Abstract
This study investigates the mechanism of protein particle formation during ultrafiltration/diafiltration (UF/DF), finding that agitation drives particle formation by promoting protein-interface adsorption and desorption. Low conductivity and the presence of surfactant reduced the level of particle formation in small-scale stirring studies, and the same trends were observed in pumping and UF/DF. Polysorbate 80 (PS80) and hydroxypropyl-β-cyclodextrin (HPβCD) reduced particle formation in UF/DF by factors of 15 and 4, respectively. Measurements of conformational stability, colloidal stability, and surface tension demonstrated that PS80 protects against particle formation by preventing protein-interface adsorption, low conductivity improves the colloidal stability of the protein, and the mechanism of action of HPβCD remains unclear. This work demonstrates that interfacial adsorption–desorption of the protein during UF/DF is the principal cause of particle formation, that the level of surfactant-free particle formation depends on the colloidal stability of the protein, and that the inclusion of surfactant greatly reduces in-process particle formation during UF/DF.
Keywords: monoclonal antibody, protein aggregation, stability, surfactants, cyclodextrins, processing
INTRODUCTION
The complex molecular architecture of protein pharmaceuticals leads to a propensity toward the formation of aggregates ranging from dimers to micron-sized particles. Although particles typically account for a small mass percentage and often do not impact drug potency, protein aggregates and particles may elicit immunogenic responses.1–3 Therefore, controlling protein particle formation is a major focus in the development of biopharmaceuticals.
There are multiple pathways to protein particle formation.4 In the simplest pathway, an aggregation-prone protein forms higher order oligomers that in some cases grow to micron-sized particles. Proteins may also undergo conformational changes that increase their susceptibility to particle formation, either because of intrinsic conformational instability or in response to an external stress. Proteins adhere to a wide range of surfaces, including air–liquid and liquid–solid interfaces, and the accumulation of proteins on these surfaces can lead to particle formation, in some cases following a surface-induced conformational change. Foreign particles can also serve as nucleation sites for the formation of protein particles.
The growing understanding of these pathways to particle formation has led to a successful approach to mitigating particle formation in which the formulation composition is selected to maximize the conformational stability of the protein and provide interfacial protection. The purification process must also be optimized to remove potential particle-nucleating impurities. However, the purification process itself presents a variety of stresses that can lead to in-process particle formation. Although in-process particle formation is sometimes disregarded with the expectation that these particles will be removed by filtration, we argue that it is essential to understand and limit in-process particle formation to establish a well-controlled process, reduce the particle burden for filtration, and reduce the risk that prefiltration stresses could lead to postfiltration particle formation.
Multiple authors have noted the formation of protein aggregates during ultrafiltration/diafiltration (UF/DF) operation,5–7 and we have observed millions of micron-sized particles per milliliter in the UF/DF products of multiple proteins prior to final filtration. The complexity of the UF/DF unit operation leads to several hypotheses for the principal mechanism of UF/DF-induced particle formation: (1) shear stress or other characteristics of the fluid flow, (2) impurities leached into the product stream from contact surfaces during extended periods of recirculation, (3) interfacial interactions, and (4) pump-specific stresses such as cavitation and tubing spallation.
First, we consider the possibility of shear stress-driven particle formation. Many studies have demonstrated that exposing a protein to shear stress can lead to protein aggregation, although the mechanism of this effect remains unclear. Some argue that the shear stress itself induces a conformational change that results in protein aggregation,8 whereas others propose that the associated fluid flow increases reactive collisions in the bulk or facilitates protein transport to and from aggregation-inducing interfaces.9 Early studies on enzyme activity reported a significant reduction in activity upon shear,10 suggesting that shear disrupted the structure and thus the function of the enzymes under study, but shear did not induce a conformational change in cytochrome C.11 Another set of studies showed that shear by a rotating disk led to protein aggregation with the amount of monomer loss depending on the shear rate,12 that this aggregation did not involve covalent modifications, and that the level of aggregation was reduced by the addition of surfactant and increased by an increase in the surface roughness of the rotating disk.13 These results suggest that shear induces protein aggregation by enhancing the transport of the protein to and from interfaces rather than by directly affecting the protein. This agrees with other studies showing that shear alone does not lead to the aggregation of monoclonal antibodies,14 that shear induces aggregation through the air–liquid interface,15 and that polysorbate prevents antibody precipitation under shear.16 One study suggested that altering the flow parameters of UF/DF operation could limit particle formation by changing the shear stress experienced by the protein.7 Although the modified parameters successfully limited aggregation and particle formation, the maximal reduction was only two-fold, suggesting that shear itself may not be the main driver of particle formation in UF/DF.
A second pathway to protein particle formation that may be involved in UF/DF-induced particle formation is nucleation on foreign particles present in solution, also referred to as heterogeneous nucleation.4,17 For example, filling pump-induced particle formation was related to the shedding of steel nanoparticles that acted as nucleation sites for protein particle formation.18 In another case, protein precipitation was induced by soluble tungsten, an impurity that can be present in prefilled syringes.19 During UF/DF operation, the repeated flow of the protein solution over various materials including the membrane, tubing, and retentate tank could result in impurities leaching into the product stream and promoting particle formation, with the portion of the tubing that is repeatedly stressed by the pump head as a particularly likely source of such impurities.
