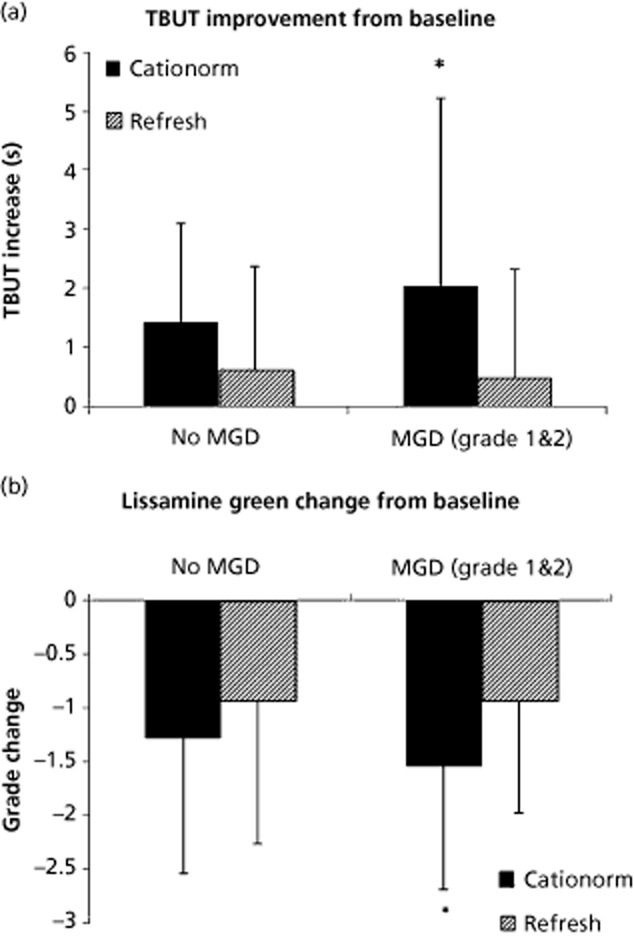
Tear film break-up time and lissamine green staining in dry eye patients with or without meibomian gland dysfunction (MGD) treated with Cationorm or Refresh. (a) Tear break-up time (TBUT) improvement over time, and (b) lissamine green change from baseline following Cationorm or Refresh treatment. *P < 0.05 (generalized estimating equation models, analysis of variance).
Thus, by mechanically stabilizing the tear film the cationic o/w nanoemulsion confirmed its benefits for the relief of mild to moderate dry eye.17 Hyperosmolarity of the tear is known to be pro-inflammatory, thus the hypoosmolarity of the aqueous phase may contribute to the management of DED signs and symptoms by transiently (upon instillation) normalizing tear osmolarity post instillation.61,62
More surprising were the beneficial effects of the cationic o/w nanoemulsion on the wound healing process. Repeated instillations of the cationic o/w nanoemulsion Cationorm were demonstrated to help the wounded corneal epithelium recover faster than following treatments with conventional artificial tears in a rabbit model of corneal abrasion.17 Both in-vitro and in-vivo data demonstrated that the CKC cationic o/w nanoemulsion promoted wound healing.55 On scraped human corneal epithelial (HCE) cells a 30 min application of the cationic o/w nanoemulsion was able to increase the pace of healing, as measured by the reduction of the size of the scraped area (Figure 6). These in-vitro data were confirmed in-vivo in a rat model of corneal scraping. Following corneal mechanical abrasion, a twice daily treatment with the CKC cationic o/w nanoemulsion allowed for a complete and almost scar-free re-epithelization of the cornea (Figure 7a).55 By opposition, treatment with a 0.02% BAK aqueous solution resulted in the formation of an opaque scar underneath the healed epithelium. These data suggested that the CKC cationic o/w nanoemulsion was able to promote a safe healing process, without the formation of opaque scar tissue within the cornea, as if the CKC cationic o/w nanoemulsion was able to manage the inflammation that resulted from the initial mechanical corneal abrasion. The number of inflammatory cells was then determined on fixed and haematoxylin-eosin stained rat corneas (Figure 7b). Corneas from the CKC cationic o/w nanoemulsion-treated group presented a reduced number of inflammatory cells, while in the other groups (phosphate buffered saline- or 0.02% BAK aqueous solution-treated groups) the inflammatory cell count remained elevated. This clearly suggested that the CKC cationic o/w nanoemulsion may have harboured anti-inflammatory properties.55 The same results were obtained with Cationorm, which contains 0.002% CKC as the cationic agent, and was confirmed in the rabbit following repeated instillations of a 0.05% ciclosporin cationic o/w nanoemulsion.52,63 The mechanism underlying these observations is currently under evaluation.64
Figure 6.
