Abstract
Objective
To evaluate whether vagus nerve stimulation (VNS) as adjunct to best medical practice (VNS + BMP) is superior to BMP alone in improving long-term health-related quality of life (HRQoL).

Methods
PuLsE (Open Prospective Randomized Long-term Effectiveness) was a prospective, randomized, parallel-group, open-label, and long-term effectiveness study (conducted at 28 sites in Europe and Canada). Adults with pharmacoresistant focal seizures (n = 112) received VNS + BMP or BMP (1:1 ratio). Medications and VNS parameters could be adjusted as clinically indicated for optimal seizure control while minimizing adverse effects. Primary endpoint was mean change from baseline HRQoL (using Quality of Life in Epilepsy Inventory-89 total score; QOLIE-89). Secondary endpoints included changes in seizure frequency, responder rate (≥50% decrease in seizure frequency), Centre for Epidemiologic Studies Depression scale (CES-D), Neurological Disorders Depression Inventory-Epilepsy scale (NDDI-E), Clinical Global Impression-Improvement scale (CGI-I), Adverse Event Profile (AEP), and antiepileptic drug (AED) load. The study was prematurely terminated due to recruitment difficulties prior to completing the planned enrollment of n = 362. Results for n = 96 who had baseline and at least one follow-up QOLIE-89 assessment (from months 3-12) were included in this analysis. Mixed model repeated measures (MMRM) analysis of variance was performed on change from baseline for the primary and secondary endpoints.
Results
Significant between-group differences in favor of VNS + BMP were observed regarding improvement in HRQoL, seizure frequency, and CGI-I score (respective p-values < 0.05, 0.03, and 0.01). More patients in the VNS + BMP group (43%) reported adverse events (AEs) versus BMP group (21%) (p = 0.01), a difference reflecting primarily mostly transient AEs related to VNS implantation or stimulation. No significant difference between treatment groups was observed for changes in CES-D, NDDI-E, AEP, and AED load.
Significance
VNS therapy as a treatment adjunct to BMP in patients with pharmacoresistant focal seizures was associated with a significant improvement in HRQoL compared with BMP alone.
Keywords: Epilepsy, Health-related quality of life, QOLIE-89, Seizures, Vagus nerve stimulation
Vagus nerve stimulation (VNS) was approved in 1997 for use as an adjunctive therapy in patients with pharmacoresistant epilepsy.1–3 Since then, VNS therapy has been provided to >70,000 patients worldwide, and its beneficial effects in reducing seizure frequency have been reported in multiple long-term open-label studies.4 Other potentially relevant benefits that have been reported include decreased severity and duration of ictal or postictal phases, and improved mood, vigilance, communication, cognition, and possibility of reducing antiepileptic drugs (AEDs) and associated adverse effects.5–10 Such benefits could have a significant impact on health-related quality of life (HRQoL), in addition to reduction in seizure frequency, and may partially explain the observation that >70% of patients choose to continue receiving VNS therapy once their battery needs to be replaced, an average of 6 years after implantation (data on file; Cyberonics, Inc., 2009; Houston, TX, U.S.A.). However, apart from seizure frequency, none of the above VNS outcomes were assessed in patients with epilepsy in the setting of a randomized controlled trial.
The PuLsE (Open Prospective Randomized Long-term Effectiveness study was designed to assess whether VNS as a treatment adjunct to best medical practice (VNS + BMP) is superior to BMP alone in improving HRQoL in patients with pharmacoresistant focal seizures.
Methods
PuLsE was an international, multicenter, prospective, randomized, parallel-group, open-label, and long-term (2 years) effectiveness study (Fig. 1; ClinicalTrials.gov identifier: NCT00522418). The primary objective was to demonstrate superiority over time in health outcomes of BMP with adjunctive VNS therapy compared with BMP alone in patients with pharmacoresistant focal seizures. A total of 28 sites across Europe and Canada participated in the study.
Figure 1.
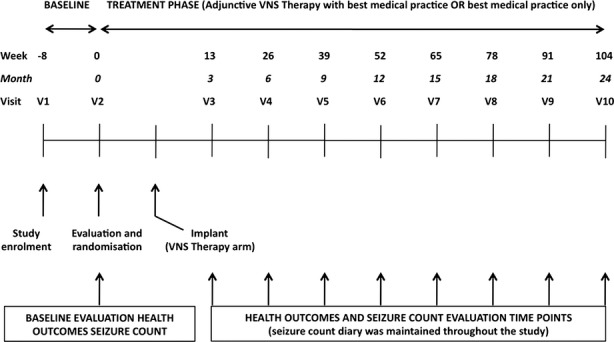
Study time line showing the timing of the various study visits and efficacy assessments.