Third, proteins come into contact with a variety of interfaces during UF/DF operation, including solid–liquid and air–liquid interfaces, and interfacial adsorption could lead to protein particle formation. Protein-interface adsorption is often based on hydrophobic interactions, but electrostatic interactions also play an important role.4 Surface-induced particle formation may involve a conformational rearrangement of the protein to maximize its area of interaction with the surface or to minimize the energy of that interaction,4,20 or the surface may instead promote protein–protein association without any conformational event.16 Surfactants in biopharmaceutical formulations protect against particle formation by competing with protein molecules for interfacial adsorption, preventing protein adsorption to interfaces.21 For example, surfactants protect proteins from exposure to the air–liquid interface in static vials during long-term storage, and also provide protection during short time-scale perturbations of that interface such as during product shipping. Surfactants can also exert effects through direct interactions with the protein.22 Proteins commonly adsorb on solid interfaces, and solid materials such as stoppers and tubing have been implicated in particle formation.23,24 UF/DF operation presents multiple solid interfaces (tank, tubing, and membrane surfaces) as well as an air–liquid interface, suggesting that interfacial interactions could play a role in UF/DF-driven particle formation.
Fourth, pump-specific stresses are often implicated in protein particle formation, and pump-driven particle formation has been suggested as a route to particle formation in UF/DF.6 Pumping itself represents a complex stress encompassing shear stress, surface interactions, potential introduction of impurities through leaching or tubing spallation, and potential cavitation. Filling pumps have been implicated in protein particle formation,25 and pump-induced cavitation has been suggested as the cause of UF/DF-induced protein aggregation.6
The goal of this study is to identify which of the possible mechanisms described above is the dominant factor in protein particle formation during UF/DF operation: shear stress, impurities, interfacial interactions, or some combination of these factors present in the pump or the overall unit operation. We accomplish this by breaking the UF/DF unit operation into its constituent parts, examining the effects of solution conditions and additives on agitation-induced particle formation, and studying the biophysical properties of the protein in these solutions. The results of this work indicate a primarily interfacially driven mechanism of particle formation in UF/DF and suggest several viable particle mitigation strategies.
MATERIALS AND METHODS
Proteins
This study used the four in-house IgG molecules mAb A, B, C, and D (A, B, and C are IgG 1 and D is IgG 2), and the proteins lysozyme, ovalbumin, and α-chymotrypsinogen (Sigma, St. Louis, Missouri). The proteins were dialyzed or diafiltered into the appropriate buffers for experimentation, and in all cases were filtered through a 0.22 μm filter immediately prior to the experiment. Except where noted, data in this manuscript were collected at a protein concentration of 5 mg/mL.
Particle Measurement
For all experiments, particle formation was monitored using Micro-Flow Imaging™ (model DPA-4200; Protein Simple, Santa Clara, California). Undiluted 1 mL samples were loaded on the instrument, and 600 μL were measured (or >106 particles) at a flow rate of 150 μL/min. The data reported here represent the total number of particles per milliliter with an estimated circular diameter of more than 1 μm (1 μm is the lower limit of particle size that can be detected by this instrument).
UF/DF Recirculation
Ultrafiltration/diafiltration recirculation experiments were conducted using a semiautomated tangential flow filtration (TFF) system including a tandem peristaltic pump (SciLog, Madison, Wisconsin) and a Minimate™ TFF capsule with a 50 cm2 Omega™ (modified polyethersulfone) membrane (Pall, Port Washington, New York). All UF/DF experiments used a 1 foot section of STA-PURE® tubing (Thermo Scientific, Waltham, Massachusetts) at the pump head and platinum-cured silicon tubing elsewhere. To ensure that the mixing in the retentate tank was consistent across experiments, the retentate tank was placed on a magnetic stir plate (Corning PC-410D; Corning, New York) and stirred at 300 rpm. In recirculation experiments, 100 mL of 5 mg/mL mAb was placed in the retentate vessel and both the retentate and permeate were routed back to the tank to maintain a constant volume throughout the process.
Stirring in Vials
Small-scale stirring experiments were conducted with 3 mL of protein solution in 3 mL glass vials (Schott AG #6800-0316, Mainz, Germany) with headspace, with Teflon stoppers (West Pharmaceutical, Exton, Pennsylvania) and 7 × 2 mm PTFE magnetic stir bars (VWR, Radnor, Pennsylvania), stirred at 250 rpm on a 15-position magnetic stir plate (Thermo Scientific).
Pumping
Pumping experiments were conducted using a Masterflex® L/S Easyflex II peristaltic pump (Cole Parmer, Vernon Hills, Illinois) at 50 mL/min. A 1 foot section of STA-PURE® tubing (Thermo Scientific) at the pump head was flanked by two 1.5 feet sections of size 16 Masterflex® platinum-cured silicone tubing, and 40 mL of 5 mg/mL mAb was pumped from and returned to a 50 mL centrifuge tube without stirring.
Dynamic Scanning Calorimetry
Dynamic scanning calorimetry (DSC) was performed using a MicroCal VP-Capillary DSC (GE Healthcare, Piscataway, New Jersey). After 0.22 μm filtration, 1 mg/mL mAb solutions in 10 mM histidine, pH 6.0, were heated from 20°C to 95°C at 90°C/h. Tm1 and Tm2 values corresponding to unfolding were determined as the peak temperatures of the Cp versus T graph.
