Abstract
Objective:
Studies suggest that bedtime dosing of an angiotensin-converting enzyme (ACE)-inhibitor or angiotensin receptor blocker shows a more sustained and consistent 24-h antihypertensive profile, including greater night-time blood pressure (BP) reduction. We compared the antihypertensive effects of morning (a.m.) and evening (p.m.) dosing of valsartan on 24-h BP.
Methods:
This 26-week, multicentre, randomized, double-blind study evaluated the efficacy and safety of valsartan 320 mg, dosed a.m. or p.m., versus lisinopril 40 mg (a.m.), a long-acting ACE-inhibitor, in patients with grade 1–2 hypertension and at least one additional cardiovascular risk factor. Patients (n = 1093; BP = 156 ± 11/91 ± 8 mmHg; 62 years, 56% male, 99% white) received (1 : 1 : 1) valsartan 160 mg a.m. or p.m. or lisinopril 20 mg a.m. for 4 weeks, then force-titrated to double the initial dose for 8 weeks. At Week 12, hydrochlorothiazide (HCTZ) 12.5 mg was added for 14 weeks if office BP was more than 140/90 mmHg and/or ambulatory BP more than 130/80 mmHg.
Results:
Mean 24-h ambulatory SBP change from baseline to Weeks 12 and 26 was comparable between valsartan a.m. (–10.6 and –13.3 mmHg) and p.m. (–9.8 and –12.3 mmHg) and lisinopril (–10.7 and –13.7 mmHg). There was no benefit of valsartan p.m. versus a.m. on night-time BP, early morning BP and morning BP surge. Evening dosing also did not improve BP lowering in patients requiring add-on HCTZ or in nondippers at baseline. All treatments were well tolerated.
Conclusion:
Once-daily dosing of valsartan 320 mg results in equally effective 24-h BP efficacy, regardless of dosing time.
Trial Registration:
ClinicalTrials.gov Identifier: NCT00241124.
Keywords: ambulatory blood pressure, circadian blood pressure pattern, hydrochlorothiazide, valsartan
INTRODUCTION
Blood pressure (BP) follows a circadian rhythm [1], with BP levels falling during sleep and increasing in the early morning hours in most individuals [2]. In patients with hypertension, a lack of fall in night-time BP (i.e. nondipping) and/or marked rise or surge in BP during the early morning hours is associated with a higher incidence of stroke and an increased risk for other cardiovascular complications, especially among the elderly [3]. This had led some researchers to recommend the dosing of antihypertensive agents in the evening or bedtime hours in patients with hypertension in order to preserve the normal circadian pattern of BP [4,5]. An ideal antihypertensive agent would be one that retains its efficacy throughout the 24-h period while also effectively restoring the normal circadian BP pattern (e.g. reduction in BP at night).
Several studies with angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme (ACE)-inhibitors, both agents that reduce the renin–angiotensin–aldosterone system (RAAS) activity, have reported improvements in night-time BP and a reduction in the early morning rise in BP with bedtime dosing compared with traditional dosing upon awakening [5–10]. The benefit of night-time dosing might be related to the incomplete 24-h action of a once-daily antihypertensive when given in the morning even though the agent is approved for single daily use. Better BP lowering with night-time dosing, however, has been reported for both short and longer acting ACE-inhibitors and ARBs, and hence it is not a pharmacokinetic effect [4,6–10].
The proposed mechanism for the benefit of night-time dosing of the ACE-inhibitor or ARB is ascribed to more effective RAAS blockade during the sleep and early morning periods [7]. It is also possible that the use of lower doses of the ARB/ACE-inhibitor account for the better effect, and, when a higher dose or its use in combination with a non-RAAS agent (e.g. diuretic, calcium channel blocker) is employed, this benefit of night-time dosing is minimized [11].
The purpose of this study was to evaluate whether the 24-h BP-lowering effects of valsartan, an effective once-a-day ARB [12–14] [starting dose of 160 mg, titrated to the maximum dose (320 mg once daily) with optional titration of hydrochlorothiazide (HCTZ)], is similarly effective, regardless of when it is administered, as either a morning or an evening dose, in patients with hypertension (grade 1–2 hypertension) and additional cardiovascular risk factors. The antihypertensive effects of valsartan were also compared with the long-acting ACE-inhibitor lisinopril to evaluate relative effectiveness on 24-h BP lowering.
MATERIALS AND METHODS
Study design
This was a multicentre, randomized, double-blind, double-dummy, active-controlled, parallel-group study evaluating the efficacy and safety of valsartan 320 mg, administered as either a morning or an evening dose, compared with a morning dose of lisinopril 40 mg in hypertensive patients with additional cardiovascular risk factors. We included lisinopril, a long-acting ACE-inhibitor with an established 24-h BP-lowering efficacy, in the study to serve as the standard for assessing the overall BP-lowering efficacy of valsartan. The study was conducted in 94 centres in five countries (Germany, Spain, France, Italy and the Netherlands).
The study comprised a 1-week washout [for patients on any prior antihypertensive medication(s)], a 2-week single-blind placebo run-in prerandomization period and a 26-week double-blind, active-treatment period. At the end of the prerandomization period, patients who had a 24-h mean ambulatory BP (maBP) more than 130/80 mmHg and one additional cardiovascular risk factor were randomized (1 : 1 : 1) to receive valsartan 160 mg as a morning dose (a.m.), valsartan 160 mg as an evening dose (p.m.) or lisinopril 20 mg as a morning dose for 4 weeks. Following this, the treatment regimens were force-titrated to valsartan 320 mg in the morning, valsartan 320 mg in the evening or lisinopril 40 mg in the morning, respectively, for the subsequent 8 weeks. At Week 12, in patients with uncontrolled BP (mean sitting BP >140/90 mmHg and/or 24-h maBP >130/80 mmHg), open-label HCTZ 12.5 mg (morning dosing) could be added to the treatment regimen at the discretion of the investigator and continued for another 14 weeks. This was performed not only as an additional safety measure to avoid uncontrolled BP in high-risk patients for a prolonged period but also to compare the BP-lowering effects of combination therapy with morning versus evening administration of valsartan.