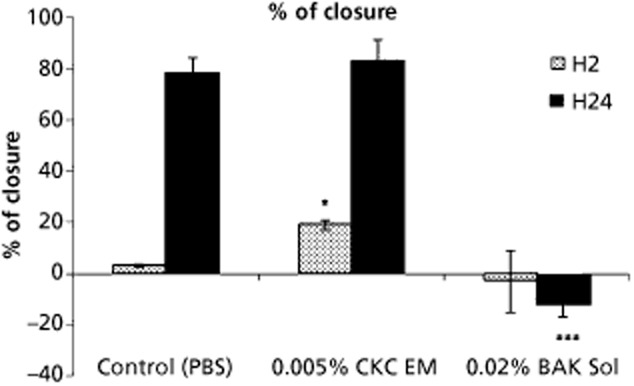
In-vitro scraping assay (human corneal epithelial cells) measuring the pace of the healing process following a 30-min treatment with 1/10 dilutions of 0.005% cetalkonium chloride cationic oil-in-water nanoemulsion (0.005% CKC EM) or 0.02% benzalkonium chloride solution (0.02% BAK Sol). *P < 0.05 against phosphate buffered saline (PBS); ***P < 0.01 against PBS (two-way analysis of variance followed by Fisher adjustment). H2, two hours; H24, 24 hours.
Adapted from Liang et al.55.
Figure 7.
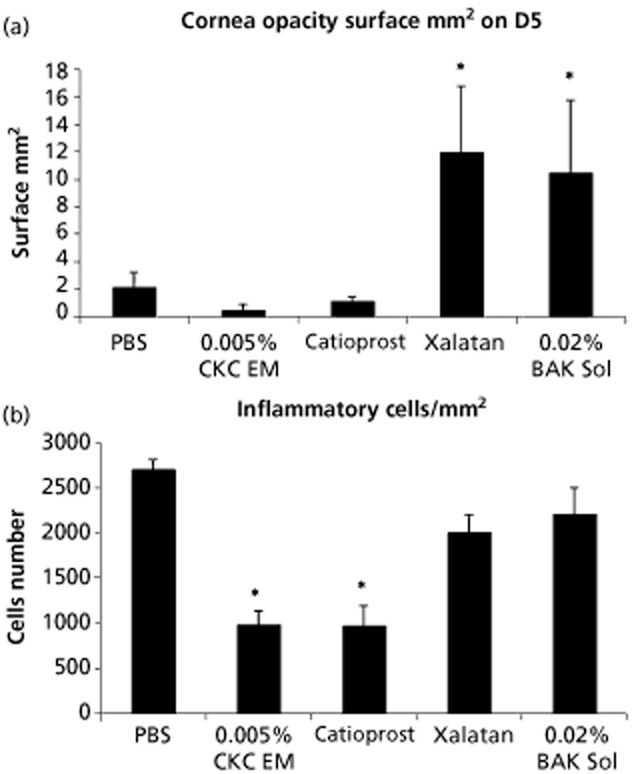
(a) Scar size and (b) inflammatory cell count in the rat cornea at day 5 (D5), after corneal scraping and five days treatment with cationic oil-in-water nanoemulsion. 0.02% Benzalkonium chloride solution (0.02% BAK Sol); 0.005% cetalkonium chloride cationic oil-in-water nanoemulsion (0.005% CKC EM). *P < 0.05 against phosphate buffered saline (PBS) (two-way analysis of variance followed by Fisher adjustment).
Adapted from Liang et al.55.
Conclusions
Cationic o/w nanoemulsions represent a new development strategy to improve ocular drug delivery of lipophilic compounds. The main issue in the development of these products was the choice of the cationic agent. CKC was found to be the best cationic agent to produce stable unpreserved cationic o/w nanoemulsions with unexpected beneficial biological activity for the ocular surface. The use of CKC over BAK as cationic agent appears obvious (Table 3) when comparing the physicochemical properties of these compounds. In the continuous effort to improve ocular drug delivery a CKC cationic o/w nanoemulsion was developed to improve the precorneal residence time and the spreading properties on negatively charged ocular surface cells of the nanoemulsions. This better spreading and improved residence time of the CKC cationic o/w nanoemulsion translated into a twofold increase in ciclosporin ocular bioavailability over anionic ciclosporin nanoemulsions. This new type of vehicle was demonstrated to be perfectly safe and well tolerated by the ocular surface. While BAK in conventional aqueous eye drops has a preservative role, thanks to its detergent action, CKC in the cationic o/w nanoemulsion exhibits neither detergent effect nor preservative role. Consequently, CKC cationic o/w nanoemulsion does not exhibit any of the observed ocular side effects related to BAK in aqueous eye drops (tear film instability, ocular surface damage, mucus removal). In addition to the improved bioavailability of the loaded drug, these CKC cationic o/w nanoemulsions have ocular surface protective properties through the restoration of a healthy tear film and by favouring the corneal wound healing process through the promotion of re-epithelization and inflammation management.