Dynamic Light Scattering
Dynamic light scattering (DLS) of 0.22 μm filtered mAb solutions from 1 to 10 μM in 10 mM histidine, pH 6.0, was carried out using a DynaPro® Plate Reader (Wyatt Technology, Santa Barbara, California). Diffusivity values based on a cumulants fit were averaged across six wells. Three independent dilution series were tested for each condition, and a linear regression of the average D versus [mAb] data was performed in GraphPad Prism (GraphPad Software, La Jolla, California). The kD was determined by dividing the slope of the best-fit line by the y-intercept.26
Surface Tensiometry
The surface tension of 0.22 μm filtered 5 mg/mL mAb solutions in 10 mM histidine, pH 6.0, was determined using a K100C surface tensiometer (Kruss, Hamburg, Germany) and a Wilhelmy plate. The reported values represent the equilibrium surface tension after 5 h of measurements, with data points collected every 60 s.
UF/DF Concentration and Filterability Measurement
In bench-scale UF/DF concentration experiments, approximately 5 g mAb was concentrated from roughly 5 to 40 mg/mL, diafiltration was conducted at 40 mg/mL for six diavolumes, and then a final concentration was performed to 120 mg/mL. These experiments were conducted using an 88 cm2 Pellicon 3 Biomax polyethersulfone membrane (Millipore, Billerica, Massachusetts). After bench-scale UF/DF concentration, the filterability of the final solution was measured by diluting the concentrated solution to the target protein concentration of 100 mg/mL and then conducting constant pressure filtration through a 0.22 μm PES filter using a FilterTec Normal Flow Filtration system (SciLog). As a linear relationship was observed between time/volume and time, the gradual pore plugging model was applied (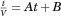 , or
, or 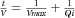 , where t is time, V is volume filtered at time t, Vmax represents the maximum volume that can be filtered at infinite time, and Qi is the instantaneous initial flow).27 Thus, the Vmax was calculated as the inverse of the slope of the time/volume versus time plot, which was then divided by the surface area of the filter to obtain filterability (L/m2).
, where t is time, V is volume filtered at time t, Vmax represents the maximum volume that can be filtered at infinite time, and Qi is the instantaneous initial flow).27 Thus, the Vmax was calculated as the inverse of the slope of the time/volume versus time plot, which was then divided by the surface area of the filter to obtain filterability (L/m2).
Statistical Analysis
Statistical comparisons were carried out in GraphPad Prism (GraphPad Software) by performing an ANOVA followed by a t-test. p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
On the basis of a previous report that particle formation during UF/DF operation could be controlled by the transmembrane pressure (TMP) and feed flow rate because of the relationship between these parameters and the shear stress across the UF/DF membrane,7 we explored the effect of these parameters on particle formation in the UF/DF of mAb A. In these experiments, 100 mL of mAb A at 5 mg/mL were recirculated in the UF/DF unit over 4 h while the solution in the retentate vessel was mixed with a stir bar. Although Figure1a does demonstrate variability among experiments with different combinations of feed flow and TMP, no consistent trend in particle formation was observed with respect to feed flow rate or TMP.
Figure 1.
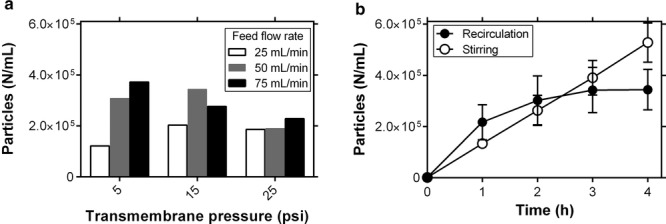
Monitoring protein particle formation during UF/DF recirculation. (a) Particles per milliliter of mAbA after 4 h recirculation of 100 mL of a 5 mg/mL solution in 10 mM histidine, 150 mM NaCl, pH 6.0, with various combinations of feed flow rate and TMP (n = 1). (b) Comparing the effects of recirculation (with stirring) and stirring alone on protein particle formation (n = 3, mean ± SE). In both cases, particles were counted using microflow imaging.
In a separate experiment, the retentate tank was disconnected from the rest of the unit and particle formation was monitored in response to stirring alone. As shown in Figure 1b, stirring in the retentate tank alone induced particle formation at a similar rate as UF/DF recirculation with stirring. The decrease in the rate of particle formation at later time points in the recirculation experiment could be because of particle trapping by the UF/DF membrane. This result suggests that the mechanism of particle formation is not specific to peristaltic pumping, interactions with the membrane, or the flow environment across the membrane. Although stirring itself is a complex stress and could lead to particle formation via shear stress or interfacial interactions, this finding focuses our attention away from stresses specific to the rest of the UF/DF system and toward general agitation stresses of the kind experienced in stirring. In particular, these results suggest that pump-specific stresses, impurities leaching into the product stream because of repeated pump–tubing interactions, shear stresses experienced in the UF/DF membrane flow channel, and interactions between the protein and membrane or tubing surfaces are not the dominant factors causing UF/DF-induced particle formation. Of the four original hypotheses, this finding leaves shear stress and interfacial interactions as the remaining possibilities.
On the basis of the relationship between UF/DF-induced particle formation and stirring-induced particle formation, solution conditions were varied in small-scale stirring experiments in vials as a way to understand the factors governing stirring-induced particle formation. Figure 2a shows that decreasing the NaCl concentration from 150 to 0 mM NaCl reduces particle formation over 1 h of stirring in vials. These results agree with previous findings from mAb A demonstrating that low ionic strength allowed for electrostatic repulsion, reducing turbidity and increasing viscosity at high concentrations.28 Note that this reduction in particle formation is only observed at very low NaCl concentrations. Figure 2b demonstrates the effect of interfacial protection on stirring-induced particle formation through the addition of polysorbate 80 (PS80). Adding PS80 to high salt solutions achieves a partial reduction in particle formation, whereas combining PS80 and low salt leads to a near-complete inhibition of stirring-induced particle formation.
