Abstract
Objective:
The objective of this study is to assess the effect of maternal HIV and Mycobacterium tuberculosis (Mtb) infection on cellular responses to bacille Calmette-Guérin (BCG) immunization.
Design:
A mother–infant cohort study.
Methods:
Samples were collected from mother–infant pairs at delivery. Infants were BCG-vaccinated at 6 weeks of age and a repeat blood sample was collected from infants at 16 weeks of age. BCG-specific T-cell proliferation and intracellular cytokine expression were measured by flow cytometry. Secreted cytokines and chemokines in cell culture supernatants were analysed using a Multiplex assay.
Results:
One hundred and nine (47 HIV-exposed and 62 HIV-unexposed) mother–infants pairs were recruited after delivery and followed longitudinally. At birth, proportions of mycobacteria-specific proliferating T cells were not associated with either in-utero HIV exposure or maternal Mtb sensitization. However, in-utero HIV exposure affected infant-specific T-cell subsets [tumour necrosis factor-alpha (TNF-α) single positive proliferating CD4+ T cells and interferon-gamma (IFN-γ), TNF-α dual-positive CD4+ T cells]. Levels of TNF-α protein in cell culture supernatants were also significantly higher in HIV-exposed infants born to Mtb-sensitized mothers. In the presence of maternal Mtb sensitization, frequencies of maternal and newborn BCG-specific proliferating CD4+ T cells were positively correlated. Following BCG vaccination, there was no demonstrable effect of HIV exposure or maternal Mtb infection on infant BCG-specific T-cell proliferative responses or concentrations of secreted cytokines and chemokines.
Conclusion:
Effects of maternal HIV and Mtb infection on infant immune profiles at birth are transient only, and HIV-exposed, noninfected infants have the same potential to respond to and be protected by BCG vaccination as HIV-unexposed infants.
Keywords: bacille Calmette-Guérin, HIV infection, HIV-exposed, immunogenicity, Mycobacterium tuberculosis infection, uninfected infants, vaccination
Introduction
Many studies have reported that HIV-exposed infants have increased rates of morbidity and mortality compared with infants not exposed to HIV in utero[1–10] including high rates of tuberculosis (TB) early in life, even if they do not acquire HIV infection themselves [11]. A number of factors might contribute to this observation, not least social considerations, including immunological differences, some of which have been shown to persist into childhood. However, their direct impact on clinical outcomes is not understood [12–22].
The developing immune system can be primed in utero by sensitization to maternal infections [23–26]. Published data show that a proportion of infants display cytokine responses to mycobacterial antigens already at birth, prior to apparent exposure to Mycobacterium tuberculosis (Mtb), bacille Calmette-Guérin (BCG) vaccination or environmental mycobacteria [27–29].
We hypothesized that in-utero exposure of infants to HIV and/or mycobacterial antigens might impact their immune responses to BCG vaccination, as considerable heterogeneity has been observed. We conducted a comprehensive analysis of immune responses in paired maternal and infant blood samples at delivery and following infant BCG vaccination with the live attenuated strain of Mycobacterium bovis.
Materials and methods
Study setting
The study was conducted between March 2009 and June 2011 in Khayelitsha, Western Cape, South Africa. The TB incidence in Khayelitsha was 1389 per 100 000 and the HIV prevalence amongst pregnant women registering for antenatal care was 33.1% in 2010 [30]. The comprehensive HIV prevention of mother-to-child transmission (PMTCT) programme at the study site has been described previously [31].
Recruitment of participants and study measures
The study was approved by the Universities of Cape Town (382/2008) and Stellenbosch (N08/10/278) and the National Research Ethics Service, England (07/H0720/178) and local health authorities. Following antenatal provision of study-specific information, eligible postpartum mothers and their infants were recruited and a blood sample collected from mothers and infants within 24 h of delivery. Women with active TB were excluded from the study (see figure, Supplemental Digital Content 1), and full eligibility criteria have been previously reported [31]. All HIV-exposed infants were tested at 4 weeks to exclude HIV infection using standardized HIV PCR (Amplicor HIV-a DNA kit, Version 1.5; Roche, Branchburg, New Jersey, USA) BCG vaccine (unadjuvanted Danish strain 1331; Statens Serum Institut, Copenhagen, Denmark) was administered at 6 weeks of age to HIV-uninfected infants, as per WHO recommendations [32]. A second blood sample was collected from infants at 16 weeks of age to assess immune responses to BCG vaccination.
Data were analysed for four groups of mothers and infants, based on maternal antenatal HIV results with confirmatory testing at delivery (Abbott Determine HIV-1/2; Abbott, Tokyo, Japan) and QuantiFERON-TB Gold In-Tube (QFN: Qiagen, Hilden, Germany) results as a measure of Mtb sensitization.
Laboratory assays
Six-day whole blood Ki67 lymphoproliferation assay
Whole blood (125 μl, diluted 1 : 10 with warm RPMI 1640) was incubated with 5 × 105 CFU/ml BCG vaccine, or 1 μg/ml Staphylococcal enterotoxin B or media alone as positive and negative controls, respectively, in 24-well plates at 37°C, 5% CO2, 80% humidity for 6 days, following a method adapted from Soares et al.[33]. BCG was used to stimulate mycobacterial recall responses in newborns and mothers and to assess the response to BCG vaccination in 16-week-old infants. After 24 h, 150 μl of supernatant was collected and stored at -80°C for cytokine analysis. On day 6, 2.5 μg/ml Brefeldin A, 26 ng/ml Phorbol 12-myristate 13-acetate (PMA) and 2.6 μg/ml Ionomycin (Sigma-Aldrich, St Louis, Missouri, USA) were added for the last 4 h of culture. Cells were harvested with EDTA, red cells lysed and white cells stained with LIVE/DEAD Fixable Violet Dead Cell Stain Kit (Invitrogen, Eugene, Oregon, USA). Fixed white cells were stored at -80°C in 10% DMSO.