To maintain blinding, patients were instructed to take one capsule each on awakening and at bedtime for the first 4 weeks, followed by two capsules at the respective dosing times for the subsequent 22 weeks. For ambulatory BP monitoring (ABPM), the patients had to come to the investigator site 1 day before the day of measurement (7 : 00 a.m. to 10 : 00 a.m.) and had to return 25–26 h later to have the devices removed. The study was conducted in accordance with the International Conference on Harmonization–Good Clinical Practice, Declaration of Helsinki, and applicable local regulations. The study received approval from an Institutional Review Board or Ethical Review Committee and all patients provided written informed consent.
Study population
Male and female patients (aged ≥18 years) with mean sitting SBP (msSBP) at least 150 mmHg if untreated and more than 140 mmHg if pretreated (uncontrolled on their present regimen), but with BP not exceeding 160/95 mmHg, were enrolled into the washout and placebo run-in periods. Patients were randomized if they had a 24-h maBP more than 130/80 mmHg and at least one additional cardiovascular risk factor such as age at least 65 years, controlled type 2 diabetes mellitus (T2DM), currently treated hypercholesterolemia, metabolic syndrome, peripheral vascular disease, history of stroke, transient ischemic attack, myocardial infarction (MI), coronary artery bypass surgery (CABS), percutaneous transluminal coronary angioplasty (PCTA) greater than 1 year or left ventricular hypertrophy. The primary exclusion criteria were as follows: patients with msSBP at least 180 mmHg and/or mean sitting DBP (msDBP) at least 110 mmHg at any time during the prerandomization phase, inability to discontinue prior medications safely for a period of 3 weeks, BMI more than 40 kg/m2, untreated hypercholesterolemia, type 1 diabetes mellitus, uncontrolled T2DM, secondary hypertension, unstable angina pectoris or arrhythmia, women of child-bearing potential and history of CABS, PCTA, stroke or MI within 12 months prior to screening visit.
Efficacy variables
Twenty-four hour ABPM devices (Spacelabs, Redmond, Washington, USA) were set to take readings every 15 min during the day and every 30 min at night. Office BP was recorded three times at each visit, and an average of the three readings was recorded for analysis. The primary efficacy analyses were BP control rate, defined as the percentage of patients at Week 26 with a 24-h maBP 130/80 mmHg or less and change from baseline to Week 12 in mean ambulatory SBP (maSBP). The secondary efficacy variables at Weeks 12 and 26 were office mean sitting BP (msBP), daytime (>8 a.m. to ≤10 p.m.) BP, night-time (>12 a.m. to ≤6 a.m.) BP, daytime/night-time BP ratio, early morning BP (6 a.m. to 8 a.m.), morning BP surge (defined as the last 2 h of the morning BP period minus the lowest night-time BP), overall BP variability (average of the individual 24-h standard deviation), maSBP in responders and poor responders [defined as those whose Week 12 BP was uncontrolled (msBP ≥140/90 mmHg and/or maBP ≥130/80 mmHg) and required add-on HCTZ (12.5 mg) therapy], and nondipper (percentage of patients with <10% decline in night-time maSBP compared with daytime maSBP) and dipper patients night-time maSBP.
Safety assessments
Safety assessments included regular monitoring and recording of all adverse events, serious adverse events (SAEs) and prespecified adverse events of particular attention.
Statistical analysis
To demonstrate BP efficacy of valsartan for once-daily dosing, the sample size calculation was based on a noninferiority comparison of the 320 mg valsartan-based treatment strategy to the 40 mg lisinopril-based treatment strategy by a clinically accepted margin (delta) of 4 mmHg. Lisinopril is a long-acting ACE inhibitor with effective 24-h BP lowering with once-daily dosing. A sample size of 264 patients per arm was required to have at least 90% power [standard deviation (SD) = 13 mmHg, α-level for statistical significance of 0.0125 (one-sided) following a Bonferroni adjustment]. All statistical analyses for the primary and secondary endpoints were performed using two-sided tests with a significance level of 5%. For all efficacy variables, the intent-to-treat population was used for the analyses. The safety population consisted of patients who received at least one dose of double-blind study drug and had at least one postbaseline safety assessment. The randomization visit was used as the baseline measurement period for all efficacy analyses. The BP control rates were compared using logistic regression with treatment, sex and statin use at baseline as fixed factors and age as a covariate. Changes in 24-h maSBP from baseline at Weeks 12 and 26 were analysed using analysis of covariance (ANCOVA) with baseline as covariate and considering a noninferiority margin of 4 mmHg. The same ANCOVA model was applied for the analyses of the secondary variables. Descriptive analyses for nondipper rates, daytime/night-time SBP and DBP ratio, early morning BP lowering, morning surge, BP variability and BP response in responders versus poor responders and dippers versus nondippers to therapy at Weeks 12 and 26 were performed.
RESULTS
Patient disposition and baseline characteristics
A total of 1291 patients were enrolled into the placebo run-in phase for 2 weeks, of whom 1093 patients received valsartan a.m. (n = 366), valsartan p.m. (n = 370) or lisinopril as a morning dose (n = 357). Of these, 950 (86.9%) patients completed the study, with the proportion of completers being similar among the three groups. The primary reasons for discontinuation were distributed similarly across the three groups, with adverse events, withdrawal of consent, unsatisfactory therapeutic effect and protocol violations being the most frequently reported. Most patients in the three treatment groups were white (∼99%) and male (55.6%). The mean age was 61.5 years, with 45.5% patients aged at least 65 years. At baseline, the maSBP and maDBP were 143.0 and 84.1 mmHg, respectively. The treatment groups were well balanced at baseline with respect to duration of hypertension, prior antihypertensive treatments, including ACE inhibitors or ARBs, presence of isolated systolic hypertension, cardiovascular risk factors and baseline ambulatory BP measures (Tables 1 and 2).