The design was dictated primarily by the need to ensure a relatively long duration of follow-up, in order to obtain a clinically meaningful assessment of long-term changes in HRQoL. This precluded on ethical grounds the use of a double-blind design, and required that the treating physicians be allowed to modify the regimen of AEDs as clinically indicated. Accordingly, BMP was defined as the individualized therapy judged optimal by investigators at each visit for each patient, which could include a change in dosage or type of AEDs (including their withdrawal). In a similar way, clinicians were allowed to adjust VNS stimulation parameters throughout the study. This approach has the advantage of reflecting routine clinical practice, thereby increasing the external validity of the study.
Study participants
Eligible participants were 16–75 years old with at least a 2-year history of focal seizures not adequately controlled by ongoing AED therapy. Additional eligibility criteria were (1) previous failure of at least three AEDs used alone or in combination; (2) treatment with at least one AED with a regimen that was stable for at least 1 month prior to study entry; and (3) at least one focal seizure with a motor component per month during the 2 months prior to study entry. Patients with psychogenic nonepileptic seizures or genetic (idiopathic) generalized epilepsies were not eligible for the study. Prior to randomization, all participants provided written informed consent approved by the ethics committees at each study site.
Endpoints
The primary endpoint was the mean change from baseline in the 89-item Quality of Life in Epilepsy Inventory (QOLIE-89) total score.11 Secondary endpoints included QOLIE-89 composite subscores (Epilepsy-targeted, Cognition, Mental Health, and Physical Health), 50% responder rate (proportion of patients with ≥50% decrease in seizure frequency vs. baseline), scores on the Centre for Epidemiologic Studies Depression scale (CES-D),12 Neurological Disorders Depression Inventory in Epilepsy scale (NDDI-E),13 Clinical Global Impression of Improvement scale (CGI-I),14 and Adverse Event Profile (AEP),15,16 and change from baseline in AED load (defined as the sum of the Prescribed Daily Dose (PDD)/Defined Daily Dose (DDD) ratios for each AED included in the treatment regimen of each individual patient).17 Safety and tolerability were evaluated based on spontaneously reported adverse events (AEs) and premature withdrawals.
Study conduct
After a prestudy screening (visit 1), patients fulfilling the eligibility criteria entered an 8-week prospective baseline, which was used to determine baseline seizure frequency and other health outcomes, all of which were recorded at visit 2 at completion of the 8-week period (Fig. 1). During visit 2, patients who continued to meet the eligibility criteria were randomized to VNS + BMP or BMP alone (1:1 ratio) through a centralized voice-based randomization service. All treatments were prescribed and delivered according to the procedures routinely used in clinical practice in each center. In particular, centers were responsible for covering the costs involved in the acquisition of the VNS therapy device. Study visits were scheduled at 3-month intervals over a 24-month assessment period. The database for the PuLsE study was originally held by the Bonn epilepsy center (Germany). Upon closure of the study, the database was transferred to Cyberonics, where data analysis was conducted by one of the coauthor (P. Raman, employee of Cyberonics).
Statistical analyses
The initial plan was to enroll 362 patients and to follow each patient for 2 years. The original statistical analysis plan included the intent-to-treat and per-protocol populations, but such analysis was not possible because of early study termination due to low enrollment rates, requiring revision of the statistical plan. Only patients with a baseline QOLIE-89 score and at least one postbaseline assessment were included in the statistical analysis of data from 3, 6, 9, and 12 months of follow-up.
For longitudinal data collected at different visits post-baseline, we performed a mixed model repeated measures (MMRM) analysis of variance (ANOVA) using the SAS GLIMMIX (generalized linear mixed model) procedure to assess trend differences between the two treatment groups. The fitted model included fixed effects of treatment group (VNS + BMP vs. BMP), visit month (3, 6, 9 and 12 months following randomization), and interaction of treatment and visit month. The evaluated response endpoints included the primary endpoint of mean change from baseline in QOLIE-89 total scores, and changes in the following secondary endpoints: seizure frequency, 50% responder rate, CES-D, NDDI-E, CGI-I, AEP, proportion of patients reporting AEs, and AED load. An additional change in QOLIE-89 total scores was assessed in patients who had no changes in their baseline AEDs. The secondary endpoints are partially redundant with the primary endpoint; therefore, p-values from secondary endpoint analyses are being reported here for exploratory analysis purposes and correction of multiple comparisons were not conducted. When inferential statistics were conducted based on MMRM analyses, the least squares means and related standard errors were summarized. Inferential statistical analyses were not conducted for the visit-wise data, as there were limited numbers of observations, instead the visit-wise data are summarized using descriptive statistics including means, percentages, medians, standard deviation, and p-values.