Thawed white cells were permeabilized and stained with optimized concentrations of fluorescent-conjugated mAbs (BD Biosciences, San Jose, California, USA); anti-CD8 PerCP-Cy5.5 (RPA-T8), anti-IFN-γ Alexa Fluor 700 (B27), anti-IL-2 FITC (MQ1-17H12), anti-TNF-α PE-Cy7 (MAb11), anti-Ki67 PE (B56), anti-IL-17A Alexa Fluor 647 (N49-853) and anti-CD3 Qdot 605 (UCHT1; Invitrogen). Ki67 is a nuclear protein expressed during active phases of the cell cycle, but absent in resting cells, making it a useful marker of proliferation [34].
PMA and ionomycin were used to assess the capacity of antigen-specific T cells to produce cytokine by restimulating cells for the last 4 h of culture. However, PMA and ionomycin strongly downregulate the surface expression of CD4+ on T cells; therefore, the CD3+ CD8− T-cell subset was used in place of the CD4+ T-cell subset. CD8− T cells are subsequently considered equivalent to CD4+ T cells, consistent with previous publications [33,35].
Multiparameter flow cytometry and controls
A BD LSRFortessa Flow Cytometer (model 649225B7; five lasers, 19 detectors) using FACSDiva software (BD Biosciences) was used to acquire the entire sample. Optimal photomultiplier tube voltages were established for this study. Single-stained antimouse or antirat beads immunoglobulin, κ, beads were used to calculate compensation in FACSDiva. Dead cells were excluded using viability stain.
Multiparameter flow cytometry data analysis
Data were analysed using FlowJo v 9.4.11 (TreeStar, Ashland, Oregon, USA) by a single operator using a predetermined gating strategy and template (see figure, Supplemental Digital Content 2). Boolean gating was used to determine combinations of antigen-specific cytokine-producing cells. Background subtraction was performed using Pestle v 1.7 (VRC, NIH) and display of multiple combination of cells was performed using SPICE v 5.22 [36]. Samples were excluded from analysis if there was no distinct population of live cells or contained less than 1000 live CD3+ T cells.
Multiplex analysis
Customized plates (MILLIPLEX MAP; Millipore, Billerica, Massachusetts, USA) were used to quantify 20 cytokines and chemokines in supernatants collected after 24 h of culture (Supplemental Digital Content 3) following manufacturer's instructions. Beads were analysed on a Bio-Plex array reader (Bio-Rad, Hercules, California, USA). The standard curve for all analytes ranged from 3.2 to 10 000 pg/ml. Unstimulated sample values were subtracted from stimulated sample values. Values less than the lower limit of detection of the assay were assigned a value of 1.6 pg/ml and values greater than the upper limit were assigned a value of 10 000 pg/ml.
Statistical analysis
Statistical analysis was performed using SPSS (version 20), GraphPad Prism (version 5.0a, 2008) and Statistica (v10). A two-way analysis of variance (ANOVA) or Kruskal–Wallis test was used to test the interaction of maternal HIV and QFN status with frequencies of specific T cells. Bonferroni adjustment was applied to correct for multiple comparisons; the corrected P value is reported. A mixed ANOVA was used to analyse paired infant responses. Multiplex data was analysed using a three-way repeated measures ANOVA with mixed models or a generalized estimating equations (GEEs) model, depending on the distribution of data. Spearman's rank order correlation was used to assess the association of maternal and infant immune responses.
Results
Participant characteristics
One hundred and nine mother–infant pairs were enrolled, of which 47 women (43%) were HIV-infected and 62 (57%) HIV-uninfected. Of this cohort, 95 infants (87%) were followed up to 16 weeks of age (Supplemental Digital Content 1). Of these, 39 infants (41%) were HIV-exposed and 56 (59%) were HIV-unexposed. One infant (1%) was found to be HIV-infected at 4 weeks of age and was referred for rapid initiation of antiretroviral treatment (mother–infant pair subsequently excluded from analysis). There was no significant difference in gestation and birth weight between HIV-exposed and unexposed infants (Supplemental Digital Content 4).
The mean CD4+ cell count among HIV-infected women was 474 cells/μl (SD 252); the median viral load was 730 copies/ml [interquartile range (IQR) 357–3925]. Antenatal zidovudine or HAART was received by 41 (89%) women; 30 (65%) women received intrapartum nevirapine or HAART.
Data were analysed in four groups, according to maternal HIV and Mtb sensitization status: HIV–QFN– (n = 27), HIV–QFN+ (n = 35), HIV+QFN– (n = 25) and HIV+ QFN+ (n = 20). One HIV-infected mother had an indeterminate QFN result; maternal and infant data were used when considering the effect of HIV status alone, but not when analysing all four groups.
In-utero HIV exposure affected T-cell subsets in newborn infants; however, overall mycobacteria-specific proliferative T-cell responses were unaffected by HIV-exposure or maternal Mycobacterium tuberculosis sensitization
There were no differences in the frequency of BCG-specific Ki67+CD4+ or CD8+ T cells between the four groups of infants at birth (P = 0.25 and P = 0.73), or in the total expression of intracellular cytokines in these cells between the four groups of infants (see table, Supplemental Digital Content 5). However, some differences were observed amongst T-cell subsets. At birth, HIV-exposed, uninfected infants displayed increased frequencies of BCG-specific TNF-α single-positive Ki67+CD4+ T cells (median = 0.34%, IQR 0.17–1.89), compared with infants born to HIV-uninfected mothers (median = 0.12%, IQR 0.00–0.49), P = 0.04. Exposed infants also showed an increased frequency of BCG-specific Ki67+CD4+ IFN-γ+TNF-α+ T cells compared with unexposed infants (median 0.2%, IQR 0.04–0.99 vs. 0.00%, IQR 0.00–0.08), P = 0.007. Frequencies of TNF-α single-positive CD8+ T cells (0.17, IQR 0.00–0.99) were increased in HIV-exposed infants compared with undetectable frequencies in unexposed infants, P = 0.007. IL-2 single-positive CD8+ T cells were increased in HIV-exposed compared with unexposed infants (0.25%, IQR 0.09–0.96 vs. 0.11%, IQR 0.00–0.89, P = 0.007).