TABLE 1.
Demographic and baseline characteristics of the patient population at randomization (Day 1)
| Valsartan a.m. n = 359 | Valsartan p.m. n = 367 | Lisinopril n = 356 | |
| Age (years) – mean (SD) | 61.6 (10.59) | 61.2 (10.36) | 61.7 (10.35) |
| ≥65 years – n (%) | 165 (46.0) | 170 (46.3) | 157 (44.1) |
| Male – n (%) | 200 (55.7) | 208 (56.7) | 194 (54.5) |
| Whites – n (%) | 355 (98.9) | 365 (99.5) | 355 (99.7) |
| BMI (kg/m2) – mean (SD) | 28.6 (4.1) | 28.7 (4.2) | 28.6 (4.1) |
| msSBP (mmHg) – mean (SD) | 155.6 (10.4) | 155.7 (10.5) | 155.5 (10.8) |
| msDBP (mmHg) – mean (SD) | 90.9 (8.2) | 90.9 (7.5) | 90.7 (7.6) |
| Duration of hypertension (years) – mean (SD) | 7 (7.3) | 7.6 (7.9) | 7.4 (7.9) |
| Currently treated for hypertension – n (%) | 255 (71) | 265 (72) | 265 (74) |
| Prior ACEI or ARB treatment – n (%) | 182 (50.7) | 189 (51.5) | 198 (55.6) |
| Isolated systolic hypertensiona – n (%) | 123 (34.3) | 128 (34.9) | 125 (35.1) |
| Poor-respondersb – n (%) | 148 (44.2) | 156 (46.3) | 136 (42.2) |
| Nondipper ratec – n (%) | 182 (55.2) | 167 (49.4) | 165 (50.5) |
| Currently smoking – n (%) | 59 (16.4) | 60 (16.3) | 50 (14.0) |
| Cardiovascular risk factors – n (%) | |||
| Controlled T2DM | 96 (26.7) | 103 (28.1) | 98 (27.5) |
| Metabolic syndromed – n (%) | 43 (12.0) | 46 (12.5) | 40 (11.2) |
| Hypercholesterolemia/hypolipidemic treatment | 190 (52.9) | 201 (54.8) | 195 (54.5) |
| MI/PTCA/CABS/stroke | 13 (3.6) | 9 (2.5) | 12 (3.4) |
| Left ventricular hypertrophy | 41 (11.4) | 33 (9.0) | 35 (9.8) |
| Peripheral vascular disease | 2 (0.6) | 1 (0.3) | 4 (1.1) |
ACEI, angiotensin-converting enzyme-inhibitor; ARB, angiotensin receptor blocker; CABS, coronary artery bypass surgery; MI, myocardial infarction; msDBP, mean sitting DBP; msSBP, mean sitting SBP; PTCA, percutaneous transluminal coronary angioplasty; SD, standard deviation; T2DM, type 2 diabetes mellitus.
aIsolated systolic hypertension was defined as msSBP >140 mmHg and msDBP <90 mmHg at baseline (Day 1).
bPoor responders were defined as those patients whose office BP was >140/90 mmHg or ambulatory BP was >130/80 mmHg at Week 12 and were given 12.5 mg HCTZ from Week 12 to Week 26.
cNondipper was defined as <10% decline in night-time maSBP compared with daytime maSBP.
dMetabolic syndrome was defined if a patient met two of the following criteria: plasma glucose ≥6.1 mmol/l (patients with diabetes were disregarded for the metabolic syndrome); BMI >30 kg/m2; serum triglycerides ≥1.7 mmol/l (patients treated either with statins or with fibrates automatically fulfilled this criterion); high-density lipoprotein <1.04 mmol/l for males and <1.29 mmol/l for females.
TABLE 2.
Baseline ambulatory blood pressure measures at randomization (Day 1)
| Valsartan a.m. (n = 330) | Valsartan p.m. (n = 338) | Lisinopril (n = 327) | |
| 24-h maSBP (mmHg) – mean (SD) | 143.4 (11.8) | 142.5 (12.2) | 143.1 (11.6) |
| 24-h maDBP (mmHg) – mean (SD) | 84.1 (8.9) | 83.9 (8.9) | 84.3 (8.0) |
| Daytime maSBP (mmHg) – mean (SD) | 147.5 (12.3) | 147.1 (13.0) | 147.7 (12.3) |
| Daytime maDBP (mmHg) – mean (SD) | 87.5 (9.6) | 87.5 (9.9) | 88.0 (8.9) |
| Night-time maSBP (mmHg) – mean (SD) | 133.9 (15.0) | 132.3 (14.3) | 132.7 (14.2) |
| Night-time maDBP (mmHg) – mean (SD) | 76.8 (9.8) | 76.4 (9.7) | 76.6 (8.7) |
| Day-night maSBP ratio – mean (SD) | 1.11 (0.09) | 1.11 (0.08) | 1.11 (0.09) |
| Day-night maDBP ratio – mean (SD) | 1.15 (0.1) | 1.15 (0.11) | 1.15 (0.1) |
| Early morning maSBP (mmHg) – mean (SD) | 150.1 (14.4) | 147.9 (15.1) | 148.8 (15.3) |
| Early morning maDBP (mmHg) – mean (SD) | 89.2 (10.7) | 88.4 (10.6) | 88.9 (10.7) |
| Morning maSBP surge (mmHg) – mean (SD) | 29.8 (13.7) | 29.0 (13.6) | 29.7 (14.4) |
| Morning maDBP surge (mmHg) – mean (SD) | 23.1 (9.5) | 22.6 (9.7) | 23.1 (10.1) |
| 24-h maSBP variability – mean (SD) | 13.1 (3.9) | 13.4 (3.7) | 13.6 (4.1) |
| 24-h maDBP variability – mean (SD) | 9.9 (2.6) | 9.9 (2.8) | 10.2 (2.9) |
Daytime hours 8 a.m. to 10 p.m.; night-time hours 12 a.m. to 6 a.m.; early morning hours 6 a.m. to 8 a.m.; morning surge defined as the early morning BP minus the lowest night-time BP. maDBP, mean ambulatory DBP; maSBP, mean ambulatory SBP.