Figure 2.
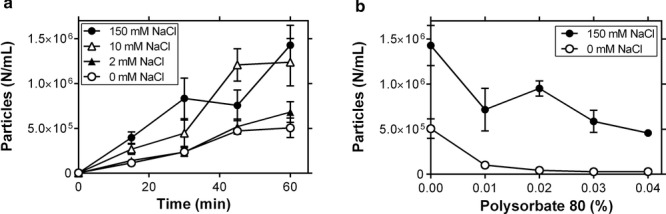
Effects of solution conditions on stirring-induced particle formation in 3 mL vials. (a) Time course of stirring-induced particle formation of 5mg/mL mAbA in 10 mM histidine, pH 6.0, with various concentrations of NaCl (n = 6, mean ± SE). (b) Particle formation after 1h stirring in 10 mM histidine, pH 6.0, with or without 150 mM NaCl and with a range of PS80 concentrations (n = 3, mean ± SE).
Together, these results suggest that stirring accelerates the transport of proteins to and from interfaces that then induce particle formation. Typical agitation experiments involving shaking or rotation continuously deform the air–liquid interface, leading to turnover in the population of proteins at the interface, inducing aggregation and particle formation. Although our stirring experiments were designed to avoid surface deformation, the fluid flow similarly refreshes the population of proteins at both air–liquid and liquid–solid interfaces. PS80 presumably reduces particle formation by competing with the protein for particle-inducing interfaces, but this protection against particle formation is far greater in low-conductivity solutions. The fact that PS80 reduces agitation-induced particle formation argues against a shear-driven pathway, as experiments with PS80 involve the same shear stress to the protein.
Low NaCl concentrations likely protect against particle formation by enabling electrostatic repulsion between protein molecules,28 but it remains unclear how this repulsion reduces interfacially driven particle formation. This effect could indicate that in addition to interfacial interactions, stirring promotes protein–protein collisions in the bulk, and that low ionic strength reduces the efficacy of these collisions in forming particles. Alternatively, the electrostatic protein–protein repulsion at low NaCl concentrations could affect protein-interface interactions in one of the two ways: low NaCl could reduce the propensity of the protein to adsorb to the interface, or it could reduce the propensity of proteins at the interface to form particles. These possibilities are addressed in part by the biophysical characterization reported later in the manuscript, but further experiments should be conducted to better understand this phenomenon.
The results thus far suggest that UF/DF-induced particle formation can be thought of as a particular form of the general category of “agitation” stresses. To clarify this point, pumping and UF/DF recirculation experiments were conducted under the same solution conditions as tested for stirring. As seen in Figure 3a, peristaltic pumping of mAb A solutions leads to rapid particle formation, and both pumping-induced (Fig. 3a) and UF/DF-induced (Fig. 3b) particle formation are reduced at low NaCl concentration or in the presence of PS80. The consistency of these effects among stirring, pumping, and UF/DF strengthens the argument that particle formation during UF/DF is not driven by factors specific to the membrane or pump such as the membrane surface, the membrane geometry or flow pattern, pump-induced cavitation, or pump-driven introduction of impurities. Instead, this process is driven by the transport of protein molecules to and from particle-inducing interfaces that can result from stirring, pumping, or recirculation in the UF/DF unit. The greater relative effect of PS80 addition to a high salt solution in the recirculation experiment as compared with the stirring experiment suggests that interfacial interactions are the predominant factor in actual UF/DF operation.
Figure 3.
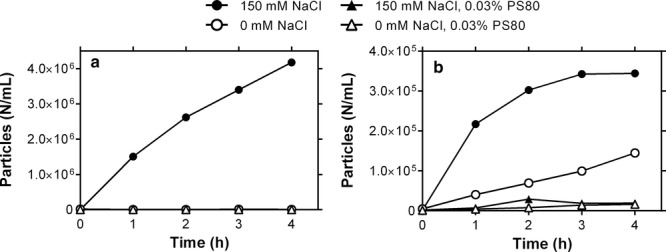
Effects of NaCl and PS80 on particle formation in pumping and recirculation experiments. (a) Pumping of 5mg/mL mAbA in 10 mM histidine, pH 6.0, with 1 foot of STA-PURE® tubing at the pump head connected with 3feet of platinum-cured silicone tubing (n = 1). (b) Recirculation of 100 mL of 5mg/mL mAbA at constant volume in a UF/DF unit with STA-PURE® tubing at the pump head (n = 3).
Additional proteins (see Table 1) were studied to determine the generality of the phenomena observed here. mAb B was subjected to the same stresses (stirring, pumping, and recirculation) under the same solution conditions (high/low NaCl, +/− PS80) as mAb A, whereas mAb C and D and three additional proteins were subjected to stirring. As shown for the stirring data in Figure 4, although different proteins yield different total particle numbers, the trends are consistent. All four mAbs and two out of three non-mAbs tested here form particles in response to stirring (lysozyme is omitted from the graph as <2000 particles/mL were formed in each condition), and both mAb A and B form particles upon pumping and UF/DF recirculation. Further, all of these proteins form fewer particles at lower NaCl concentrations and in the presence of PS80. Although these results do not ensure that all proteins will exhibit the same behavior, they suggest that UF/DF-induced particle formation results from interfacial interactions rather than molecule-specific characteristics. Furthermore, although different mAbs exhibit different propensities to form particles in response to these stresses, the same mitigation strategies are likely to benefit a wide range of molecules.