Consistent with the results from flow cytometric data, in-utero HIV exposure and maternal Mtb sensitization were also associated with significant differences in BCG-specific secreted cytokines and chemokines. At birth, HIV-exposed, uninfected infants had significantly higher concentrations of TNF-α in response to BCG antigens than unexposed infants (P = 0.03). When comparing the four groups of infants, maternal HIV infection only had an effect on infant TNF-α and granulocyte macrophage colony-stimulating factor responses in the presence of maternal Mtb sensitization (Fig. 1). A higher IFN-γ concentration to BCG antigens was also seen in HIV-unexposed infants born to Mtb-sensitized mothers than HIV-exposed infants born to Mtb-unsensitized mothers (Fig. 1). Maternal Mtb sensitization was associated with higher sCD40L responses to BCG antigens in HIV-unexposed, but not in HIV-exposed infants (Fig. 1).
Fig. 1.
Concentration of tumour necrosis factor-alpha, GM-CSF, sCD40L and interferon-gamma in vitro in response to bacille Calmette-Guérin antigens in newborn infant samples.
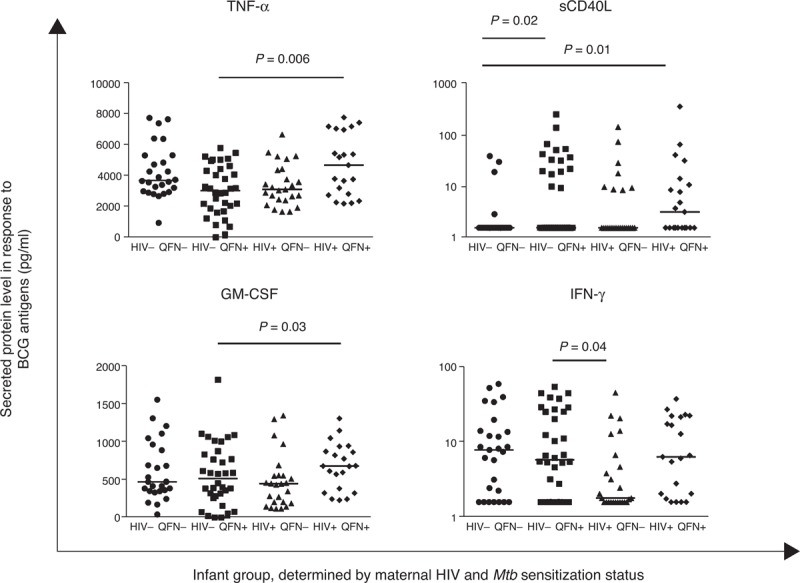
Horizontal lines represent median values. Only comparisons with P < 0.05 are given. IFN-γ and sCD40L data are presented using a log scale.
Positive association between some mycobacteria-specific responses in mothers and their infants at birth
We used identical immunological assays to determine association between maternal HIV infection and Mtb sensitization with maternal responses to BCG antigens at delivery. Amongst HIV-infected women, Mtb-sensitized participants had higher frequencies of BCG-specific CD4+ and CD8+ Ki67+ T cells than Mtb-unsensitized women (Fig. 2). Mtb-sensitized, HIV-infected women also had significantly higher frequencies of BCG-specific proliferating CD4+ T cells expressing IFN-γ, TNF-α and IL-2 than Mtb-unsensitized, HIV-infected women (Fig. 2). In agreement with these findings, the concentration of IFN-γ in BCG-stimulated cell culture supernatants was also significantly higher in Mtb-sensitized than Mtb-unsensitized women (Fig. 3). Interestingly, such differences were not observed amongst HIV-uninfected women. In addition, the frequency of CD8+ T cells expressing TNF-α (P = 0.006), IL-2 (P = 0.03) and IL-17 (P = 0.01) differed significantly between the four maternal groups, Supplemental Digital Content 6.
Fig. 2.
Maternal T-cell responses to BCG antigens.
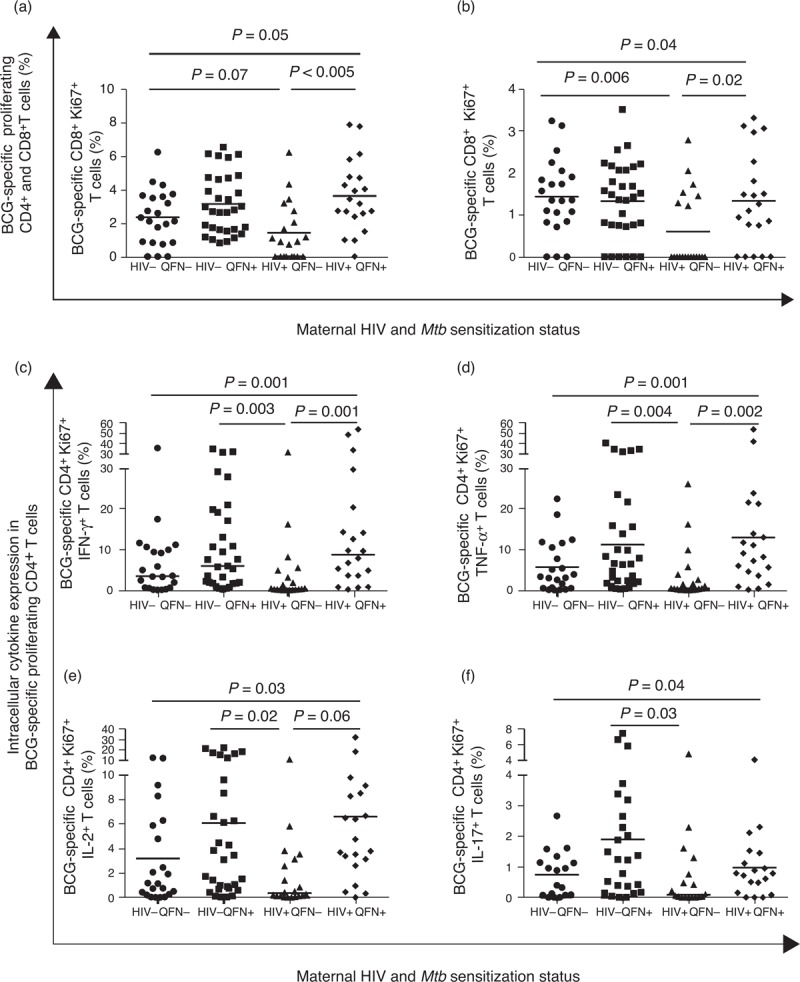
CD4+ (a) and CD8+ (b) T cell proliferation in response to bacille Calmette-Guérin stimulation and frequencies of BCG-specific CD4+ Ki67+ T cells expressing IFN-γ (c), TNF-α (d), IL-2 (e) or IL-17 (f) in women at delivery. Groups of mothers: HIV–QFN– (n = 22, n = 18 for IL-17); HIV–QFN+ (n = 31, n = 25 for IL-17); HIV+QFN− (n = 20, n = 18 for IL-17) and HIV+ QFN+ (n = 20, n = 19 for IL-17). Square root transformed data are presented in (a) and (b); horizontal lines represent mean response. Only comparisons with adjusted P < 0.05 are given.