Efficacy results
There was no difference in the BP control rate at the end of the study (Week 26) between patients receiving valsartan 320 mg as a morning dose (40.7%) or evening dose (39.7%) or when compared with lisinopril 40 mg (43.3%, P = 0.4989 for lisinopril versus valsartan a.m.) (Table 3). Likewise, the percentage of patients achieving BP control at Week 12 was similar irrespective of the time of dosing of valsartan (36.7% a.m. versus 35.0% p.m., P = 0.6752) or when compared with lisinopril treatment (39.5%, P = 0.4641 versus valsartan a.m.). The change in maSBP from baseline to Week 12 showed no difference between the valsartan a.m. and p.m. groups [−10.6 versus − 9.8, P = 0.4219, 95% confidence interval (CI): _2.30 to 0.96, with no adjustment for multiplicity] and between the valsartan a.m. and lisinopril groups (_10.6 versus _10.7, P = 0.8966, 97.5% CI, i.e. adjusted for multiplicity: −1.76 to 1.98). The CI limits were well within the noninferiority margins of ±4 mmHg. The mean hourly SBP and DBP values were also comparable among the three treatment groups at Week 12, with the circadian BP profile being similar for the morning and evening dosing of valsartan (Fig. 1). In addition, the change from baseline in maSBP and maDBP at the end of the study (Week 26) was also similar between the valsartan a.m. and p.m. dosing groups (Table 3).
TABLE 3.
Ambulatory blood pressure control rates (<130/80 mmHg), change in 24-h mean ambulatory blood pressure, mean office (sitting) blood pressure, daytime mean ambulatory blood pressure, night-time mean ambulatory blood pressure, daytime/night-time mean ambulatory blood pressure ratio, early morning mean ambulatory blood pressure, morning surge mean ambulatory blood pressure, mean ambulatory blood pressure variability, poor responders and responders mean ambulatory blood pressure and nondipper and dipper night-time mean ambulatory blood pressure from baseline at Weeks 12 and 26 (LOCF)
| Value (mean ± SD) | Valsartan a.m. | Valsartan p.m. | Lisinopril | Valsartan a.m. | Valsartan p.m. | Lisinopril |
| Week 12 | Week 26 (LOCF) | |||||
| No. of patients | 316 | 323 | 314 | 327 | 338 | 326 |
| Ambulatory BP control (%) | 36.7 | 35.0 | 39.5 | 40.7 | 39.7 | 43.3 |
| 24-h maSBP (mmHg) | −10.6 ± 11.5 | −9.8 ± 11.2 | −10.7 ± 11.3 | −13.3 ± 12.3 | −12.3 ± 12.5 | −13.7 ± 11.6 |
| 24-h maDBP (mmHg) | −6.2 ± 7.0 | −5.6 ± 6.8 | −6.7 ± 7.4 | −7.4 ± 7.4 | −7.3 ± 7.9 | −7.9 ± 7.7 |
| Office msSBP (mmHg) | −14.7 ± 15.2 | −15.3 ± 13.5 | −15.1 ± 14.1 | −16.2 ± 15.1 | −16.7 ± 14.5 | −16.0 ± 15.5 |
