Abstract
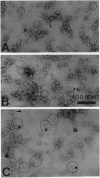
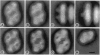
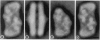
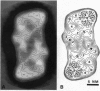
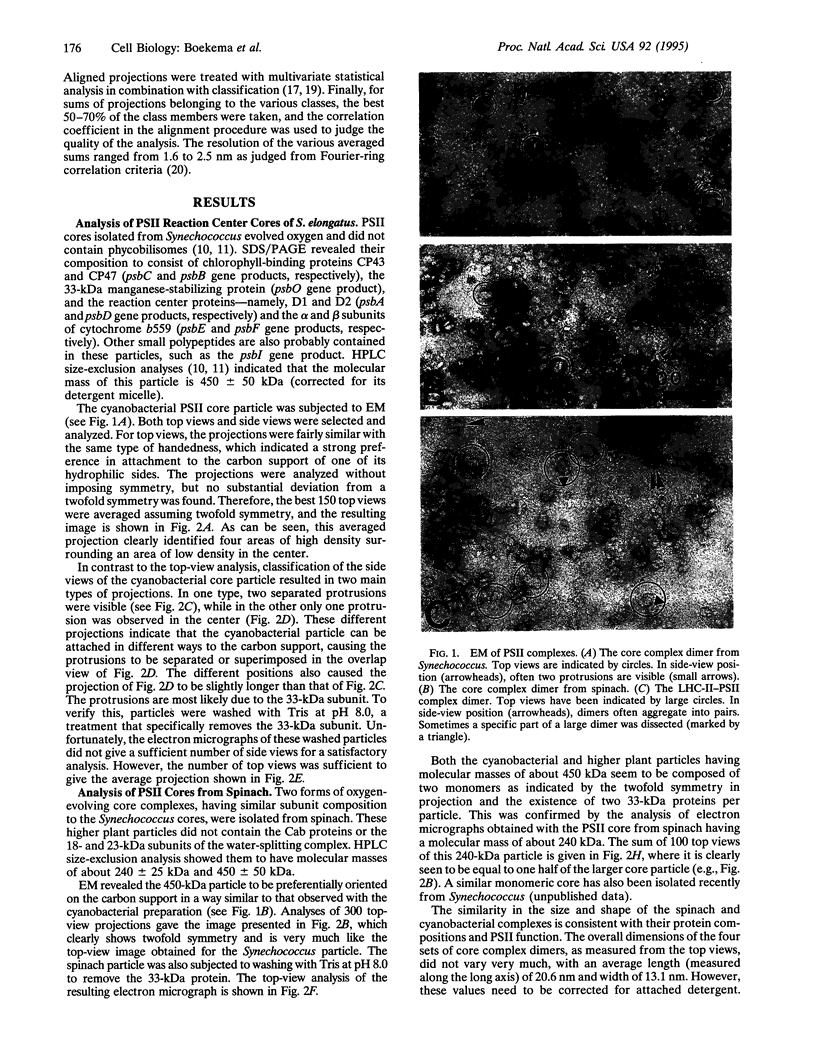
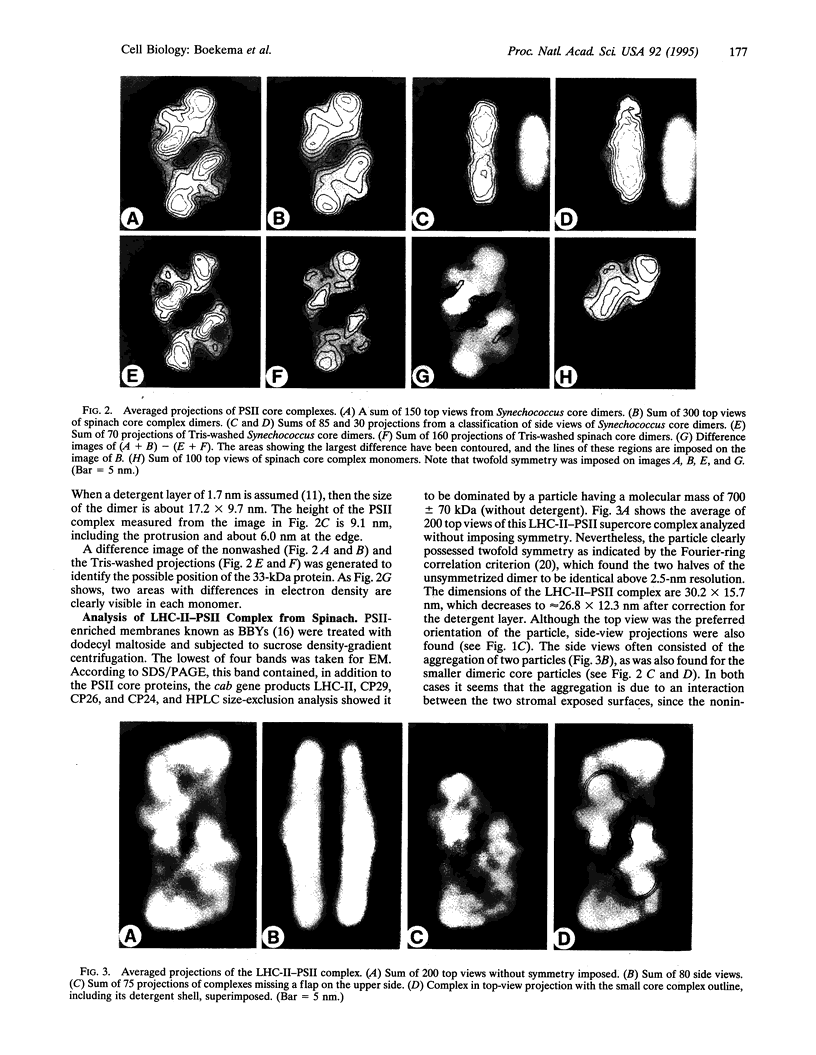
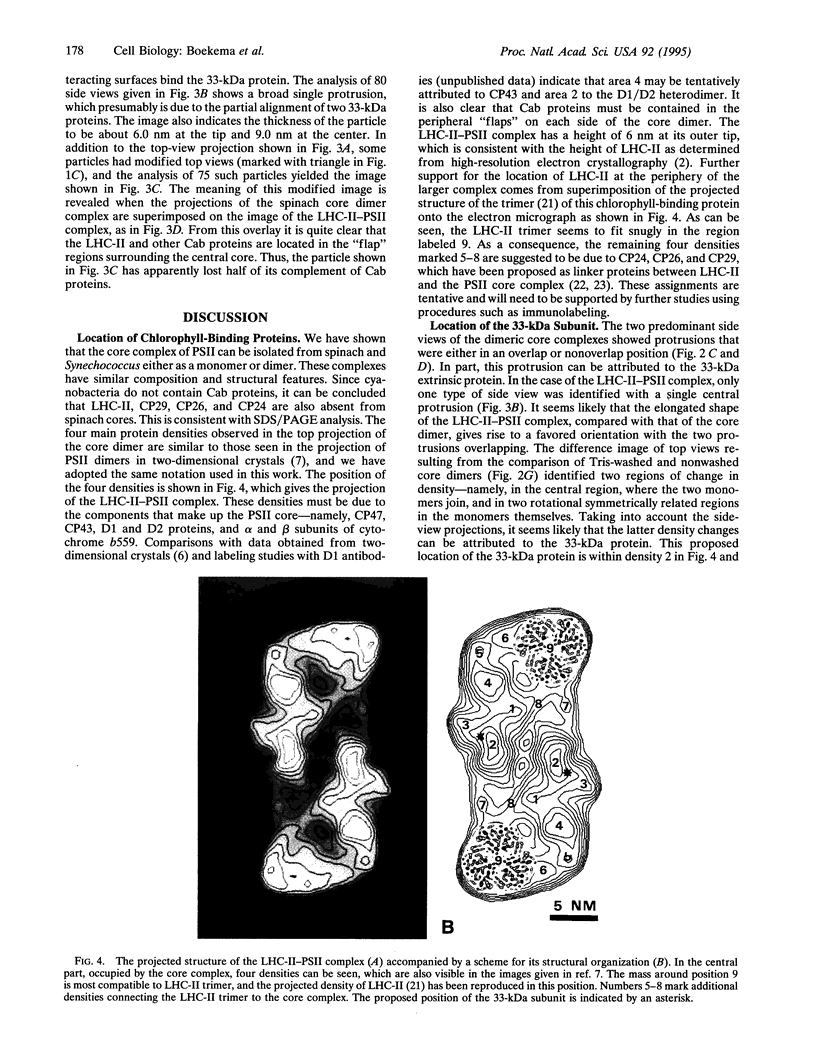
Photosystem II (PSII) complexes, isolated from spinach and the thermophilic cyanobacterium Synechococcus elongatus, were characterized by electron microscopy and single-particle image-averaging analyses. Oxygen-evolving core complexes from spinach and Synechococcus having molecular masses of about 450 kDa and dimensions of approximately 17.2 x 9.7 nm showed twofold symmetry indicative of a dimeric organization. Confirmation of this came from image analysis of oxygen-evolving monomeric cores of PSII isolated from spinach and Synechococcus having a mass of approximately 240 kDa. Washing with Tris at pH 8.0 and analysis of side-view projections indicated the possible position of the 33-kDa extrinsic manganese-stabilizing protein. A larger complex was isolated that contained the light-harvesting complex II (LHC-II) and other chlorophyll a/b-binding proteins, CP29, CP26, and CP24. This LHC-II-PSII complex had a mass of about 700 kDa, and electron microscopy revealed it also to be a dimer having dimensions of about 26.8 and 12.3 nm. From comparison with the dimeric core complex, it was deduced that the latter is located in the center of the larger particle, with additional peripheral regions accommodating the chlorophyll a/b-binding proteins. It is suggested that two LHC-II trimers are present in each dimeric LHC-II-PSII complex and that each trimer is linked to the reaction center core complex by CP24, CP26, and CP29. The results