When MMRM analysis results indicated significant treatment-group trend differences (p < 0.05), post hoc visit-wise analyses were performed using patient data at each visit. p-Values based on means were generated using analysis of variance (F-test) for continuous data. p-Values based on medians were generated using the Wilcoxon rank-sum test for continuous data. p-Values were generated using the chi-square test for categorical data.
Results
This study was conducted between February 2006 and July 2008 and was prematurely terminated by the sponsor due to a low enrollment rate and not as a result of a safety or efficacy signal. Low enrollment resulted primarily from the strong positive or negative views about the value of VNS therapy expressed by most study candidates, leading to only a minority of them accepting to participate in the study. As a result of early study termination, only a few patients (n = 7) achieved 2-year follow-up. The VNS therapy devices were not removed from the participating patients following study termination, and the patients continued to use their devices as part of routine medical care, as clinically indicated.
A total of 131 patients were screened and 122 were randomized to receive VNS + BMP or BMP alone. Data from one study site (including 10 randomized patients) were removed from the analysis datasets, as inadvertently a centrally approved informed consent form was used without the additional mandatory approval of the site's local ethics committee. The remaining 112 randomized patients were included in the safety analyses. Of these, 96 (83%)—including 48 patients allocated to VNS + BMP and 48 patients allocated to BMP—had baseline data and at least one post baseline follow-up QOLIE-89 assessment, and were thus included in the efficacy analyses. Sixty of these 96 patients had completed their 1-year follow-up visit by the time the study was terminated, including 55 patients with QOLIE-89 data available at each visit (assessments at 0, 3, 6, 9, and 12 months; 28 in the VNS + BMP group and 27 in the BMP group).
Of the eligible randomized patients, 16 were not included in the statistical analysis, as their study participation ended prior to collection of baseline data and at least one post-baseline follow-up QOLIE-89 assessment for the following reasons: 9 patients due to premature study termination (two from VNS + BMP group and seven from BMP group), 2 patients due to consent withdrawal (one from each treatment group), 2 patients due to compliance issues (one from each treatment group), 2 patients who withdrew early for reasons not listed (both from the VNS + BMP group), and one patient in the BMP group who withdrew early due to lack of efficacy.
Patients in the two treatment groups were comparable at baseline in terms of gender, age, age at onset of epilepsy, proportion with structural or metabolic versus unknown etiology, seizure frequency, AED load, and mean baseline scores from QOLIE-89, AEP, CES-D, NDDI-E, and CGI-I assessments (Table 1). There were no significant differences between the two treatment groups for any of the recorded baseline characteristics (p ≥ 0.05).
Among patients in the VNS + BMP arm, there was a median interim period of 48 days (range 8–162) between randomization and implantation surgery. The duration of the preoperative waiting period varied in relation to regional and national regulations for approving reimbursement of VNS therapy in individual subjects, and the local waiting time for nonurgent neurosurgical procedures. Treatment assessments for the VNS + BMP arm were started at the initiation of VNS treatment, and each patient began VNS dose titration according to protocol-specified guidelines.
At the 12-month follow-up visit, the median VNS parameters were 1.8 mA output current (range 0.8–2.8 mA), 500 μs pulse width, 30 s signal ON time, 5 min signal OFF time, and 147.8 mC of total charge delivered per day (range 40.3–420.0 mC/day) (see Table S1 for detailed values at each follow-up visit).
HRQoL evaluation
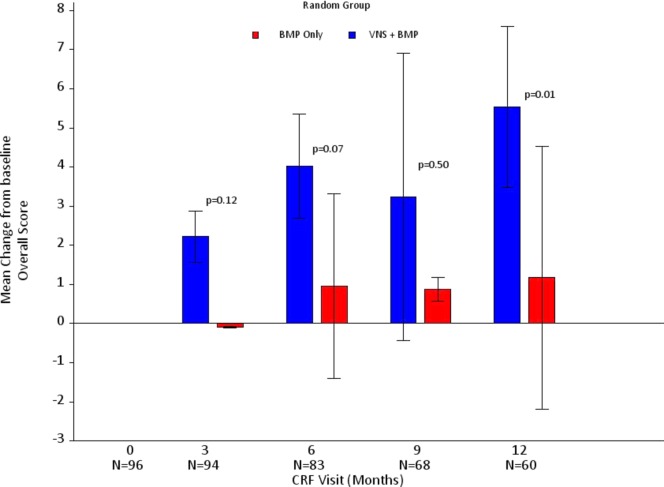
MMRM analysis of change from baseline in QOLIE-89 score over time showed a significant difference between the two groups (48 VNS + BMP patients and 48 control patients), with a greater improvement in patients allocated to the VNS + BMP group (p < 0.05) (Table S2). Visit-wise ANOVA showed that the benefit of VNS + BMP was maximal at 12 months (p = 0.01), with a mean (± standard deviation [SD]) improvement of 5.5 (±7.2) in patients allocated to VNS + BMP (n = 31) compared with 1.2 (±6.9) in those allocated to BMP alone (n = 29) (Fig. 2). The visit-wise ANOVA data for the other time points were as follows: 3 months (p = 0.12; 47 VNS + BMP patients and 47 BMP patients), 6 months (p = 0.07; 38 VNS + BMP patients and 45 BMP patients), and 9 months (p = 0.50; 33 VNS + BMP patients and 35 BMP patients).