Fig. 3.
Interferon-gamma, interleukin-1ra and MDC responses to bacille Calmette-Guérin antigens in cell culture supernatants in mothers at delivery.
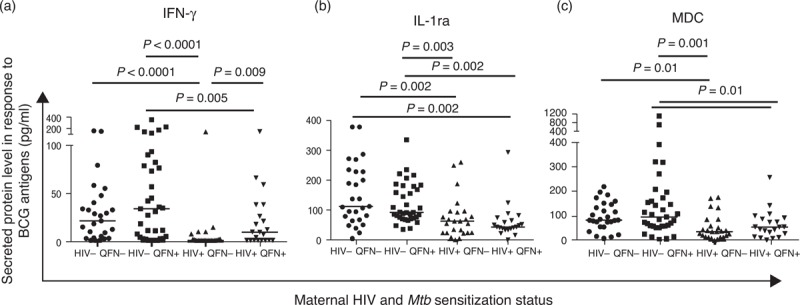
Horizontal lines represent median values. Only comparisons with P < 0.05 are given.
We then correlated the maternal responses with paired infant responses. In the presence of maternal Mtb sensitization, frequencies of maternal and newborn infant BCG-specific proliferating CD4+ T cells were positively correlated, rs = 0.43, P = 0.03; however, no association was observed in the absence of maternal Mtb sensitization, rs = 0.02, P = 0.92. This association did not persist following infant BCG vaccination. Moreover, at birth, the frequency of BCG-specific Ki67+CD4+ and CD8+ IL-2+ T cells was associated with the frequency of the same cells in mothers (Table 1). At 16 weeks, this correlation persisted in the CD8+, but not the CD4+ compartment, and association was strongest in HIV-infected, Mtb-sensitized mothers and their infants, rs = 0.65, P = 0.01. Maternal and newborn infant CD4+Ki67+ TNF-α+ T-cell frequencies were also positively correlated; this association was strongest in the context of maternal Mtb sensitization, but it did not persist at 16 weeks of age. An association of maternal and infant TNF-α concentration was also observed at birth and persisted in 16-week-old infants born to HIV-uninfected, Mtb-sensitized mothers, rs = 0.43, P = 0.01 (Supplemental Digital Content 7). Further strong associations between concentrations of maternal and newborn infant cytokines (IL-1β, IL-1Ra, IL-6 and IL-10) were observed (Supplemental Digital Content 7).
Table 1.
Association between maternal and infant bacille Calmette-Guérin specific CD4+ and CD8+ Ki67+ cytokine+ T-cell frequencies at birth.
| CD4+Ki67+ | CD8+Ki67+ | ||||||||
| Group | IFN-γ+ | IL-2+ | TNF-α + | IL-17+ | IFN-γ+ | IL-2+ | TNF-α+ | IL-17+ | |
| Overall cohort (n = 49) | rs | 0.27 | 0.49 | 0.39 | 0.23 | 0.14 | 0.45 | 0.22 | −0.17 |
| P | 0.07 | <0.0005 | 0.006 | 0.15 | 0.36 | 0.001 | 0.13 | 0.29 | |
| HIV-negative (n = 32) | rs | 0.29 | 0.53 | 0.43 | 0.28 | 0.37 | 0.50 | 0.12 | −0.03 |
| P | 0.11 | 0.002 | 0.02 | 0.16 | 0.04 | 0.004 | 0.66 | 0.88 | |
| HIV-positive (n = 17) | rs | 0.25 | 0.37 | 0.30 | 0.06 | −0.18 | 0.29 | 0.30 | −0.42 |
| P | 0.33 | 0.14 | 0.24 | 0.85 | 0.49 | 0.26 | 0.09 | 0.16 | |
| QFN− (n = 22) | rs | −0.05 | 0.45 | 0.02 | −0.14 | −0.24 | 0.44 | −0.24 | −0.32 |
| P | 0.84 | 0.03 | 0.93 | 0.60 | 0.28 | 0.04 | 0.28 | 0.23 | |
| QFN+ (n = 26) | rs | 0.32 | 0.49 | 0.52 | 0.48 | 0.36 | 0.50 | 0.47 | −0.10 |
| P | 0.11 | 0.01 | 0.007 | 0.02 | 0.07 | 0.01 | 0.02 | 0.65 | |
| HIV–QFN− (n = 14) | rs | −0.05 | 0.56 | 0.20 | −0.08 | 0.02 | 0.58 | −0.12 | −0.22 |
| P | 0.88 | 0.04 | 0.49 | 0.82 | 0.94 | 0.03 | 0.70 | 0.54 | |
| HIV–QFN+ (n = 18) | rs | 0.44 | 0.49 | 0.56 | 0.52 | 0.59 | 0.44 | 0.55 | 0.09 |
| P | 0.07 | 0.04 | 0.02 | 0.31 | 0.01 | 0.06 | 0.02 | 0.74 | |
| HIV+ QFN− (n = 8) | rs | −0.10 | 0.17 | −0.12 | −0.22 | −0.40 | 0.00 | −0.51 | −0.48 |
| P | 0.81 | 0.70 | 0.78 | 0.68 | 0.33 | 1.00 | 0.20 | 0.33 | |
| HIV+ QFN+ (n = 8) | rs | −0.23 | −0.17 | −0.10 | 0.25 | 0.19 | 0.63 | 0.04 | −0.70 |
| P | 0.59 | 0.69 | 0.82 | 0.59 | 0.65 | 0.09 | 0.93 | 0.08 | |
Data were square root transformed and analysed using Spearman's rank order correlation, P < 0 .05 were considered significant and are highlighted in bold. IFN-γ, interferon-gamma; IL, interleukin; TNF-α, tumour necrosis factor-alpha.