also suggest that PSII may exist as a dimer in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. A., Loehr T. M., Cotton T. M., Simpson D. J., Smith K. M. Spectroscopic analysis of chlorophyll model complexes: methyl ester ClFe(III)pheophorbides. Biochim Biophys Acta. 1989 May 8;974(2):163–179. doi: 10.1016/s0005-2728(89)80369-x. [DOI] [PubMed] [Google Scholar]
- Bassi R., Dainese P. A supramolecular light-harvesting complex from chloroplast photosystem-II membranes. Eur J Biochem. 1992 Feb 15;204(1):317–326. doi: 10.1111/j.1432-1033.1992.tb16640.x. [DOI] [PubMed] [Google Scholar]
- Bioenergetics. Dedicated to Professor E.C. Slater on the occasion of his 75th birthday. Biochim Biophys Acta. 1992 Jan 16;1098(2):131–274. [PubMed] [Google Scholar]
- Boekema E. J., Boonstra A. F., Dekker J. P., Rögner M. Electron microscopic structural analysis of Photosystem I, Photosystem II, and the cytochrome b6/f complex from green plants and cyanobacteria. J Bioenerg Biomembr. 1994 Feb;26(1):17–29. doi: 10.1007/BF00763217. [DOI] [PubMed] [Google Scholar]
- Dekker J. P., Betts S. D., Yocum C. F., Boekema E. J. Characterization by electron microscopy of isolated particles and two-dimensional crystals of the CP47-D1-D2-cytochrome b-559 complex of photosystem II. Biochemistry. 1990 Apr 3;29(13):3220–3225. doi: 10.1021/bi00465a011. [DOI] [PubMed] [Google Scholar]
- Haag E., Irrgang K. D., Boekema E. J., Renger G. Functional and structural analysis of photosystem II core complexes from spinach with high oxygen evolution capacity. Eur J Biochem. 1990 Apr 20;189(1):47–53. doi: 10.1111/j.1432-1033.1990.tb15458.x. [DOI] [PubMed] [Google Scholar]