| Office msDBP (mmHg) | −7.0 ± 8.6 | −7.8 ± 8.1 | −7.5 ± 8.6 | −7.8 ± 9.1 | −8.4 ± 9.0 | −8.3 ± 9.2 |
| Daytime maSBP (mmHg) | −10.7 ± 12.4 | −9.5 ± 12.5 | −10.5 ± 12.6 | −13.3 ± 13.1 | −12.4 ± 13.8 | −13.5 ± 13.1 |
| Daytime maDBP (mmHg) | −6.2 ± 7.6 | −5.4 ± 8.1 | −6.6 ± 8.6 | −7.6 ± 8.3 | −7.4 ± 9.0 | −7.9 ± 8.7 |
| Night-time maSBP (mmHg) | −11.2 ± 14.2 | −10.3 ± 13.5 | −11.5 ± 13.1 | −13.7 ± 15.6 | −12.7 ± 14.1 | −14.0 ± 13.2 |
| Night-time maDBP (mmHg) | −6.5 ± 9.1 | −5.9 ± 8.1 | −7.1 ± 8.4 | −7.4 ± 9.2 | −7.6 ± 9.0 | −8.0 ± 8.7 |
| Daytime/night-time maSBP ratio | 0.01 ± 0.09 | 0.02 ± 0.09 | 0.02 ± 0.09 | 0.01 ± 0.1 | 0.01 ± 0.09 | 0.02 ± 0.1 |
| Daytime/night-time maDBP ratio | 0.01 ± 0.11 | 0.02 ± 0.12 | 0.02 ± 0.12 | 0.01 ± 0.12 | 0.02 ± 0.12 | 0.02 ± 0.12 |
| Early morning (600–800 h) maSBP (mmHg) | −11.5 ± 16.6 | −10.4 ± 14.8 | −10.3 ± 16.1 | −14.2 ± 16.8 | −12.1 ± 16.4 | −13.6 ± 16.4 |
| Early morning (600–800 h) maDBP (mmHg) | −6.5 ± 10.8 | −5.8 ± 9.4 | −6.7 ± 11.2 | −7.5 ± 11.2 | −7.3 ± 11.2 | −7.8 ± 11.5 |
| Morning surge maSBP (mmHg) | −0.9 ± 15.3 | −0.8 ± 15.2 | 0.6 ± 15.2 | −0.9 ± 15.8 | −0.3 ± 14.8 | −0.5 ± 16.4 |
| Morning surge maDBP (mmHg) | −0.6 ± 11.2 | −0.5 ± 10.8 | −0.1 ± 11.4 | −0.3 ± 11.2 | −0.6 ± 11.0 | −0.2 ± 11.6 |
| maSBP variability (mmHg) | −0.1 ±± 4.1 | 0.0 ± 4.2 | 0.2 ± 4.3 | 0.2 ± 4.7 | −0.2 ± 4.1 | 0.0 ± 4.5 |
| maDBP variability (mmHg) | 0.0 ± 2.7 | 0.0 ± 3.1 | 0.0 ± 3.1 | 0.1 ± 3.2 | −0.2 ± 3.0 | −0.1 ± 3.2 |
| Poor responders: maSBP | −7.0 ± 12.1 | −5.2 ± 10.3 | −5.0 ± 10.5 | −13.7 ± 13.9 | −14.0 ± 12.6 | −14.8 ± 13.4 |
| Responders: maSBP | −13.8 ± 10.1 | −13.7 ± 10.7 | −14.7 ± 9.4 | −13.4 ± 10.9 | −11.3 ± 12.4 | −13.2 ± 10.0 |
| Nondipper: night-time maSBP | −12.8 ± 14.3 | −12.0 ± 13.9 | −13.7 ± 13.4 | −16.8 ± 15.6 | −14.9 ± 14.1 | −16.4 ± 13.3 |
| Dipper: night-time maSBP | −8.4 ± 11.7 | −8.5 ± 11.8 | −9.1 ± 11.3 | −9.4 ± 12.9 | −10.1 ± 13.8 | −11.9 ± 12.5 |
BP, blood pressure; maDBP, mean ambulatory DBP; maSBP, mean ambulatory SBP; msDBP, mean sitting DBP; msSBP, mean sitting SBP.
FIGURE 1.
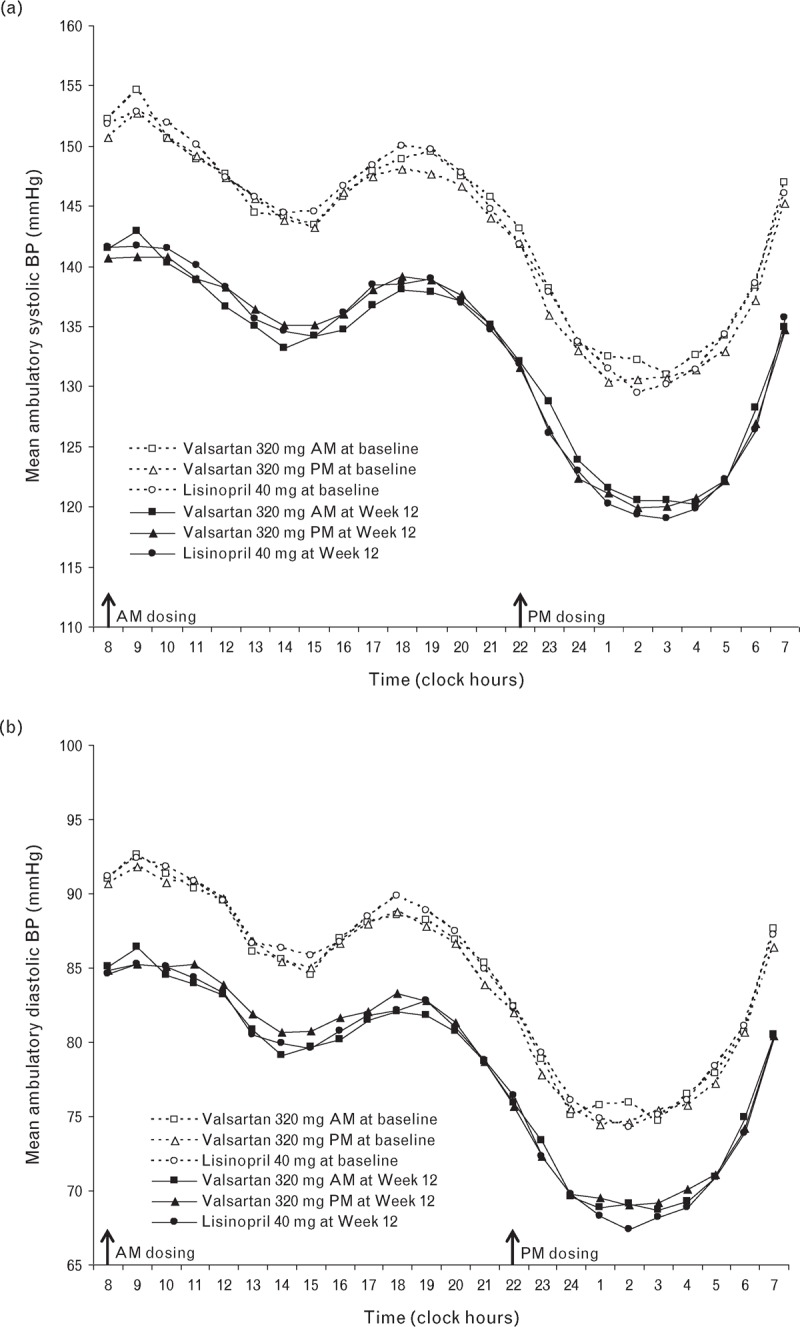
Mean hourly ambulatory BP profile for the three treatment groups at baseline and Week 12 for SBP (a) and DBP (b).
The office BP values in the treatment groups at Weeks 12 and 26 were also not different among the three treatment groups (Table 3). Changes in daytime and night-time BP, daytime/night-time BP ratio, early morning BP, morning BP surge and BP variability were similar across the three treatment groups (Table 3).