Responses to bacille Calmette-Guérin vaccination in infants at 16 weeks of age were robust and independent of maternal HIV or Mycobacterium tuberculosis status
In order to determine the potential impact of infant responses at birth on the subsequent immunogenicity of delayed BCG vaccination at 6 weeks of age, identical assays were repeated in infants at 16 weeks of age.
BCG vaccination induced strong immune responses in all infants: there was a significant increase in the frequency of CD4+Ki67+ T cells in infants following vaccination (mean 36.84%, SD 3.50) compared with prevaccination (mean 3.35%, SD 1.5), P < 0.0005. CD8+Ki67+ T cells also significantly increased (mean prevaccination 2.19%, SD 1.18, mean postvaccination 10.18% SD 1.80), P < 0.0005. Similarly, post-BCG vaccination, there was a highly significant increase in BCG-specific CD4+ and CD8+ Ki67+ IFN-γ+, TNF-α+, IL-2+ and IL-17+ T cells, P < 0.0005 (P = 0.003 for CD8+ IL-17+ T cells). The expansion of these cells was similar for all infants, with no statistically significant differences between groups of infants (Supplemental Digital Content 8).
At 16 weeks of age, all infants displayed similar frequencies of BCG-specific CD4+ and CD8+ proliferating T cells, irrespective of maternal HIV and Mtb infection status (Fig. 4). Furthermore, there was no effect of maternal HIV infection or Mtb sensitization on the frequency of BCG-specific proliferating CD4+ T cells expressing intracellular IFN-γ, TNF-α, IL-2 or IL-17, Fig. 4, or CD8+ T cells expressing intracellular IFN-γ (P = 0.77), TNF-α (P = 0.83), IL-2 (P = 0.37) or IL-17 (P = 0.20), see figure, Supplemental Digital Content 9.
Fig. 4.
Frequency of bacille Calmette-Guérin specific Ki67+ CD4+ and CD8+ T cells following infant BCG vaccination.
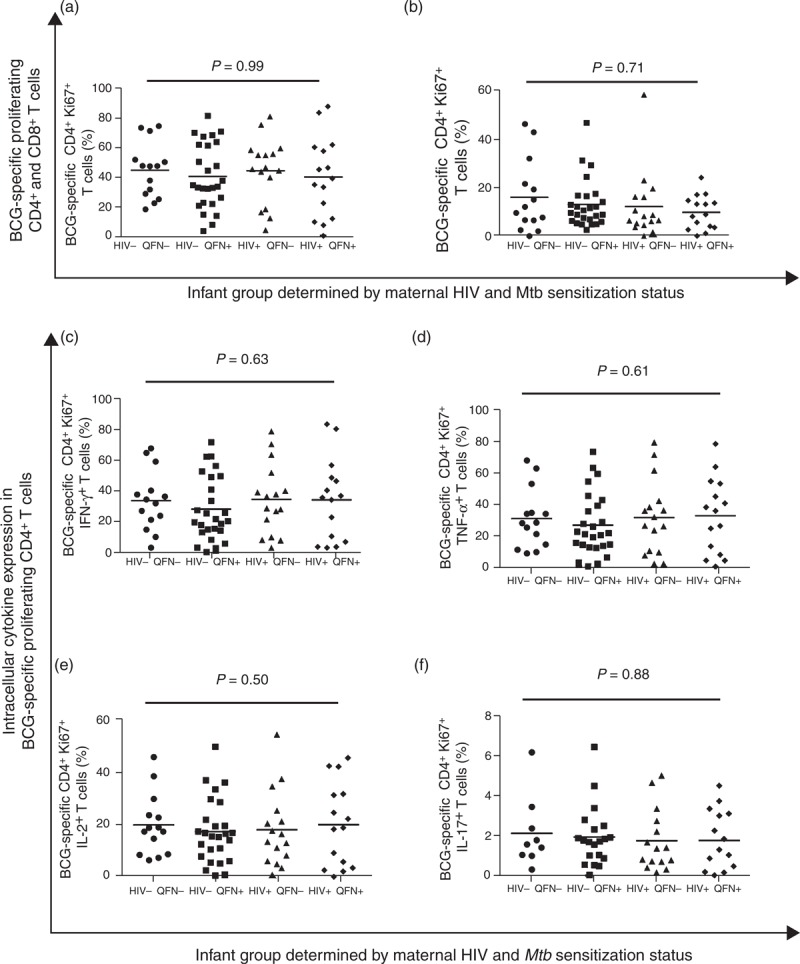
BCG-specific proliferating CD4+ T cells (a), and CD8+ T cells (b). Frequencies of BCG-specific CD4+ Ki67+ T cells expressing IFN-γ +(c), TNF-α (d), IL-2 (e) or IL-17 (f). Horizontal lines represent median values. Only comparisons with P < 0.05 are given.
We examined the quality of the CD4+ proliferative response to BCG antigens by examining distinct functional populations. The distribution of these cells were similar amongst infants, irrespective of maternal HIV (P = 0.26) or Mtb sensitization status (P = 0.76), Supplemental Digital Content 10. BCG-specific IL-2+ CD8+ T cells contributed a very small proportion of the overall response; however, the frequency was significantly different between the four groups of infants, P = 0.005. HIV-unexposed infants had higher frequencies of these cells (median = 0.18%, IQR 0.09–0.73) than HIV-exposed infants, (median = 0.04%, IQR 0.00–0.19, P = 0.002). Infants born to Mtb-sensitized mothers (median = 0.18%, IQR 0.03–0.70) also had an increased frequency of these cells compared with infants born to unsensitized mothers (median = 0.05%, IQR 0.00–0.16), P = 0.02.