Table 1.
Molecular Properties
| Molecule | Isotype | pI | MW (kDa) |
|---|---|---|---|
| mAbA | IgG1 | 7.87–8.25 | 148 |
| mAbB | IgG1 | 8.98–9.03 | 148 |
| mAbC | IgG1 | 8.6–9.1 | 145 |
| mAbD | IgG2 | 8.36–8.47 | 147 |
| lysozyme | 11.35 | 14 | |
| ovalbumin | 4.54 | 44 | |
| α-chymotrypsinogen | 8.97 | 26 |
Figure 4.
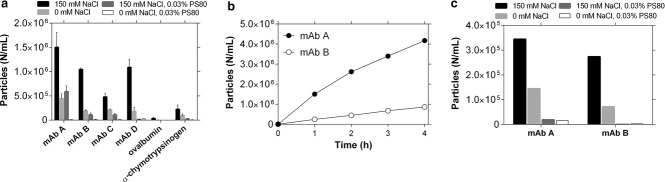
Comparison with additional proteins. (a) Particle formation after 1h stirring of 5mg/mL protein solutions in vials in 10 mM histidine, pH 6.0, with or without 150 mM NaCl and 0.03% PS80 (n = 3, mean ± SE). α-Chymotrypsinogen experiments were performed at 1 mg/mL because of the low solubility of this molecule, and ovalbumin was tested at pH 7.0 because of its pI. (b) Pumping-induced particle formation of 5mg/mL mAbA and B in 10 mM histidine, pH 6.0, 150 mM NaCl (experiments were also performed in 0 mM NaCl and 0.03% PS80 but are not plotted as all datapoints were <103). (c) Particle formation of 5mg/mL mAbA and B solutions in 10 mM histidine, pH 6.0, in UF/DF recirculation with stirring (n = 1).
These results suggest that interfacial interactions are responsible for UF/DF-induced particle formation, and that adding PS80 to the process stream and conducting UF/DF at low conductivity are potential mitigation strategies to prevent particle formation. Before testing these strategies in bench-scale concentration and diafiltration runs, we investigated whether other additives could also be used to protect against UF/DF-induced particle formation. This additive screening had the particular goal of identifying non-micelle forming additives to protect against particle formation, as micelle formation of PS80 and other surfactants with micelle sizes greater than the molecular weight cutoff of the membrane would lead to concentration of the additive along with the protein and thus difficulty in controlling the additive concentration. Thus, we tested hydroxypropyl-β-cyclodextrin (HPβCD) based on a previous study demonstrating protection against agitation-induced protein aggregation29; the hydrophobic amino acids isoleucine, leucine, and phenylalanine30; and the known protein stabilizer arginine.31
These additives were tested for their ability to protect against stirring-induced (Fig. 5a) and UF/DF-induced particle formation (Fig. 5b). Some of the hydrophobic amino acids yielded marginal reductions in stirring-induced particle formation, whereas arginine and HPβCD provided excellent protection against agitation-induced particle formation. All of the additives tested here led to some reduction in particle formation, with HPβCD as the most effective. To understand the mechanisms by which these additives affect protein particle formation, we measured the conformational and colloidal stability of mAb A in these conditions, as well as the surface tension of the solutions (Table 2).
Figure 5.
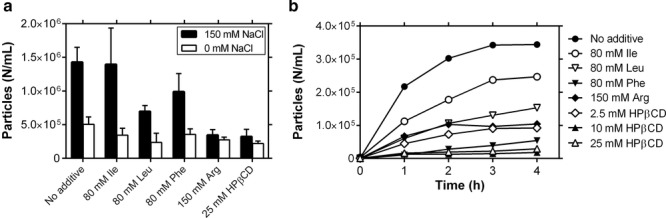
Testing additives as protectants against UF/DF-induced particle formation. (a) Particle formation after 1h stirring in 3mL vials for 5mg/mL mAbA in 10 mM histidine, pH 6.0, with or without 150 mM NaCl and the additives indicated in the legend (n = 3, mean ± SE). (b) UF/DF recirculation-induced particle formation of 5mg/mL mAbA in 10 mM histidine, 150 mm NaCl, pH 6.0, at 50 mL/min feed flow and 15psi TMP (n = 1).
Table 2.
Effects of Solution Additives on mAb Conformational and Colloidal Stability and Surface Tension
| mAb | [NaCl] (mM) | Additive | Tm1, Tm2 (°C)a | kDb | Surface Tension (mN/M)c |
|---|---|---|---|---|---|
| mAbA | 150 | – | 67.6, 78.7 | −17.4 | 54.8 ± 0.5 |
| mAbA | 0 | – | No Tm1, 78.2 | −13.1 | 54.8 ± 0.4 |
| mAbB | 150 | – | 66.5, 83.3 | −7.42 | 55.2 ± 0.2 |
| mAbB | 0 | – | 68.7, 85.4 | 43.86 | 54.4 ± 0.4 |
| mAbA | 150 | 0.03% PS80 | 67.8, 78.6 | −16.4 | 36.7 ± 0.4 |
| mAbA | 150 | 10 mM HPβCD | 68.4, 78.9 | −20.0 | 53.5 ± 1.4 |
| mAbA | 150 | 150 mM Arg | 66.8, 78.3 | −10.3 | 54.7 ± 0.3 |
| mAbA | 150 | 80 mM Phe | 66.2, 77.3 | −14.8 | 45.0 ± 0.2 |
| mAbA | 150 | 80 mM Ile | 68.1, 79.0 | −14.3 | 55.3 ± 1.0 |
| mAbA | 150 | 80 mM Leu | 68.3, 78.7 | −11.0 | 54.5 ± 0.2 |
All samples were prepared in 10 mM histidine, pH 6.0.