The proportion of patients who were uncontrolled on monotherapy and required addition of HCTZ at Week 12 (poor responders) was similar across the treatment groups (valsartan a.m., 44.2%; valsartan p.m., 46.3%; lisinopril, 42.2%). The reduction in maSBP at Week 12 in poor responders was similar between treatments, and the addition of HCTZ resulted in similar additional reductions in BP across the three treatment groups at the end of the study (Week 26) (Table 3). The addition of HCTZ in poor responders resulted in similar BP lowering at the end of the study when compared with those patients who were responders to monotherapy. Poor responders had similar baseline characteristics (age, body weight, duration of hypertension) as responders, but had higher baseline office and ambulatory BP and a higher percentage of patients were categorized as nondippers.
In patients classified as nondippers at baseline (55.2% in valsartan a.m., 49.4% in valsartan p.m. and 50.5% in lisinopril), the reduction in night-time maSBP was similar across the treatments at Week 12 and at the end of the study (Table 3). There was a reduction in the percentage of nondippers due to the effective BP lowering of the three treatments, but the nondipper rates remained similar across the three treatment groups (49.0% in valsartan a.m., 45.7% in valsartan p.m. and 42.5% in lisinopril).
Safety assessments
Both study drugs, valsartan and lisinopril, were well tolerated in the treatment groups. The overall incidence of adverse events was comparable among the valsartan a.m. (40.5%), valsartan p.m. (38.1%) and lisinopril (41.3%) groups (Table 4). Discontinuations due to adverse events were 6.7% in the lisinopril treatment group compared with 4.4 and 4.6% in the valsartan a.m. and p.m. groups, respectively. The most commonly observed adverse event was cough, which was higher in the lisinopril (8.1%) group than in the valsartan a.m. and p.m. groups (2.2 and 3.2%, respectively). Of the 29 cases of cough in the lisinopril group, 20 were suspected to be drug related, whereas in the collective valsartan group, only eight of the 20 cases were thought to be drug related by the treating physician. Nausea was more frequent in the valsartan a.m. (2.2%) group, while bronchitis was more frequent in the valsartan p.m. (4.1%) group. There were a total of 34 SAEs, with 11 (3.3%) in the valsartan a.m., 13 (3.5%) in the valsartan p.m. and 10 (2.8%) in the lisinopril groups. Three patients died during the study: two in the valsartan a.m. group and one in the valsartan p.m. group. None of the fatalities was considered to be related to the study drug by the treating physician.
TABLE 4.
Overall incidence of adverse events (≥2% in any group)
| Incidence, n (%) | |||
| Adverse event | Valsartan a.m. (n = 365) | Valsartan p.m. (n = 370) | Lisinopril (n = 356) |
| Headache | 13 (3.6) | 12 (3.2) | 12 (3.4) |
| Nasopharyngitis | 9 (2.5) | 8 (2.2) | 8 (2.2) |
| Bronchitis | 8 (2.2) | 15 (4.1) | 8 (2.2) |
| Cough | 8 (2.2) | 12 (3.2) | 29 (8.1) |
| Nausea | 8 (2.2) | 1 (0.3) | 1 (0.3) |
| Vertigo | 6 (1.6) | 6 (1.6) | 8 (2.2) |
| Upper abdominal pain | 5 (1.4) | 3 (0.8) | 8 (2.2) |
| Diarrhoea | 4 (1.1) | 4 (1.1) | 8 (2.2) |
| Back pain | 3 (0.8) | 9 (2.4) | 6 (1.7) |
| Total patients with AEs | 148 (40.5) | 141 (38.1) | 147 (41.3) |
AE, adverse event.
DISCUSSION
BP exhibits considerable variation during the day and follows a circadian rhythm, with SBP and DBP falling during sleep and rising rapidly with the start of morning activity [15]. If a once-daily antihypertensive agent is given in the morning, it is important that it maintains BP control throughout the day-time and night-time periods, particularly towards the end of the dosing interval, to cover the critical early morning hours. To better ensure coverage during the night-time and early morning periods, dosing of antihypertensive agents, particularly ARBs, at bedtime has been recommended, with a number of studies providing support [4–9]. Despite the clinical evidence, the pharmacologic rationale for this recommendation with an antihypertensive that has previously demonstrated effective 24-h BP lowering is not clear [16]. We conducted a randomized, multicentre clinical trial in over 1000 patients with hypertension to test this hypothesis. We demonstrated that it did not matter whether valsartan was given in the morning or night-time period, as it resulted in similar BP control and BP lowering in patients with grade 1–2 hypertension with additional cardiovascular risks, even when compared with the long-acting ACE-inhibitor lisinopril.
Over the 24-h period, valsartan demonstrated effective BP reductions, comparable to that produced by lisinopril 40 mg, regardless of whether valsartan was dosed in the morning or at night. This was also true for night-time BP lowering and for limiting the early morning BP rise. Other ABPM measures, including day/night BP ratio, morning BP surge and short-term BP variability, were also not improved by night-time dosing. Antihypertensive agents that maintain BP-lowering efficacy at the end of the dosing period and preserve the normal fall in night-time BP while limiting the early morning rise in BP meet the requirements of an ideal agent for reducing the risk associated with hypertension-related cardiovascular disease [17,18]. On the basis of the findings of our study, valsartan fulfils the requirements of an ideal BP-lowering agent, irrespective of whether it is given in the morning or at night. Furthermore, the BP-lowering efficacy comparing valsartan and lisinopril was similar throughout the 24-h period, suggesting that regardless of the type of RAAS blockade, via ACE inhibition or ARB, a similar 24-h BP-lowering profile is seen that is not differentially affected by circadian effects or time of day administration.