Following BCG vaccination at 6 weeks of age, there were no differences in the cytokines or chemokines detectable in cell culture supernatants amongst any group of infants, and there was no significant effect of maternal HIV or Mtb sensitization alone or in combination (data not shown).
Discussion
Our study is the first to investigate the effects of both maternal HIV infection and Mtb sensitization on BCG-induced immune responses in mother–infant pairs. Despite the differences in T cell responses between HIV-infected and HIV-uninfected mothers and some differences in their infants at birth, immune responses following BCG vaccination were strikingly similar amongst all infants by 16 weeks of age. This implies that HIV-exposed and unexposed infants have the same potential to respond to BCG immunization at 6 weeks of age, irrespective of maternal Mtb sensitization, and are as likely to benefit from vaccination. Given the high risk of Mtb exposure early in life in HIV-exposed infants, this is an important finding [11,37].
There is compelling evidence that foetal HIV-exposure can ‘prime’ the developing immune system resulting in a more activated and mature immunophenotype [13–16,19,38–40]. It has been postulated that HIV-exposed, uninfected infants may therefore be more responsive to unrelated antigens [27]. A previously unexplored hypothesis is that sensitization to mycobacteria in utero might ‘prime’ the immune system such that infants have recall responses to mycobacterial antigens after birth. In mice, it has been shown that Mtb can prime the foetal immune system, but this had not previously been explored in humans [41].
In our cohort, we did not observe any effect of HIV exposure or maternal Mtb sensitization on T-cell proliferation or total intracellular expression of cytokine in response to BCG antigens in newborn infants. However, there were some differences in frequencies of subsets of proliferating T cells expressing TNF-α IFN-γ, IL-2 in various combinations. These results were corroborated by results of secreted cytokines. It seems therefore that exposure to HIV in utero may prime specific aspects of the response to mycobacterial antigens at birth and that maternal Mtb sensitization can modulate this to some extent. The underlying mechanisms for this are not clear but could include HIV or Mtb antigen, and maternal cells or antigen-loaded microvesicles might traverse the placental barrier; alternatively, maternal cytokines or chemokines may cross the placenta and influence the immunological milieu of the developing foetus. In support of this, we observed correlations between maternal and infant cellular and cytokine responses to mycobacterial antigens at birth, a few of which persisted to 16 weeks of age.
In addition to infant responses, we measured paired maternal mycobacterial responses in order to explore possible reasons for the differences in infant immune responses at birth. We showed that HIV-infected mothers demonstrate significant differences in responses to BCG antigens dependent on prior Mtb sensitization; this effect was not observed amongst HIV-uninfected women, which was somewhat surprising. A potential explanation is that memory responses to BCG are severely impaired in HIV-infected women, but boosting occurs though cross-priming of BCG memory cells by Mtb[42]. It has been established that central memory T-cell responses to childhood vaccines are lost early in the course of adult HIV infection and remain severely impaired [43,44]. In contrast, vaccine recall responses are detectable for many years in HIV-uninfected individuals [44,45]. We speculate that in HIV-uninfected women, the pool of memory cells induced by childhood BCG vaccination is maintained and cross-priming by Mtb does not increase this population of BCG memory cells further. Experiments analysing the differential responses between BCG and Mtb-specific antigens would be required to investigate this hypothesis.
Participating HIV-infected women were more likely to reside in an informal housing structure than HIV-uninfected women. This alludes to additional socio-economic disadvantage in this group and suggests that there are likely to be unmeasured differences between the groups of women, for example in diet, maternal microbiome and microbial exposure that might act as potential confounders. Most relevant is Mtb exposure; we used the QFN test to determine Mtb sensitization and repeated clinical screening of mothers and infants to exclude TB disease. However, given the factors discussed, the intensity of exposure might be different in the different groups.
An important strength of our study was the measurement of infant responses to mycobacterial antigen pre and post-BCG vaccination in order to ascertain whether differentially affected immune responses at birth affect vaccine immunogenicity. Our data show that BCG vaccination is associated with a substantial increase in BCG-specific T-cell proliferation and intracellular cytokine expression, independent of HIV exposure and maternal Mtb sensitization. There are few studies that have examined antigen-specific responses pre and post-BCG vaccination in infants exposed to HIV in utero and none that have addressed whether maternal Mtb infection can additionally alter this response. In a different study setting, using different methodology, Van Rie et al. [27] found that the pattern of change in secreted IFN-γ response to BCG vaccination was altered in some, but not all, HIV-exposed, uninfected infants, in contrast to our own results.
Following BCG vaccination, we found no difference in the frequency of proliferating T cells, the expression of intracellular cytokines or the concentration of secreted cytokines between infants. However, HIV-exposed, uninfected infants and infants born to Mtb-sensitized mothers had significantly higher frequencies of BCG-specific IL-2 single-positive CD8+ T cells than HIV-unexposed infants. Although this small subset of CD8+ T cells may not have any relevance for the protection afforded by the BCG vaccine, these cells may reflect infant antigenic CD8+ ‘memory’. Interestingly, an association between maternal and infant CD8+ IL-2+ T cells was still observed at 16 weeks postdelivery, particularly amongst infants born to Mtb-sensitized mothers.
Mansoor et al.[46] also reported comparable BCG immunogenicity in HIV-exposed and unexposed infants. In contrast to the short-term stimulation assay used in their laboratory, we utilized a 6-day proliferation assay to assess central memory responses, which are thought to be critical for long-term vaccine-induced protection [47]. Our data are also consistent with a study from Uganda, which showed no difference in secreted cytokines in response to mycobacterial antigens amongst BCG-vaccinated infants, irrespective of maternal HIV status [48]. Another study from Malawi found a tendency towards reduced proliferative capacity in response to purified protein derivative, but similar responses to BCG antigens in HIV-exposed, uninfected infants compared with HIV-unexposed infants [14]. Mazzola et al. [49], however, reported a higher proportion of proliferating CD4+ T cells and lower proportion of γδ+ cells in HIV-exposed infants than unexposed infants. Unlike our study, none of these studies also assessed the impact of maternal Mtb sensitization on infant BCG responses.