Melting temperatures indicating conformational stability were determined using DSC (n = 2, mean).
kD captures colloidal stability and was determined as the slope/intercept of the D versus [mAb] graph generated from DLS data (n = 3, with six wells measured in each experiment).
Surface tensions are reported for 5mg/mL mAb solutions (n = 3, mean ± SE).
Table 2 reports the Tm1 and Tm2 values obtained from DSC as measures of the conformational stability of mAb A, and the colloidal stability is captured by the kD parameter determined from DLS experiments, with more negative kD values representing more attractive protein–protein interactions. Comparing the data for mAb A between high and low [NaCl] conditions, decreasing [NaCl] leads to two effects: conformational stabilization, as demonstrated by the lack of a Tm1 corresponding to the unfolding of the CH2 domain, and an improvement in colloidal stability. No change in surface tension was observed, suggesting that rather than reducing the amount of protein that adsorbs to the air–water interface, low NaCl concentrations reduce the ability of the interface to induce particle formation. mAb B exhibits a greater colloidal stability than mAb A at both high and low [NaCl], which could explain the lower level of particle formation observed for mAb B. These results, along with those for all four amino acids, suggest that agitation-induced protein particle formation is related to colloidal stability in the absence of interfacial protection, with less attractive protein–protein interactions leading to a lower level of particle formation.
Although PS80 is sometimes suggested to affect protein stability through direct PS80–protein interactions in solution, in this case we observe no difference in the protein's conformational or colloidal stability in the presence of PS80. The significantly lower surface tension in the presence of PS80 (p < 0.001) demonstrates that PS80 coats the air–liquid interface, preventing protein-interface adsorption because of its higher surface activity. Although HPβCD has been proposed to protect against agitation-induced aggregation and particle formation by providing interfacial protection,29 we observe no effect of HPβCD on any of the three parameters measured here. This observation is in agreement with recent findings that HPβCD does not prevent protein-interface adsorption in the same way as PS80, and the mechanism by which it protects against interfacially induced particle formation remains under investigation.32
Next, the potential mitigation strategies were investigated in bench-scale concentration and diafiltration experiments at low conductivity or with PS80, HPβCD, or arginine added to the UF/DF product stream. As we expected PS80 to be concentrated along with the protein because of its propensity for micelle formation, 0.0016% PS80 was added so that the final concentration (measured as 0.015%) would remain below the formulation concentration of 0.03%. The results in Figure 6a demonstrate that low NaCl concentration, the addition of PS80, and the addition of HPβCD all led to statistically significant decreases in protein particle formation during UF/DF. PS80 reduced in-process particle formation by about 15-fold, whereas reducing NaCl concentration and adding HPβCD led to roughly four-fold reductions. Further, as noted in a previous publication,28 electrostatic repulsion at low NaCl concentrations leads to a high viscosity at high protein concentrations, making the final concentration difficult at low NaCl. Arginine exhibited very little effect on particle formation in UF/DF. Experiments at 150 and 500 mM arginine revealed no change in the level of in-process particle formation, and these results are omitted from Figure 6 as n = 1. This finding highlights the importance of the interface in particle formation in UF/DF: although PS80 and potentially HPβCD provide protection against interfacially induced particle formation, arginine is thought to protect against protein–protein interactions by binding to the protein surface, leading to a crowding effect and increasing the energetic barrier to self-association.33 In this case, arginine improves the colloidal stability of the mAb (Table 2). As the effect of arginine is believed to rely on arginine–protein binding, the arginine to protein concentration ratio may affect its ability to suppress aggregation. Thus, at the low protein concentration used in the stirring and recirculation experiments (5 mg/mL), 150 mM arginine may induce sufficient crowding to significantly reduce particle formation, even in the absence of interfacial protection. In actual UF/DF operation, however, the arginine concentration remains the same while the protein concentration increases, potentially decreasing the ability of this additive to protect against particle formation because of the lower arginine to protein ratio. In contrast, interfacial protectants are not dependent on the additive-to-protein concentration ratio, and thus remain effective throughout the concentration experiment.
Figure 6.
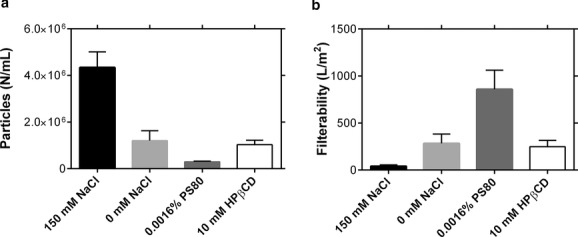
Testing mitigation strategies for UF/DF-induced particle formation. (a) Particle counts for the final concentrated material. Approximately 5 g of mAbA was concentrated from 5 to 120mg/mL with six diavolumes at 40mg/mL. (b) Filterability of the final concentrated solution adjusted to 100mg/mL (both n = 3, mean ± SE).
Although particles formed during UF/DF can be filtered away, in some cases without significant effects on yield or postfiltration stability, limiting particle formation during UF/DF operation has the additional benefit of improving the filterability of the concentrated product as shown in Figure 6b. Of the three conditions with reduced levels of particle formation in Figure 6a, two (0 mM NaCl and 0.03% PS80) exhibited notable improvements in filterability. Although HPβCD led to a five-fold reduction in particle formation, it only led to a two-fold increase in filterability, compared with a 10-fold increase in the presence of polysorbate. Thus, these results demonstrate that controlling particle formation can significantly improve the ease of processing downstream of the UF/DF step.