Figure 2.
Primary outcome measure: Mean change in QOLIE-89 overall score from baseline (Month 0; n = 96) to Months 3 (n = 94), 6 (n = 83), 9 (n = 68), and 12 (n = 60).
Similar improvements in QOLIE-89 score were observed for patients in the VNS + BMP subgroups who had no change in their number or type of AEDs (n = 42; p = 0.03), or in their AED drug load albeit not significant (n = 32; p = 0.08), compared with the entire BMP group (n = 48) (Table S2).
MRMM analysis of each QOLIE-89 subscales showed more improvement in patients allocated to VNS + BMP than in those receiving BMP alone; however, the differences were not significant: Epilepsy-targeted score (p = 0.06), Cognitive (p = 0.20), Mental Health (p = 0.33), and Physical Health (p = 0.17) (Table S2; visit-wise ANOVA data are provided in Table S3).
Seizure control and CGI-I, CES-D, and NDDI-E outcomes
MMRM analysis of the change from baseline in total number of seizures per week was significantly greater in the VNS + BMP group than in the BMP group (p = 0.03). Median percent change in seizure frequency from baseline to 12 months confirms an increasing improvement in seizure control for the VNS + BMP group versus the BMP group over time, although differences at individual time points failed to reach statistical significance (Fig. 3).
Figure 3.
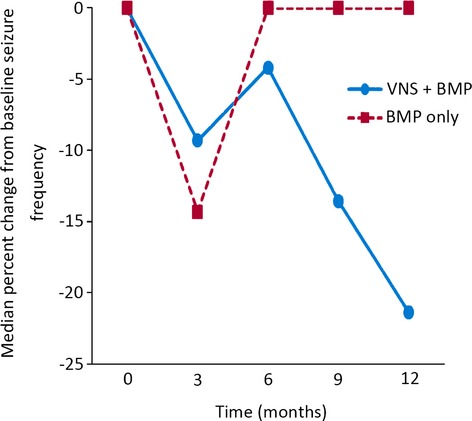
Median percent change in total seizure frequency from baseline (Month 0; n = 95) to Months 3 (n = 93), 6 (n = 80), 9 (n = 67), and 12 (n = 60). p-Values at baseline, and 3, 6, 9, and 12 months were 0.94, 0.77, 0.35, 0.12, and 0.13, respectively.
MMRM analysis of the 50% responder rates did not differ significantly between the VNS + BMP group (n = 10/31; 32%) and control group (n = 7/29; 24%) at month 12 (p = 0.49) (data not shown).
MMRM analysis of changes over time in CGI-I score demonstrated a significant difference between the two groups, with greater improvement in patients allocated to the VNS + BMP group (p = 0.01) (Table 2). Visit-wise ANOVA showed that the benefit of VNS + BMP was significant for patients allocated to VNS + BMP, compared with those allocated to BMP alone at 3 and 12 months (p-values of 0.01 and 0.03, respectively), and a trend was observed at 9 months (p = 0.05) but not at 6 months (p = 0.49) (Fig. 4).
Figure 4.
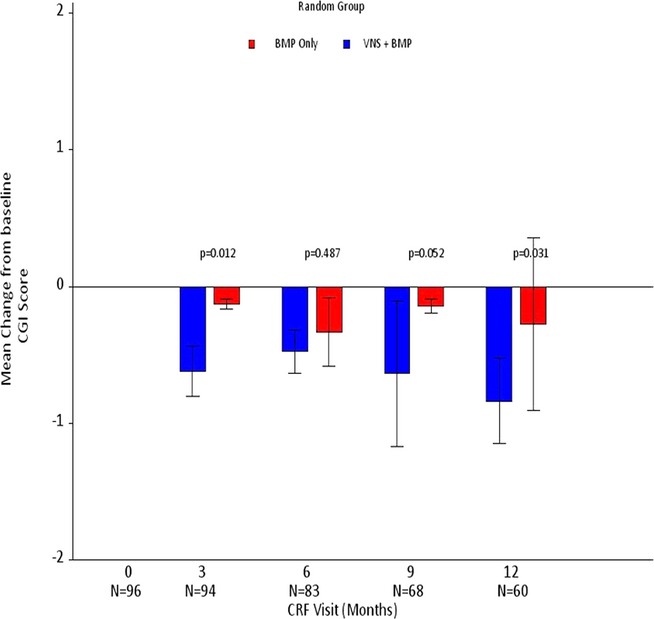
Mean change in CGI-I score from baseline (Month 0; n = 96) to Months 3 (n = 94), 6 (n = 83), 9 (n = 68), and 12 (n = 60).