We have previously reported that concentrations of specific antibody are influenced by in-utero HIV-exposure, but that antibody responses to non-BCG vaccines were unaffected [31]. Our findings of cellular immune responses to BCG vaccination reported here mirror these observations.
A limitation of our study was enrolment of modest numbers of mother–infant pairs at a single centre. Enrolment was consecutive and representative of the women delivering at the community health facility, but the cohort may not be representative of other areas of the resource-poor world. We were unable to correlate BCG-induced responses with protection against TB, which would require very large cohorts, making the detailed immunological studies challenging.
In summary, despite the effect of maternal HIV and Mtb sensitization on maternal mycobacteria-specific responses and infant responses at birth, our data suggest that HIV-exposed infants have the same potential to respond to BCG immunization administered at 6 weeks of age as HIV-unexposed infants and are therefore as likely to benefit from this vaccine.
Acknowledgements
This work was supported by the European Society for Pediatric Infectious Diseases (C.E.J.), the Thrasher Research Foundation (C.E.J.), British Society of Infection (C.E.J.). B.K. receives support from the Wellcome Trust (GR 077273), Medical Research Council (MR/K007602/1; MR/K011944/1; MC_UP_A900/115) and the National Institute for Health Research, UK. R.J.W. receives support from the Wellcome Trust (084323, 088316), Medical Research Council (U1175.02.0002.00014), European Union (FP7-PEOPLE-2011-IRSES and FP7 HEALTH-F3–2012–305578) and from EDCTP (IP.07.32080.002).
C.E.J., B.K., A.C.H., R.J.W., T.J.S. did the conception or design of the work. C.E.J., B.K., A.C.H., N.G.T.-C., M.K., N.N.C. did the acquisition, analysis or interpretation of data. C.E.J., B.K., A.C.H., R.J.W., T.J.S., N.G.T.-C., M.K., N.N.C. did the drafting of the manuscript or revising it critically it for important intellectual content. C.E.J., B.K., A.C.H., R.J.W., T.J.S., N.G.T.-C., M.K., N.N.C. provided the final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: C.E.J., B.K., A.C.H., R.J.W., T.J.S., N.G.T.-C., M.K., N.N.C.
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Material
References
- 1.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J 2007; 26:519–526. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro RL, Lockman S, Kim S, Smeaton L, Rahkola JT, Thior I, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis 2007; 196:562–569. [DOI] [PubMed] [Google Scholar]
- 3.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr 2006; 41:504–508. [DOI] [PubMed] [Google Scholar]
- 4.Nakiyingi JS, Bracher M, Whitworth JA, Ruberantwari A, Busingye J, Mbulaiteye SM, et al. Child survival in relation to mother's HIV infection and survival: evidence from a Ugandan cohort study. AIDS 2003; 17:1827–1834. [DOI] [PubMed] [Google Scholar]
- 5.Newell M-L, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–1243. [DOI] [PubMed] [Google Scholar]
- 6.Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J 2011; 30:45–51. [DOI] [PubMed] [Google Scholar]
- 7.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet 2007; 369:1440–1451. [DOI] [PubMed] [Google Scholar]
- 8.Otieno RO, Ouma C, Ong’echa JM, Keller CC, Were T, Waindi EN, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS 2006; 20:275–280. [DOI] [PubMed] [Google Scholar]
- 9.Epalza C, Goetghebuer T, Hainaut M, Prayez F, Barlow P, Dediste A, et al. High incidence of invasive group B streptococcal infections in HIV-exposed uninfected infants. Pediatrics 2010; 126:e631–e638. [DOI] [PubMed] [Google Scholar]
- 10.Torre P, Zeldow B, Hoffman HJ, Buchanan A, Siberry GK, Rice M, et al. Hearing loss in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents. Pediatr Infect Dis J 2012; 31:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhi SA, Nachman S, Violari A, Kim S, Cotton MF, Bobat R, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med 2011; 365:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schramm DB, Kuhn L, Gray GE, Tiemessen CT. In vivo effects of HIV-1 exposure in the presence and absence of single-dose nevirapine on cellular plasma activation markers of infants born to HIV-1-seropositive mothers. J Acquir Immune Defic Syndr 2006; 42:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 2000; 96:3866–3871. [PubMed] [Google Scholar]
- 14.Miles DJC, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guérin (BCG) vaccine in HIV-uninfected infants. Immunology 2010; 129:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen SD, Jeppesen DL, Kolte L, Clark DR, Sørensen TU, Dreves AM, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood 2001; 98:398–404. [DOI] [PubMed] [Google Scholar]
- 16.Rich KC, Siegel JN, Jennings C, Rydman RJ, Landay AL. Function and phenotype of immature CD4+ lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol 1997; 4:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Embree J, Bwayo J, Nagelkerke N, Njenga S, Nyange P, Ndinya-Achola J, et al. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. Pediatr Infect Dis J 2001; 20:397–403. [DOI] [PubMed] [Google Scholar]
- 18.Borges-Almeida E, Milanez HM, Vilela MMS, Cunha FG, Abramczuk BM, Reis-Alves SC, et al. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed noninfected newborns. BMC Infect Dis 2011; 11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono E, Nunes dos Santos AM, de Menezes Succi RC, Machado DM, de Angelis DSA, Salomão R, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res 2008; 41:700–708. [DOI] [PubMed] [Google Scholar]
- 20.Velilla PA, Montoya CJ, Hoyos A, Moreno ME, Chougnet C, Rugeles MT. Effect of intrauterine HIV-1 exposure on the frequency and function of uninfected newborns’ dendritic cells. Clin Immunol 2008; 126:243–250. [DOI] [PubMed] [Google Scholar]