The results of this study suggest two strategies to prevent protein particle formation during UF/DF: adding PS80 or HPβCD. As most processes have UF/DF as the final step, additives to prevent particle formation must be acceptable as formulation excipients. Polysorbates are common biopharmaceutical excipients but will be concentrated during UF/DF, necessitating careful planning and in-process concentration testing and adjustment to ensure that the final polysorbate level is within the target range. HPβCD overcomes this limitation as it does not form micelles and can be added to the UF/DF step at the desired formulation concentration. Although it is less common than PS80, HPβCD has been approved as a formulation excipient for small molecule drugs34 and has been studied as a biopharmaceutical formulation excipient, suggesting that this may be a viable strategy to prevent in-process particle formation.
CONCLUSIONS
Although the strength of this study's conclusions are limited by the small number of proteins examined and the apparent complexity of this phenomenon, this work improves our understanding of the mechanism of protein particle formation in UF/DF operation and offers several mitigation strategies that can be used to limit particle formation during production operations. Particle formation during UF/DF operation is shown to be driven by the transport of protein molecules to and from hydrophobic interfaces that then induce particle formation. Adding a surface-active molecule to the product pool successfully mitigates particle formation during UF/DF operation, and solution conditions that improve colloidal stability also reduce the level of particle formation. Polysorbates are good candidates as interfacial protectants as they are common biopharmaceutical formulation excipients, but their concentration cannot be easily controlled because of their propensity for micelle formation. HPβCD presents the opportunity to limit particle formation while maintaining control over the excipient concentration.
Acknowledgments
This study was sponsored by MedImmune. The authors thank Haibin Luo, Justin Weaver, Jared Bee, and Kripa Ram for helpful discussion and manuscript review.
REFERENCES
- 1.Rosenberg AS. Effects of protein aggregates: An immunologic perspective. AAPS J. 2006;8(3):E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G, Fan YX, Kirshner S, Verthelyi D, Kozlowski S, Clouse KA, Swann PG, Rosenberg A, Cherney B. Overlooking subvisible particles in therapeutic protein products: Gaps that may compromise product quality. J Pharm Sci. 2009;98(4):1201–1205. doi: 10.1002/jps.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh SK, Afonina N, Awwad M, K Bechtold-Peters, Blue JT, Chou D, Cromwell M, Krause HJ, Mahler HC, Meyer BK, Narhi L, Nesta DP, Spitznagel T. An industry perspective on the monitoring of subvisible particles as a quality attribute for protein therapeutics. J Pharm Sci. 2010;99(8):3302–3321. doi: 10.1002/jps.22097. [DOI] [PubMed] [Google Scholar]
- 4.Philo JS, Arakawa T. Mechanisms of protein aggregation. Curr Pharm Biotech. 2009;10:348–351. doi: 10.2174/138920109788488932. [DOI] [PubMed] [Google Scholar]
- 5.Cromwell MEM, Hilario E, Jacobson F. Protein aggregation and bioprocessing. AAPS J. 2006;8(3):E572–E579. doi: 10.1208/aapsj080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Reis R, Zydney A. Bioprocess membrane technology. J Mem Sci. 2007;297:16–50. [Google Scholar]
- 7.Rosenberg E, Hepbildikler S, Kuhne W, Winter G. Ultrafiltration concentration of monoclonal antibody solutions: Development of an optimized method minimizing aggregation. J Mem Sci. 2009;342:50–59. [Google Scholar]
- 8.Bekard IB, Asimakis P, Bertolini J, Dunstan DE. The effects of shear flow on protein structure and function. Biopolymers. 2011;95(11):733–745. doi: 10.1002/bip.21646. [DOI] [PubMed] [Google Scholar]
- 9.Biddlecombe JG, Smith G, Uddin S, Mulot S, Spencer D, Gee C, Fish BC, Bracewell DG. Factors influencing antibody stability at solid–liquid interfaces in a high shear environment. Biotechnol Prog. 2009;25(5):1499–1507. doi: 10.1002/btpr.211. [DOI] [PubMed] [Google Scholar]
- 10.Charm SE, Wong BL. Enzyme inactivation with shearing. Biotechnol Bioeng. 1970;12:1103–1109. doi: 10.1002/bit.260120615. [DOI] [PubMed] [Google Scholar]
- 11.Jaspe J, Hagen SJ. Do protein molecules unfold in a simple shear flow? Biophys J. 2006;91:3415–3424. doi: 10.1529/biophysj.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biddlecombe JG, Craig AV, Zhang H, Uddin S, Mulot S, Fish BC, Bracewell DG. Determining antibody stability: Creation of solid–liquid interfacial effects within a high shear environment. Biotechnol Prog. 2007;23:1218–1222. doi: 10.1021/bp0701261. [DOI] [PubMed] [Google Scholar]
- 13.Biddlecombe JG, Smith G, Uddin S, Mulot S, Spencer D, Gee C, Fish BC, Bracewell DG. Factors influencing antibody stability at solid–liquid interfaces in a high shear environment. Biotechnol Prog. 2009;25(5):1499–1507. doi: 10.1002/btpr.211. [DOI] [PubMed] [Google Scholar]