The lack of a nocturnal fall in BP (i.e. nondipper) has been gaining importance as a predictor of cardiovascular risk, with nondippers being susceptible to a higher rate of stroke or MI [19]. Although we demonstrated effective night-time BP lowering with valsartan, irrespective of dosing time, we further evaluated those patients who had a blunted nocturnal BP response (i.e. nondippers) at baseline. Previous studies suggested improvements specifically in night-time BP lowering in nondippers when valsartan was administered at the bedtime rather than on awakening [6]. In our study, the reduction in night-time SBP in nondippers was similar when valsartan was dosed at night or in the morning; likewise, the proportion of patients who converted from nondippers to dippers was equally improved by night-time or morning dosing. Further, the BP-lowering benefit of added HCTZ to poor responders to valsartan therapy was not enhanced in those patients given valsartan at night compared with a morning administration, despite previous studies suggesting a benefit of night-time dosing of the RAAS blocker when combining it with a diuretic or calcium channel blocker [20,21]. Interestingly, at the end of the study, the reductions in BP became similar between the poor responders and the responders in all treatment groups, indicating that morning dosing of HCTZ in the poor responders corrected for the differences in BP lowering with responders and was not influenced by the time of dosing. Overall, in patients who had a blunted nocturnal BP response at baseline or did not adequately respond to monotherapy and required add-on HCTZ, night-time dosing of valsartan did not affect the BP-lowering response compared with morning dosing.
This study confirms the once-daily use of valsartan for BP lowering, irrespective of whether it is administered as a morning or as an evening dose. This is in accordance with earlier reports that showed similar effects of valsartan 160 mg on nocturnal BP and 24-h SBP and DBP, independent of dosing time [13]. The findings, however, are contrary to previous studies with valsartan that reported improvements in BP lowering, particularly with nocturnal BP in nondippers (i.e. increase the proportion of nondippers to dippers and increase daytime to night-time BP ratio) with night-time dosing [4,6,20,21]. The differing findings may be explained by the use of 320 mg valsartan, the maximum approved daily dose, as the BP-lowering effect in nondippers in our study was similar, irrespective of whether valsartan was administered as a morning or as an evening dose.
The pharmacologic rationale for more effective BP lowering with evening dosing of antihypertensive therapy presupposes the inability of morning dosing to effectively lower BP at night and during the early morning period. This cannot be supported, however, as studies that evaluated morning versus evening administration of amlodipine, a long-acting calcium channel blocker (circulating half-life >24 h), reported no benefit of evening versus morning dosing [22]. Perhaps dosing of the antihypertensive agent is important. The question remains, did the use of higher doses of valsartan (160, 320 mg) in this study help to maintain the 24-h BP-lowering effect, whereas the use of lower or suboptimal doses would have exposed a weakness in morning dosing? Support for this comes from a study wherein less-effective BP lowering at the end of the dosing period was reported with either morning or evening administration when using a low dose of a RAAS blocker [23]. Alternatively, the benefit of evening dosing of antihypertensive therapy, particularly for ARBs, may be related to the need for more effective RAAS blockade during the evening period as RAAS activity is increased during the night [9]. For example, a previous study demonstrated more effective night-time control of BP with evening administration of quinapril and was associated with a more sustained decline in ACE activity [24]. In contrast, a study with valsartan, even though it was not in patients with hypertension, demonstrated similar levels of RAAS blockade at the end of the dosing period in patients with heart failure, irrespective of whether valsartan 320 mg was given as a single morning dose or divided into a 160 mg morning and evening dose [25]. Because we did not observe any BP differences with regard to morning or evening administration, it can be concluded that once-daily use of valsartan at 320 mg effectively inhibits the RAAS for the full 24-h period.
The main limitation of this study was the inability to precisely determine when the patients went to bed and when they awoke, as patient diaries were not maintained. In order to correct for imprecise daytime and night-time periods, we used narrower windows for the daytime (8 a.m. to 10 p.m.) and night-time (12 a.m. to 6 a.m.) periods. Further, comparisons between morning and evening dosing of valsartan were limited to the maximum dose (320 mg), and comparisons of lower doses of valsartan may have shown a similar response provided the drugs are given in doses that have a complete 24-h duration of action.
In conclusion, in patients with grade 1–2 hypertension, treatment with valsartan can be given once a day, regardless of the dosing time, to effectively lower BP throughout the 24-h period, including the night-time and early morning periods.
ACKNOWLEDGEMENTS
The authors thank Venkateswara Prasad Gali (Novartis, India) for editorial and writing assistance on this manuscript and Dr Yan Jia, Statistician, Novartis Pharmaceuticals, for additional statistical analyses.
Conflicts of interest
There are no conflicts of interest.
Reviewers’ Summary Evaluations
Reviewer 2
The use of valsartan 320 mg produced similar effects on office, daytime and night-time blood pressures, as well as in nocturnal fall when administered in the morning or at bedtime, in contrast to previous reports suggesting a more pronounced effect when administered at bedtime.
The strength is the large number of patients included, thus minimizing a possible bias due to differences in the conditions under which ambulatory blood pressure was monitored. The main limitation is that assessment was performed only with valsartan at doses of 320 mg, thus providing no information with lower doses of valsartan.
Reviewer 3
The study is well designed and studies whether an ARB given in a dose that has a complete 24-h duration of action has different effects when given in the morning or evening. The results clearly show similar effects as would be expected if plasma concentrations are similar throughout the 24-h interval. This effect cannot however be implied to occur with lower doses of these agents. With doses that do not have a complete 24-h duration of action (Valsartan 160 mg) the results may have been different.