MMRM analysis of changes in CES-D and NDDI-E scores did not show significant differences between groups (p-values were 0.90 and 0.13, respectively) (Table 2). Change from baseline values at follow-up time points are provided in Table S4.
A summary of changes in underlying AED treatment is provided in Table S5. Both treatment groups had similar AED loads from baseline to follow-up time points. When change from baseline AED load was evaluated by MMRM analysis, a nonsignificant trend was observed between the groups (p = 0.08) with a greater increase in the BMP group (means ± standard error (SE): 0.18 ± 0.05) than in the VNS + BMP group (means ± SE: 0.06 ± 0.05) (data not shown).
AEP scores and AEs
Changes from baseline AEP values at 3, 6, 9, and 12 months are provided in Table S4. MMRM analysis of least-squares mean score (SE) was −3.7 (±1.0) in the VNS + BMP group and −1.3 (±1.0) in the BMP group, but the difference was not significant (p = 0.08) (Table 2).
At least one AE was reported in 23 patients (43%) in the VNS + BMP group and in 12 patients (21%) in the control group (p = 0.01). The majority of AEs reported in the VNS + BMP group were related to VNS therapy, that is, device implantation (n = 12; 22%) and electrode stimulation (n = 11; 20%). Specific AEs reported at a frequency of >5% were reported only in the VNS + BMP group and included dysphonia (n = 8; 15%) and chest pain, headache, hypoesthesia, and depression, each reported in 3 patients (6%). Of these AEs, chest pain (n = 3) and hypoesthesia (n = 3) were considered related to VNS device implantation; and dysphonia (n = 7) was considered related to device stimulation. In addition, one patient experienced localized infection related to device implantation.
Serious AEs were reported in five (9%) patients in the VNS + BMP group and in three (5%) patients in the BMP group. In the VNS + BMP group, these included transient vocal cord paralysis in two patients (considered to be related to the implantation procedure; both completely resolved); brief respiratory arrest of moderate severity in one patient from postoperative laryngospasm (considered related to implantation procedure and AED treatment; resolved on the same day); fall, convulsion, head injury, and worsened seizures in one subject (considered related to VNS stimulation and AED treatment); and prostatic cancer and suicide attempt in one patient each (not considered related to study treatment). None of the serious AEs reported in the BMP group were considered related to AED treatment. The majority of study discontinuations in either treatment group were due to premature termination of the study by the sponsor (VNS + BMP group: 46/54, 85% and BMP group: 47/58, 81%; data on file at Cyberonics, Inc.). No deaths were reported in this study, and there were no discontinuations due to an AE in either treatment group.
Discussion
This randomized controlled trial—designed to reflect clinical practice—demonstrated that adjunctive VNS therapy after 12-month follow-up is associated with significantly greater improvement in HRQoL over BMP alone (control group) in patients with pharmacoresistant focal epilepsy.
Compared with previous studies assessing long-term outcome of VNS therapy, our study has significant strengths in using a randomized controlled design and a robust primary endpoint such as HRQoL, whose improvement is the ultimate goal of any therapeutic intervention. It is important to note that the study endpoint was determined with an instrument, the QOLIE-89 inventory, which has been validated in many different settings and languages worldwide (including all languages used by our patients) and represents the most comprehensive epilepsy-specific measure of HRQoL currently available.18 On the other hand, an important study limitation relates to the smaller sample size and shorter duration of follow-up than initially planned. These were a consequence of a low enrollment rate that led to the early study termination by the sponsor. Despite this limitation, and the necessary revision of the statistical plan, the results supported the primary hypothesis by showing significantly greater improvement in HRQoL in patients receiving VNS compared with BMP alone.
The low enrollment rate resulted primarily from the fact that most study candidates had strong views (either positive or negative) about the value of VNS therapy and therefore were reluctant to be randomized. As a consequence, recruited patients are expected to be less biased than those who refused to participate, which might strengthen rather than limit the external validity of a study having quality of life as primary outcome.