- 21.Chougnet C, Kovacs A, Baker R, Mueller BU, Luban NL, Liewehr DJ, et al. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis 2000; 181:1590–1597. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn L, Coutsoudis A, Moodley D, Mngqundaniso N, Trabattoni D, Shearer GM, et al. Interferon-gamma and interleukin-10 production among HIV-1-infected and uninfected infants of HIV-1-infected mothers. Pediatr Res 2001; 50:412–416. [DOI] [PubMed] [Google Scholar]
- 23.Adegnika AA, Köhler C, Agnandji ST, Chai SK, Labuda L, Breitling LP, et al. Pregnancy-associated malaria affects toll-like receptor ligand-induced cytokine responses in cord blood. J Infect Dis 2008; 198:928–936. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, et al. Helminth- and Bacillus Calmette-Guérin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol 1999; 162:6843–6848. [PubMed] [Google Scholar]
- 25.Malhotra I, Ouma J, Wamachi A, Kioko J, Mungai P, Omollo A, et al. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J Clin Invest 1997; 99:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis 2012; 12:330–340. [DOI] [PubMed] [Google Scholar]
- 27.Van Rie A, Madhi SA, Heera JR, Meddows-Taylor S, Wendelboe AM, Anthony F, et al. Gamma interferon production in response to Mycobacterium bovis BCG and Mycobacterium tuberculosis antigens in infants born to human immunodeficiency virus-infected mothers. Clin Vaccine Immunol 2006; 13:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kampmann B, Tena GN, Mzazi S, Eley B, Young DB, Levin M. Novel human in vitro system for evaluating antimycobacterial vaccines. Infect Immun 2004; 72:6401–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Biggelaar AHJ, Prescott SL, Roponen M, Nadal-Sims MA, Devitt CJ, Phuanukoonnon S, et al. Neonatal innate cytokine responses to BCG controlling T-cell development vary between populations. J Allergy Clin Immunol 2009; 124:544.e2–550.e2. [DOI] [PubMed] [Google Scholar]
- 30.City of Cape Town Department of Health. City health HIV, AIDS, STI and TB plan 2012/2013. Available from: http://www.capetown.gov.za/en/IDP/Documents/Statutory%20compliance%20plans%20201213/AnnexureI_HIV_Aids_STI_TB_Plan_2012_2013.pdf [accessed 28 November 2014]. [Google Scholar]
- 31.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 2011; 305:576–584. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Revised BCG vaccination guidelines for infants at risk for HIV infection. Wkly Epidemiol Rec 2007; 82:193–196. [PubMed] [Google Scholar]
- 33.Soares A, Govender L, Hughes J, Mavakla W, De Kock M, Barnard C, et al. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods 2010; 362:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000; 182:311–322. [DOI] [PubMed] [Google Scholar]
- 35.Kemp K, Bruunsgaard H. Identification of IFN-gamma-producing CD4+ T cells following PMA stimulation. J Interferon Cytokine Res 2001; 21:503–506. [DOI] [PubMed] [Google Scholar]
- 36.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of postcytometric complex multivariate datasets. Cytometry A 2011; 79:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotton MF, Schaaf HS, Lottering G, Weber HL, Coetzee J, Nachman S, et al. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis 2008; 12:225–227. [PubMed] [Google Scholar]
- 38.Schramm DB, Meddows-Taylor S, Gray GE, Kuhn L, Tiemessen CT. Low maternal viral loads and reduced granulocyte-macrophage colony-stimulating factor levels characterize exposed, uninfected infants who develop protective human immunodeficiency virus type 1-specific responses. Clin Vaccine Immunol 2007; 14:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Economides A, Schmid I, Anisman-Posner DJ, Plaeger S, Bryson YJ, Uittenbogaart CH. Apoptosis in cord blood T lymphocytes from infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol 1998; 5:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resino S, Seoane E, Gutiérrez MDG, León JA, Muñoz-Fernández MA. CD4(+) T-cell immunodeficiency is more dependent on immune activation than viral load in HIV-infected children on highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2006; 42:269–276. [DOI] [PubMed] [Google Scholar]
- 41.Rahman MJ, Dégano IR, Singh M, Fernández C. Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model. PLoS One 2010; 5:e9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weir RE, Black GF, Nazareth B, Floyd S, Stenson S, Stanley C, et al. The influence of previous exposure to environmental mycobacteria on the interferon-gamma response to bacille Calmette-Guérin vaccination in southern England and northern Malawi. Clin Exp Immunol 2006; 146:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puissant-Lubrano B, Combadiere B, Duffy D, Wincker N, Frachette M-J, Ait-Mohand H, et al. Influence of antigen exposure on the loss of long-term memory to childhood vaccines in HIV-infected patients. Vaccine 2009; 27:3576–3583. [DOI] [PubMed] [Google Scholar]
- 44.Elrefaei M, McElroy MD, Preas CP, Hoh R, Deeks S, Martin J, et al. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol 2004; 173:2184–2189. [DOI] [PubMed] [Google Scholar]
- 45.Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, Bricaire F, et al. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J Exp Med 2004; 199:1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mansoor N, Scriba TJ, De Kock M, Tameris M, Abel B, Keyser A, et al. HIV-1 infection in infants severely impairs the immune response induced by Bacille Calmette-Guérin vaccine. J Infect Dis 2009; 199:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanekom WA, Dockrell HM, Ottenhoff THM, Doherty TM, Fletcher H, Mcshane H, et al. Immunological outcomes of new tuberculosis vaccine trials: WHO panel recommendations. PLoS Med 2008; 5:e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elliott AM, Mawa PA, Webb EL, Nampijja M, Lyadda N, Bukusuba J, et al. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine 2010; 29:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzola TN, da Silva MTN, Abramczuk BM, Moreno YMF, Lima SCBS, Zorzeto TQ, et al. Impaired Bacillus Calmette-Guérin cellular immune response in HIV-exposed, uninfected infants. AIDS 2011; 25:2079–2087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