Declarations
Conflict of interest
The authors are employees of Novagali Pharma SAS.
Funding
This review received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Kaur IP, et al. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269:1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 3.du Toit LC, et al. Ocular drug delivery – a look towards nanobioadhesives. Expert Opin Drug Deliv. 2011;8:71–94. doi: 10.1517/17425247.2011.542142. [DOI] [PubMed] [Google Scholar]
- 4.Abdelkader H, Alany RG. Controlled and continuous release ocular drug delivery systems: pros and cons. Curr Drug Deliv. 2012;9:421–430. doi: 10.2174/156720112801323125. [DOI] [PubMed] [Google Scholar]
- 5.Muchtar S, et al. A submicron emulsion as ocular vehicle for delta-8-tetrahydrocannabinol: effect on intraocular pressure in rabbits. Ophthalmic Res. 1992;24:142–149. doi: 10.1159/000267160. [DOI] [PubMed] [Google Scholar]
- 6.Naveh N, et al. Pilocarpine incorporated into a submicron emulsion vehicle causes an unexpectedly prolonged ocular hypotensive effect in rabbits. J Ocul Pharmacol. 1994;10:509–520. doi: 10.1089/jop.1994.10.509. [DOI] [PubMed] [Google Scholar]
- 7.Ding S, et al. Nonirritating Emulsions for Sensitive Tissues, I; Patent No.: 5474979; US: Allergan; 1995. [Google Scholar]
- 8.Vandamme TF. Microemulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retin Eye Res. 2002;21:15–34. doi: 10.1016/s1350-9462(01)00017-9. [DOI] [PubMed] [Google Scholar]
- 9.Tamilvanan S, Benita S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur J Pharm Biopharm. 2004;58:357–368. doi: 10.1016/j.ejpb.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Royle L, et al. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25:763–773. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovich-Guilatt L, et al. Cationic vectors in ocular drug delivery. J Drug Target. 2004;12:623–633. doi: 10.1080/10611860400015910. [DOI] [PubMed] [Google Scholar]
- 12.Klang S, et al. The stability of piroxicam incorporated in a positively-charged submicron emulsion for ocular administration. Int J Pharm. 1996;132:33–44. [Google Scholar]
- 13.Klang S, et al. Influence of emulsion droplet surface charge on indomethacin ocular tissue distribution. Pharm Dev Technol. 2000;5:521–532. doi: 10.1081/pdt-100102035. [DOI] [PubMed] [Google Scholar]
- 14.Tamilvanan S, et al. Ocular delivery of cyclosporin A. I. Design and characterization of cyclosporin A-loaded positively-charged submicron emulsion. S.T.P. Pharma Sci. 2001;11:421–426. [Google Scholar]
- 15.Benita S, Elbaz E. Oil- in-Water Emulsions of Positively Charged Particles. Yisum Research Development Company of the Hebrew University of Jerusalem; Patent No.: US 6007826 A.1993. [Google Scholar]
- 16.Campbell PI. Toxicity of some charged lipids used in liposome preparations. Cytobios. 1983;37:21–26. [PubMed] [Google Scholar]
- 17.Lallemand F, et al. Successfully improving ocular drug delivery using the cationic nanoemulsion novasorb. J Drug Deliv. 2012;2012:604204. doi: 10.1155/2012/604204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becher P. Emulsions: Theory and Practice. 3. New York: Oxford University Press; 2001. [Google Scholar]
- 19.Rubino JT. The influence of charged lipids on the flocculation and coalescence of oil-in-water emulsions. I: kinetic assessment of emulsion stability. J Parenter Sci Technol. 1990;44:210–215. [PubMed] [Google Scholar]
- 20.Opawale FO, Burgess DJ. Influence of interfacial properties of lipophilic surfactants on water-in-oil emulsion stability. J Colloid Interface Sci. 1998;197:142–150. doi: 10.1006/jcis.1997.5222. [DOI] [PubMed] [Google Scholar]
- 21.Constantinides PP, Scalart J-P. Formulation and physical characterization of water-in-oil microemulsions containing long- versus medium-chain glycerides. Int J Pharm. 1997;158:57–68. [Google Scholar]
- 22.Hunter RJ. Zeta Potential in Colloid Science: Principles and Applications. London: Academic Press; 1988. [Google Scholar]