- Harauz G., Boekema E., van Heel M. Statistical image analysis of electron micrographs of ribosomal subunits. Methods Enzymol. 1988;164:35–49. doi: 10.1016/s0076-6879(88)64033-x. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N., Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994 Feb 17;367(6464):614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Lyon M. K., Marr K. M., Furcinitti P. S. Formation and characterization of two-dimensional crystals of photosystem II. J Struct Biol. 1993 Mar-Apr;110(2):133–140. doi: 10.1006/jsbi.1993.1014. [DOI] [PubMed] [Google Scholar]
- Peter G. F., Thornber J. P. Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J Biol Chem. 1991 Sep 5;266(25):16745–16754. [PubMed] [Google Scholar]
- Santini C., Tidu V., Tognon G., Ghiretti Magaldi A., Bassi R. Three-dimensional structure of the higher-plant photosystem II reaction centre and evidence for its dimeric organization in vivo. Eur J Biochem. 1994 Apr 1;221(1):307–315. doi: 10.1111/j.1432-1033.1994.tb18742.x. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Seibert M., DeWit M., Staehelin L. A. Structural localization of the O2-evolving apparatus to multimeric (tetrameric) particles on the lumenal surface of freeze-etched photosynthetic membranes. J Cell Biol. 1987 Nov;105(5):2257–2265. doi: 10.1083/jcb.105.5.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. N., Kühlbrandt W. High-resolution electron crystallography of light-harvesting chlorophyll a/b-protein complex in three different media. J Mol Biol. 1991 Feb 20;217(4):691–699. doi: 10.1016/0022-2836(91)90526-c. [DOI] [PubMed] [Google Scholar]
- van Heel M., Frank J. Use of multivariate statistics in analysing the images of biological macromolecules. Ultramicroscopy. 1981;6(2):187–194. doi: 10.1016/0304-3991(81)90059-0. [DOI] [PubMed] [Google Scholar]