Footnotes
Abbreviations: ABPM, ambulatory blood pressure monitoring; ACE, angiotensin-converting enzyme; ANCOVA, analysis of covariance; ARB, angiotensin receptor blocker; CABS, coronary artery bypass surgery; CI, confidence interval; HCTZ, hydrochlorothiazide; maDBP, mean ambulatory DBP; maSBP, mean ambulatory SBP; MI, myocardial infarction; msDBP, mean sitting DBP; msSBP, mean sitting SBP; PCTA, percutaneous transluminal coronary angioplasty; RAAS, renin–angiotensin–aldosterone system; SAE, serious adverse event; SD, standard deviation; T2DM, type 2 diabetes mellitus
REFERENCES
- 1.Anwar YA, White WB. Chronotherapeutics for cardiovascular disease. Drugs 1998; 55:631–643. [DOI] [PubMed] [Google Scholar]
- 2.Gosse P, Lasserre R, Minifié C, Lemetayer P, Clementy J. Blood pressure surge on rising. J Hypertens 2004; 22:1113–1118. [DOI] [PubMed] [Google Scholar]
- 3.Kario K. Vascular damage in exaggerated morning surge in blood pressure. Hypertension 2007; 49:771–772. [DOI] [PubMed] [Google Scholar]
- 4.Hermida RC, Ayala DE, Calvo C. Optimal timing for antihypertensive dosing: focus on valsartan. Ther Clin Risk Manag 2007; 3:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermida RC, Ayala DE, Fernandez JR, Calvo C. Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 2008; 51:69–76. [DOI] [PubMed] [Google Scholar]
- 6.Hermida RC, Calvo C, Ayala DE, Fernandez JR, Covelo M, Mojon A, López JE. Treatment of nondipper hypertension with bedtime administration of valsartan. J Hypertens 2005; 23:1913–1922. [DOI] [PubMed] [Google Scholar]
- 7.Hermida RC, Ayala DE. Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: improved blood pressure control with bedtime dosing. Hypertension 2009; 54:40–46. [DOI] [PubMed] [Google Scholar]
- 8.Hermida RC, Ayala DE, Fernández JR, Calvo C. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension 2007; 50:715–722. [DOI] [PubMed] [Google Scholar]
- 9.Smolensky MH, Hermida RC, Portaluppi F. Comparison of the efficacy of morning versus evening administration of olmesartan in uncomplicated essential hypertension. Chronobiol Int 2007; 24:171–181. [DOI] [PubMed] [Google Scholar]
- 10.Tofé Povedano S, García De La Villa B. 24-h and night-time blood pressures in type 2 diabetic hypertensive patients following morning or evening administration of olmesartan. J Clin Hypertens (Greenwich) 2009; 11:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzu T, Sakaguchi M, Yokomaku Y, Kume S, Kanasaki M, Isshiki K, et al. Effects of high sodium intake and diuretics on the circadian rhythm of blood pressure in type 2 diabetic patients treated with an angiotensin II receptor blocker. Clin Exp Nephrol 2009; 13:300–306. [DOI] [PubMed] [Google Scholar]
- 12.Lasko BH, Laplante A, Hébert D, Bonnefis-Boyer S. Canadian valsartan study in patients with mild-to-moderate hypertension. Blood Press Monit 2001; 6:91–99. [DOI] [PubMed] [Google Scholar]
- 13.Hermida RC, Calvo C, Ayala DE, Domínguez MJ, Covelo M, Fernández JR, et al. Administration time-dependent effects of valsartan on ambulatory blood pressure in hypertensive subjects. Hypertension 2003; 42:283–290. [DOI] [PubMed] [Google Scholar]
- 14.Neutel J, Weber M, Pool J, Smith D, Fitzsimmons S, Chiang Y-T, et al. Valsartan, a new angiotensin II antagonist: antihypertensive effects over 24 h. Clin Ther 1997; 19:447–458. [DOI] [PubMed] [Google Scholar]
- 15.Smolensky MH, Haus E. Circadian rhythm and clinical medicine with applications to hypertension. Am J Hypertens 2001; 14:280S–290S. [DOI] [PubMed] [Google Scholar]
- 16.Palatini P, Mugellini A, Spagnuolo V, Santonastaso M, Ambrosia GB, Caiazza A, Malacco E. Investigators Group. Comparison of the effects on 24-h ambulatory blood pressure of valsartan and amlodipine, alone or in combination with a low-dose diuretic, in elderly patients with isolated systolic hypertension (Val-syst Study). Blood Press Monit 2004; 9:91–97. [DOI] [PubMed] [Google Scholar]
- 17.White WB. Importance of blood pressure control over a 24-hr period. J Manag Care Pharm 2007; 13 (8 Suppl B):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 19.Staessen JA, Thijs L, Fagard R. Predicting cardiovascular risk using conventional versus ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999; 282:539–546. [DOI] [PubMed] [Google Scholar]
- 20.Hermida RC, Ayala DE, Mojón A, Fontao MJ, Fernández JR. Chronotherapy with valsartan/hydrochlorothiazide combination in essential hypertension: improved sleep-time blood pressure control with bedtime dosing. Chronobiol Int 2011; 28:601–610. [DOI] [PubMed] [Google Scholar]
- 21.Hermida RC, Ayala DE, Mojón A, Smolensky MH, Portaluppi F, Fernández JR. Sleep-time ambulatory blood pressure as a novel therapeutic target for cardiovascular risk reduction. J Hum Hypertens 2014; 28:567–574. [DOI] [PubMed] [Google Scholar]
- 22.Qiu YG, Chen JZ, Zhu JH, Yao XY. Differential effects of morning or evening dosing of amlodipine on circadian blood pressure and heart rate. Cardiovasc Drugs Ther 2003; 17:335–341. [DOI] [PubMed] [Google Scholar]
- 23.Morgan T, Anderson A, Jones E. The effect on 24 h blood pressure control of an angiotensin converting enzyme inhibitor (perindopril) administered in the morning or at night. J Hypertens 1997; 15:205–211. [DOI] [PubMed] [Google Scholar]
- 24.Palatini P, Racioppa A, Raule G, Zaninotto M, Penzo M, Pessina AC. Effect of timing of administration on the plasma ACE inhibitory activity and the antihypertensive effect of quinapril. Clin Pharmacol Ther 1992; 52:378–383. [DOI] [PubMed] [Google Scholar]
- 25.Anand IS, Deswal A, Kereiakes DJ, Purkayastha D, Zappe DH. Comparison of once-daily versus twice-daily dosing of valsartan in patients with chronic stable heart failure. Vasc Health Risk Manag 2010; 6:449–455. [PMC free article] [PubMed] [Google Scholar]