- 14.Bee JS, Stevenson JL, Mehta B, Svitel J, Pollastrini J, Platz R, Freund E, Carpenter JF, Randolph TW. Response of a concentrated monoclonal antibody formulation to high shear. Biotech Bioeng. 2009;103(5):936–943. doi: 10.1002/bit.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maa YH, Hsu CC. Protein denaturation by combined effect of shear and air–liquid interface. Biotechnol Bioeng. 1996;54(6):503–512. doi: 10.1002/(SICI)1097-0290(19970620)54:6<503::AID-BIT1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Patapoff TW, Esue O. Polysorbate 20 prevents the precipitation of a monoclonal antibody during shear. Pharm Dev Tech. 2009;14(6):659–664. doi: 10.1080/10837450902911929. [DOI] [PubMed] [Google Scholar]
- 17.Bee JS, Randolph TW, Carpenter JF, Bishop SM, Dimitrova MN. Effects of surfaces and leachables on the stability of biopharmaceuticals. J Pharm Sci. 2011;100(10):4158–4170. doi: 10.1002/jps.22597. [DOI] [PubMed] [Google Scholar]
- 18.Tyagi AK, Randolph TW, Dong A, Maloney KM, Hitscherich C, Carpenter J. IgG particle formation during filling pump operation: A case study of heterogeneous nucleation on stainless steel nanoparticles. J Pharm Sci. 2009;98(1):94–104. doi: 10.1002/jps.21419. [DOI] [PubMed] [Google Scholar]
- 19.Bee JS, Nelson SA, Freund E, Carpenter JF, Randolph TW. Precipitation of a monoclonal antibody by soluble tungsten. J Pharm Sci. 2009;98(9):3290–3301. doi: 10.1002/jps.21707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brych SR, Gokarn YR, Hultgen H, Stevenson RJ, Rajan R, Matsumura M. Characterization of antibody aggregation: Role of buried, unpaired cysteines in particle formation. J Pharm Sci. 2010;99(2):764–781. doi: 10.1002/jps.21868. [DOI] [PubMed] [Google Scholar]
- 21.Bee JS, Schwartz DK, Trabelsi S, Freund E, Stevenson JL, Carpenter JF, Randolph TW. Production of particles of therapeutic proteins at the air-water interface during compression/dilation cycles. Soft Matter. 2012;8:10329–10335. [Google Scholar]
- 22.Deechongkit S, Wen J, Narhi LO, Jiang Y, Park SS, Kim J, Kerwin BA. Physical and biophysical effects of polysorbate 20 and 80 on darbepoeitin alfa. J Pharm Sci. 2009;98(9):3200–3217. doi: 10.1002/jps.21740. [DOI] [PubMed] [Google Scholar]
- 23.Sluzky V, Tamada JA, Klibanov AM, Langer R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc Natl Acad Sci USA. 1991;88(21):9377–9381. doi: 10.1073/pnas.88.21.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzannis ST, Hrushesky WJM, Wood PA, Przybycien TM. Adsorption of a formulated protein on a drug delivery device surface. J Coll Int Sci. 1997;189:216–228. [Google Scholar]
- 25.Nayak A, Colandene J, Bradford V, Perkins M. Characterization of subvisible particle formation during the filling pump operation of a monoclonal antibody solution. J Pharm Sci. 2011;100(10):4198–4204. doi: 10.1002/jps.22676. [DOI] [PubMed] [Google Scholar]
- 26.Saluja A, Fesinmeyer RM, Hogan S, Brems DN, Gokarn YR. Diffusion and sedimentation interaction parameters for measuring the second virial coefficient and their utility as predictors of protein aggregation. Biophys J. 2010;99(8):2657–2665. doi: 10.1016/j.bpj.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grace HP. Structure and performance of filter media. I. The internal structure of filter media. AIChE J. 1956;2(3):307–315. [Google Scholar]
- 28.Salinas BA, Sathish HA, Bishop SM, Harn N, Carpenter JF, Randolph TW. Understanding and modulating opalescence and viscosity in a monoclonal antibody formulation. J Pharm Sci. 2010;99(1):82–93. doi: 10.1002/jps.21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serno T, Carpenter JF, Randolph TW, Winter G. Inhibition of agitation-induced aggregation of an IgG-antibody by hydroxypropyl-β-cyclodextrin. J Pharm Sci. 2010;99(3):1193–1206. doi: 10.1002/jps.21931. [DOI] [PubMed] [Google Scholar]
- 30.Bull HB, Breese K. Surface tension of amino acid solutions: A hydrophobicity scale of the amino acid residues. Arch Biochem Biophys. 1974;161:665–670. doi: 10.1016/0003-9861(74)90352-x. [DOI] [PubMed] [Google Scholar]
- 31.Arakawa T, Ejima D, Tsumoto K, Obeyama N, Tanaka Y, Kita Y, Timasheff SN. Suppression of protein interactions by arginine: A proposed mechanism of the arginine effects. Biophys Chem. 2007;127:1–8. doi: 10.1016/j.bpc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Serno T, Härtl E, Besheer A, Miller R, Winter G. The role of polysorbate 80 and HPβCD at the air-water interface of IgG solutions. Pharm Res. 2013;30:117–130. doi: 10.1007/s11095-012-0854-x. [DOI] [PubMed] [Google Scholar]
- 33.Shukla D, Trout BL. Interaction of arginine with proteins and the mechanism by which it inhibits aggregation. J Phys Chem B. 2010;114:13426–13438. doi: 10.1021/jp108399g. [DOI] [PubMed] [Google Scholar]
- 34.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: Past, present and future. Nat Rev Drug Disc. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]