It could be argued that because of the open label and flexible design, with individual changes in AEDs possible in both groups, results may have been affected by the patient's or physician's expectations or decisions. Although this limitation is acknowledged, a double-blind design, as well as less flexibility in AED changes, could not be justified for the duration of follow-up required to demonstrate clinically meaningful long-term effects on HRQoL. Indeed, blinding would have required that patients in the control group receive a sham operation or have their VNS device turned off for the entire duration of follow-up, two options that would be difficult to justify ethically. Similarly, flexibility in AED changes in both groups was believed necessary to ensure safe and adequate long-term management of a population with pharmacoresistant focal epilepsy. Furthermore, this flexibility mirrored clinical practice and promoted the external validity of the study. It is notable that our main finding was confirmed after excluding patients from the VNS + BMP group who had changes in their AED treatment.
Changes in AEP scores, which reflect the burden of AED-related toxicity, showed a trend to have a more favorable course in the VNS + BMP group than in the BMP group. This is in contrast with the observation that AEs were reported with a higher frequency among patients treated with adjunctive VNS. This paradoxical finding reflects the fact that patients from the VNS + BMP group filled a specific questionnaire for VNS-related AEs that are not included among the AEP items. Because AED-induced AEs are a major determinant of HRQoL in patients with pharmacoresistant epilepsy,15,19 the possibility that a reduction in AED toxicity contributed to the better HRQoL outcome in VNS-treated patients needs to be considered. The difference in AEP score changes between the two groups, however, was small and unlikely to account for the significant improvement in HRQoL in the VNS-treated group.
Similar to the trend toward a lower AEP score, the greater seizure reduction in the VNS + BMP group compared with the BMP group might have contributed to the HRQoL benefits associated with VNS. The reduction in seizure frequency in the VNS + BMP group was statistically significant, but of a magnitude that previous studies have shown to affect HRQoL only minimally.19,20 Moreover, in previous studies, VNS-associated improvement in QOLIE-89 score and other measures of quality of life did not correlate with changes in seizure frequency.21 Finally, our HRQoL findings do not seem to be primarily driven by an effect of VNS on mood, because no significant differences were observed between VNS + BMP and BMP groups in the two depression scales used in this study (CES-D and NDDI-E), or in the QOLIE-89 Mental Health subscale. Based on these findings, we suggest that the VNS-related improvement in HRQoL in our patients might reflect the sum of modest benefits in multiple factors rather than a single determinant.
The improvement in QOLIE-89 total score and in seizure frequency in the VNS + BMP group compared with the BMP group increased gradually over time and reached a maximum at the end of follow-up (12 months after randomization). However, findings at 3, 6, and 9 months after randomization were not statistically significant, possibly due to differences in the populations studied at each time point. Yet, these findings are in line with the progressive ramp-up and reported time course of the effectiveness of VNS on seizure frequency,1,22 as confirmed in this study.
Overall, the results from this trial provide further evidence for the added value of VNS therapy over flexibly adjusted AED therapy in patients with pharmacoresistant focal epilepsy who are not candidates for surgical resection. Moreover, our findings demonstrate that the benefits of such therapy may extend beyond the sole reduction in seizure frequency.
Acknowledgments
The authors wish to thank the patients who participated in this study, and the contributing staff at the study sites. Additional investigators were Franz Brunnhuber (King's College Hospital, Neurology, London, United Kingdom), Hans JA Carpay (Tergooiziekenhuizen, Blaricum, The Netherlands), Philippe Derambure (Hôpital Roger Salengro, Neurologie, Lille, France), Christian Elger (Univ. Klinik für Epileptologie, Bonn, Germany), Gerard Hageman (Medisch Spectrum Twente, Neurologie, Enschede, The Netherlands), Edouard Hirsch (Hôpitaux Universitaires de Strasbourg, Service d'exploration des épilepsies, Strasbourg, France), Hrisimir Kostov (Rikshospitalet University Hospital—Dept. of Neurodiagnostics National Centre for epilepsy, Sandvika, Norway), Henk van Lambalgen (SEIN, Zwolle, The Netherlands), Benjamin Legros (Hôpital Erasme – Neurologie, Anderlecht, Belgium), Hans Lindsten (Umea University Hospital, Department of Neurology, Umea, Sweden), Thomas Mayer (Kleinwachau Sächsisches Epilepsiezentrum Radeberg, Radeberg, Germany), Andrew McEvoy (The National Hospital for Neurology and Neurosurgery, London, United Kingdom), Uwe Runge (Klinik der Ernst-Moritz-Arndt-Universität, Neurologische Klinik, Greifswald, Germany), Bernhard Steinhoff (Epilepsiezentrum Kork, Epilepsieklinik für Erwachsene, Kehl-Kork, Germany), and Arielle Crespel (CHU Gui de Chauliac, Neurology, Montpellier, France). The study was sponsored by Cyberonics, Inc. (Houston, Texas, U.S.A.; manufacturer of the VNS Therapy System). Wim Van Grunderbeek (an employee of Cyberonics) was the study manager. M. Bunker and A.K. Jayewardene are employees of Cyberonics and assisted the author P. Raman and the other authors of the study with data analysis. Karishma Manzur (an employee of Lenimen Consulting, Inc.) provided medical writing assistance and was compensated by Cyberonics.