- 23.Rabinovich-Guilatt L, et al. Extensive surface studies help to analyse zeta potential data: the case of cationic emulsions. Chem Phys Lipids. 2004;131:1–13. doi: 10.1016/j.chemphyslip.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Klang SH, et al. Physicochemical characterization and acute toxicity evaluation of a positively-charged submicron emulsion vehicle. J Pharm Pharmacol. 1994;46:986–993. doi: 10.1111/j.2042-7158.1994.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 25.Furrer P, et al. Ocular tolerance of preservatives and alternatives. Eur J Pharm Biopharm. 2002;53:263–280. doi: 10.1016/s0939-6411(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 26.Baudouin C, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86:716–726. doi: 10.1111/j.1755-3768.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 28.Debbasch C, et al. Quaternary ammoniums and other preservatives' contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest Ophthalmol Vis Sci. 2001;42:642–652. [PubMed] [Google Scholar]
- 29.Pisella PJ, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45:1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]
- 30.Jaenen N, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17:341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 31.Ammar DA, et al. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofzia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010;27:837–845. doi: 10.1007/s12325-010-0070-1. [DOI] [PubMed] [Google Scholar]
- 32.Kahook MY, Ammar DA. In vitro toxicity of topical ocular prostaglandin analogs and preservatives on corneal epithelial cells. J Ocul Pharmacol Ther. 2010;26:259–263. doi: 10.1089/jop.2010.0003. [DOI] [PubMed] [Google Scholar]
- 33.Ammar DA, Kahook MY. Effects of glaucoma medications and preservatives on cultured human trabecular meshwork and non-pigmented ciliary epithelial cell lines. Br J Ophthalmol. 2011;95:1466–1469. doi: 10.1136/bjophthalmol-2011-300012. [DOI] [PubMed] [Google Scholar]
- 34.EMEA. Emea Public Statement on Antimicrobial Preservatives in Ophthalmic Preparations for Human Use – Emea/622721/2009. 2009.
- 35.Freeman PD, Kahook M. Preservatives in topical ophthalmic medications: historical and clinical perspectives. Expert Rev Ophthalmol. 2009;4:59–64. [Google Scholar]
- 36.Liang H, et al. Reduction of quaternary ammonium-induced ocular surface toxicity by emulsions: an in vivo study in rabbits. Mol Vis. 2008;14:204–216. [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MJ, et al. Method for the measurement of the diffusion coefficient of benzalkonium chloride. Water Res. 2002;36:1423–1428. doi: 10.1016/s0043-1354(01)00356-6. [DOI] [PubMed] [Google Scholar]
- 38.Bague S, et al. Oil-in-Water Type Emulsion with Low Concentration of Cationic Agent and Positive Zeta Potential, Novagali Pharma; Patent No.: US 8298568.2006. [Google Scholar]
- 39.Rabinovich L, et al. Emulsion Compositions Containing Quaternary Ammonium Compounds, Novagali Pharma; Patent No.: US 7973081.2008. [Google Scholar]
- 40.Vlachy N, et al. Determining the cytotoxicity of catanionic surfactant mixtures on HeLa cells. Colloids Surf B Biointerfaces. 2009;70:278–280. doi: 10.1016/j.colsurfb.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 41.Kurup TRR, et al. Preservative requirements in emulsions. Pharm Acta Helv. 1992;67:204–208. [Google Scholar]
- 42.Kazmi SJ, Mitchell AG. Preservation of solubilized and emulsified systems I: correlation of mathematically predicted preservative availability with antimicrobial activity. J Pharm Sci. 1978;67:1260–1266. doi: 10.1002/jps.2600670919. [DOI] [PubMed] [Google Scholar]
- 43.Sznitowska M, et al. Physicochemical screening of antimicrobial agents as potential preservatives for submicron emulsions. Eur J Pharm Sci. 2002;15:489–495. doi: 10.1016/s0928-0987(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 44.Macha S, Mitra AK. Ocular disposition of ganciclovir and its monoester prodrugs following intravitreal administration using microdialysis. Drug Metab Dispos. 2002;30:670–675. doi: 10.1124/dmd.30.6.670. [DOI] [PubMed] [Google Scholar]