Disclosures or Conflicts of Interest
The authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors had full access to the study data, and were involved in the study data interpretation and the writing of the report. The authors approved the final version of the report, and were responsible for the decision to submit the manuscript for publication. P. Ryvlin is an employee of TIGER, CRNL, INSERM U1028, CNRS 5292 and Hospices Civils de Lyon, Lyon, France, and Claude Bernard Lyon-1 University, and he has received speaker honoraria or consultant fees from UCB Pharma, GlaxoSmithKline, Eisai, Cyberonics, and Medtronic. F. Gilliam has received grants from the National Institutes of Health (NIH) and The American Epilepsy Society, and has received speaker honoraria and research support from GlaxoSmithKline and Eisai. G. Colicchio has received grants from Ministry of Scientific Research and from Cyberonics for this study. P.A. Iudice has received grants from the Italian Ministry for Education, University, and Research, and has received speaker honoraria or consultancy fees from Eisai, GlaxoSmithKline, Janssen-Cilag and UCB Pharma. H. Stefan has received honoraria for advisory board participation and/or lecturing from Cyberonics, Electa, Janssen-Cilag, UCB Pharma, Eisai GmbH, Pfizer Pharma, GlaxoSmithKline, Desitin Arzneimttel, and Cerbomed, and has received grants from DFG (German Research Society), ELAN (University Foundation), Sander Foundation, and royalties from Cambridge University Press. P. Boon has received speaker's fees and grant support from Cyberonics, UCB, Medtronic, Eisai, Neurotech, and GlaxoSmithKline. M. Sadler is an employee of QEII Health Sciences Centre, Halifax, Nova Scotia, Canada, and Dalhousie University, and has received speaker honoraria or consultancy fees from UCB Canada. E. Perucca is an employee of the University of Pavia, has received grants from the European Union, the Italian Medicines Agency, the Italian Ministry of Health, the Italian Ministry for Education, University, and Research, and the C. Mondino National Neurological Institute, and has received speaker's or consultancy fees from Eisai, GlaxoSmithKline, Lundbeck, Medichem, Sun Pharmaceutical, Supernus Pharmaceuticals, UCB SA, Upsher-Smith, Vertex Pharmaceuticals, and ViroPharma. The other coauthors (D.K. Nguyen, P. Tinuper, N. Zamponi, U. Aguglia, L. Wagner, L. Minotti, P. Benna, and P. Raman) have nothing to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Parameters of VNS pulse generator for patients included in the VNS + BMP treatment group at Months 3 (n = 45), 6 (n = 41), 9 (n = 33), and 12 (n = 28).
Table S2. Change from baseline to Month 12 in QOLIE-89 scores.
Table S3. Change from baseline in QOLIE-89 subscale scores; the number of subjects at each time point for the VNS + BMP and BMP groups, respectively were n = 48 and 48 (at baseline), n = 47 and 47 (at Month 3), n = 38 and 45 (at Month 6), n = 33 and 35 (at Month 9), and n = 31 and 29 (at Month 12).
Table S4. Change from baseline in CES-D, NDDI-E, and AEP scores; the number of subjects at each time point for the VNS + BMP and BMP groups, respectively were n = 48 and 48 (at baseline), n = 47 and 47 (at Month 3), n = 38 and 45 (at Month 6), n = 33 and 34 (at Month 9), and n = 31 and 29 (at Month 12).
Table S5. Antiepileptic drug (AED) treatment and AED load.
References
- Elliott RE, Morsi A, Kalhorn SP, et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy Behav. 2011;20:57–63. doi: 10.1016/j.yebeh.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol. 2010;9:27–29. doi: 10.1016/S1474-4422(09)70304-7. [DOI] [PubMed] [Google Scholar]
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365:919–926. doi: 10.1056/NEJMra1004418. [DOI] [PubMed] [Google Scholar]
- Elliott RE, Morsi A, Tanweer O, et al. Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS > 10 years. Epilepsy Behav. 2011;20:478–483. doi: 10.1016/j.yebeh.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Cersosimo RO, Bartuluchi M, Fortini S, et al. Vagus nerve stimulation: effectiveness and tolerability in 64 paediatric patients with refractory epilepsies. Epileptic Disord. 2011;13:382–388. doi: 10.1684/epd.2011.0479. [DOI] [PubMed] [Google Scholar]
- Shahwan A, Bailey C, Maxiner W, et al. Vagus nerve stimulation for refractory epilepsy in children: more to VNS than seizure frequency reduction. Epilepsia. 2009;50:1220–1228. doi: 10.1111/j.1528-1167.2008.01940.x. [DOI] [PubMed] [Google Scholar]
- Renfroe JB, Wheless JW. Earlier use of adjunctive vagus nerve stimulation therapy for refractory epilepsy. Neurology. 2002;59:S26–S30. doi: 10.1212/wnl.59.6_suppl_4.s26. [DOI] [PubMed] [Google Scholar]
- Alonso-Vanegas MA, Austria-Velásquez J, López-Gómez M, et al. Chronic intermittent vagal nerve stimulation in the treatment of refractory epilepsy: experience in Mexico with 35 cases. Cir Cir. 2010;78:15–23, 24. [PubMed] [Google Scholar]
- Cersosimo RO, Bartuluchi M, De Los Santos C, et al. Vagus nerve stimulation: effectiveness and tolerability in patients with epileptic encephalopathies. Childs Nerv Syst. 2011;27:787–792. doi: 10.1007/s00381-010-1314-8. [DOI] [PubMed] [Google Scholar]
- Klinkenberg S, Majoie HJ, van der Heijden MM, et al. Vagus nerve stimulation has a positive effect on mood in patients with refractory epilepsy. Clin Neurol Neurosurg. 2012;114:336–340. doi: 10.1016/j.clineuro.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Vickrey BG, Cramer J, et al. Development of the quality of life in epilepsy inventory. Epilepsia. 1995;36:1089–1104. doi: 10.1111/j.1528-1157.1995.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Gilliam FG, Barry JJ, Hermann BP, et al. Rapid detection of major depression in epilepsy: a multicentre study. Lancet Neurol. 2006;5:399–405. doi: 10.1016/S1474-4422(06)70415-X. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; 1976. [Google Scholar]
- Gilliam FG, Fessler AJ, Baker G, et al. Systematic screening allows reduction of adverse antiepileptic drug effects: a randomized trial. Neurology. 2004;62:23–27. doi: 10.1212/wnl.62.1.23. [DOI] [PubMed] [Google Scholar]
- Perucca P, Jacoby A, Marson AG, et al. Adverse antiepileptic drug effects in new-onset seizures: a case–control study. Neurology. 2011;76:273–279. doi: 10.1212/WNL.0b013e318207b073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canevini MP, De Sarro G, Galimberti CA, et al. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia. 2010;51:797–804. doi: 10.1111/j.1528-1167.2010.02520.x. [DOI] [PubMed] [Google Scholar]
- Langfitt JT, Vickrey BG, McDermott MP, et al. Validity and responsiveness of generic preference-based HRQOL instruments in chronic epilepsy. Qual Life Res. 2006;15:899–914. doi: 10.1007/s11136-005-5231-3. [DOI] [PubMed] [Google Scholar]
- Luoni C, Bisulli F, Canevini MP, et al. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia. 2011;52:2181–2191. doi: 10.1111/j.1528-1167.2011.03325.x. [DOI] [PubMed] [Google Scholar]
- Birbeck GL, Hays RD, Cui X, et al. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia. 2002;43:535–538. doi: 10.1046/j.1528-1157.2002.32201.x. [DOI] [PubMed] [Google Scholar]
- McLachlan RS, Sadler M, Pillay N, et al. Quality of life after vagus nerve stimulation for intractable epilepsy: is seizure control the only contributing factor? Eur Neurol. 2003;50:16–19. doi: 10.1159/000070853. [DOI] [PubMed] [Google Scholar]
- Morris GL, 3rd, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53:1731–1735. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article
Parameters of VNS pulse generator for patients included in the VNS + BMP treatment group at Months 3 (n = 45), 6 (n = 41), 9 (n = 33), and 12 (n = 28).
Table S2. Change from baseline to Month 12 in QOLIE-89 scores.
Table S3. Change from baseline in QOLIE-89 subscale scores; the number of subjects at each time point for the VNS + BMP and BMP groups, respectively were n = 48 and 48 (at baseline), n = 47 and 47 (at Month 3), n = 38 and 45 (at Month 6), n = 33 and 35 (at Month 9), and n = 31 and 29 (at Month 12).
Table S4. Change from baseline in CES-D, NDDI-E, and AEP scores; the number of subjects at each time point for the VNS + BMP and BMP groups, respectively were n = 48 and 48 (at baseline), n = 47 and 47 (at Month 3), n = 38 and 45 (at Month 6), n = 33 and 34 (at Month 9), and n = 31 and 29 (at Month 12).
Table S5. Antiepileptic drug (AED) treatment and AED load.