- 45.Abdulrazik M, et al. Ocular delivery of cyclosporin A. II. Effect of submicron emulsion's surface charge on ocular distribution of topical cyclosporin A. STP Pharma Sci. 2001;11:427–432. [Google Scholar]
- 46.Daull P, et al. Distribution of cyclosporine A in ocular tissues after topical administration of cyclosporine a cationic emulsions to pigmented rabbits. Cornea. 2013;32:345–354. doi: 10.1097/ICO.0b013e31825e83f4. [DOI] [PubMed] [Google Scholar]
- 47.Daull P, et al. A comparative study of a preservative-free latanoprost cationic emulsion (Catioprost) and a BAK-preserved latanoprost solution in animal models. J Ocul Pharmacol Ther. 2012;28:515–523. doi: 10.1089/jop.2011.0245. [DOI] [PubMed] [Google Scholar]
- 48.Baudouin C, de Lunardo C. Short-term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol. 1998;82:39–42. doi: 10.1136/bjo.82.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishibashi T, et al. Comparison of the short-term effects on the human corneal surface of topical timolol maleate with and without benzalkonium chloride. J Glaucoma. 2003;12:486–490. doi: 10.1097/00061198-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Ciancaglini M, et al. Conjunctival modifications in ocular hypertension and primary open angle glaucoma: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2008;49:3042–3048. doi: 10.1167/iovs.07-1201. [DOI] [PubMed] [Google Scholar]
- 51.Amrane M, et al. The Influence on Seasonality and Subtype in Vernal Keratoconjunctivitis (Vkc) Patients in a Randomized Clinical Trial Investigating Nova22007, a Preservative-Free Cyclosporine Cationic Emulsion. Fort Lauderdale: ARVO; 2012. [Google Scholar]
- 52.Liang H, et al. Ocular safety of cationic emulsion of cyclosporine in an in vitro corneal wound healing model and an acute in vivo rabbit model. Mol Vis. 2012;18:2195–2204. [PMC free article] [PubMed] [Google Scholar]
- 53.Liang H, et al. Comparison of the ocular tolerability of a latanoprost cationic emulsion versus conventional formulations of prostaglandins: an in vivo toxicity assay. Mol Vis. 2009;15:1690–1699. [PMC free article] [PubMed] [Google Scholar]
- 54.Daull P, Garrigue JS. Preservative-free cationic nanoemulsion of latanoprost. Ophthalmol Times Eur. 2013;9:8–10. [Google Scholar]
- 55.Liang H, et al. In vitro and in vivo evaluation of a preservative-free cationic emulsion of latanoprost in corneal wound healing models. Cornea. 2012;31:1319–1329. doi: 10.1097/ICO.0b013e318255a7f8. [DOI] [PubMed] [Google Scholar]
- 56.Mundorf TK. Treat glaucoma patients globally. Adv Ocul Care. 2011:71–72. [Google Scholar]
- 57.Godfrey DA. Glaucoma: what's next? 2011. pp. 42–43. Ophthalmol Times.
- 58.Abelson MB, Lafond A. Glaucoma and dry eye: a tough combo. 2011. pp. 108–111. Rev Ophthalmol.
- 59.Amrane M, et al. The Effect of Vekacia® (Unpreserved Cyclosporine Cationic Emulsion) on Severe Corneal Involvement in Patients with Vernal Keratoconjunctivitis Participating in a Randomized, Parallel Group Controlled, Clinical Trial. Genève: SOE; 2011. [Google Scholar]
- 60.Amrane M, et al. Efficacy of Cationorm Preservative-Free Cationic Emulsion Versus Refresh in Dry Eye Disease (Ded) Patients with/without Meibomian Gland Dysfunction (Mgd) Kenchoji, Kamakura, Japan: TFOS Asia; 2012. [Google Scholar]
- 61.Luo L, et al. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31:186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 62.Li DQ, et al. Stimulation of matrix metalloproteinases by hyperosmolarity via a jnk pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 63.Daull P, et al. Comparison of the anti-inflammatory effects of artificial tears in a rat model of corneal scraping. Nice: EVER; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filion MC, Phillips NC. Anti-inflammatory activity of cationic lipids. Br J Pharmacol. 1997;122:551–557. doi: 10.1038/sj.bjp.0701396. [DOI] [PMC free article] [PubMed] [Google Scholar]