Abstract
Background
Rifabutin has been known to be effective in multidrug-resistant Helicobacter pylori-harboring patients undergoing treatment failure for H. pylori infection.
Aim
To evaluate the efficacy of 7-day treatment regimen consisting rifabutin daily but increasing the dose of amoxicillin and lansoprazole in patients who have failed first and second eradication and to assess the side effect profiles in South Korea.
Methods
From December 2007 to May 2013, 59 H. pylori-infected patients with two previous eradication failures were enrolled for this study prospectively. The eligible patients were randomly assigned to either group A or B. Group A received lansoprazole 30 mg bid, amoxicillin 1.0 g tid and rifabutin 150 mg bid during 7 days, whereas group B received lansoprazole 60 mg bid, amoxicillin 1.0 g tid and rifabutin 150 mg bid during 7 days.
Results
In group A, H. pylori eradication was achieved in 25 (78.1%) of the 32 patients in the ITT analysis and in 25 (80.6%) of the 31 patients in the PP analysis. In group B, H. pylori eradication was achieved in 26 (96.3%) of the 27 patients in the ITT analysis and in 27 (100%) of the 26 patients in the PP analysis. There was statistically significant difference between the two groups in terms of the eradication rates in PP analysis (p = .047), whereas a marginally statistical significance was found in terms of the eradication rates in ITT analysis (p = .051). Reported side effects were mild, and treatment was well tolerated. No major changes in physical examination or in standard laboratory parameters were observed after treatment.
Conclusions
Rifabutin-based high-dose proton-pump inhibitor (PPI)-combined therapy as empirical rescue treatment is more effective than standard dose PPI-combined rifabutin-based therapy, safe and best tolerable in third-line therapy in the Korean population. The key to successful rescue therapy with rifabutin–amoxicillin–PPI regimen may be to increase doses of PPI.
Keywords: Rifabutin, third-line recue therapy, high-dose proton-pump inhibitor, amoxicillin
Helicobacter pylori (H. pylori) infection is one of the most prevalent infectious diseases worldwide, which exists in almost 50% of the world's population. H. pylori infection plays an important role in gastric adenocarcinoma and the development of chronic gastritis, gastric ulcer, duodenal ulcer, an gastric mucosa-associated lymphoid tissue lymphoma 1,2. Maastricht III Consensus Report has recommended that proton-pump inhibitor (PPI), clarithromycin, and either amoxicillin or metronidazole treatment for 7–14 days is the first choice for H. pylori infection 3. Although some studies have revealed that the eradication rates of standard triple therapies are around 80% (by per-protocol analysis), most studies have demonstrated the success rate of recommended triple therapies is falling 4–8. According to recent studies, such eradication rates have plummeted to even 25–60% 9–12.
The main reasons for eradication failure are poor patient compliance, resistant bacteria, low gastric pH, drug-related side effects, and high bacterial load 4. In patients who failed initial treatment, a high proportion of H. pylori strains developed resistance to metronidazole or clarithromycin. Several salvage therapies of a second-line therapy have been recommended including a quadruple combination of PPI, bismuth, tetracycline, and metronidazole, but they still fail to eradicate the bacterium in 5–43% of the cases with average eradication rate of 76% on the basis of a pooled analysis. Infection harbors antibiotic-resistant strains that are ineradicable after several courses of treatments 13.
Recently, a standard third-line therapy will remain to be established, and European guidelines recommend culture before selection of a third-line treatment based on the microbial sensitivity to the antibiotics. The alternative candidates for third-line therapy are quinolone, tetracycline, rifabutin, and furazolidone; high-dose PPI/amoxicillin therapy is also promising 13,14. Rifabutin is a spiropiperidyl derivative of rifampin-S, an antitubercular compound and has been shown to exhibit high in vitro activity against H. pylori, and no resistant strains have been isolated from patients treated or untreated for H. pylori infection 15,16. Furthermore, rifabutin is chemically stable over a wide pH range, and its antibacterial activity is not affected by the acidic environment of the stomach 17. Previous clinical trials have suggested that rifabutin may be a promising rescue treatment of H. pylori infection, with an eradication rate of around 70% 18,19. On the other hand, Qasim et al. 20 have achieved only a 38% eradication rate. H. pylori eradication rate of rifabutin regimens as third-line treatment was 66%, results being heterogeneous 21.
To improve efficacy, modification in the dosing and duration of regimen were tried. In rescue therapy, high-dose PPI and amoxicillin therapy had advantages. For the rescue therapy, using high-dose PPI and amoxicillin therapy is advantageous, given the fact that this regimen can resolve the problems of clarithromycin and metronidazole resistance and homozygous extensive metabolizers of CYP2C19 gene polymorphisms, which are main reasons for the eradication failure. 21. The aim of this study was to evaluate the efficacy of 7-day treatment regimen consisting rifabutin 300 mg daily but increasing the dose of amoxicillin to 1 g tid and lansoprazole 30 mg bid or 60 mg bid in patients who have failed second eradication and to assess the side effect profiles.
Methods
Patients and Eradication Therapy
From December 2007 to May 2013, 59 H. pylori-infected patients with two previous eradication failures were enrolled for this study prospectively. The study design was based on a single-centered, randomized, open-label, and controlled clinical trial. The study protocol was approved by the Institutional Review Board at Yongin Severance Hospital and confirmed to the ethical guidelines of the Declaration of Helsinki, 1964, as revised in 2004. The requirement for informed consent was waived, and all subjects signed written informed consent. The intended sample of 55 recruited subjects (32 patients for group A and 27 patients for group B) provided a power of approximately 90%, assuming a significance level <.01. The dropout rate was expected to be <10%, based on the documented good tolerability of drugs. All patients received first-line eradication therapies (lansoprazole 30 mg bid, clarithromycin 500 mg bid, amoxicillin 1 g bid) for 7 days and received second-line eradication therapy (lansoprazole 30 mg bid, tripotassium-dicitrato-bismuthate 600 mg bid, metronidazole 500 mg tid, tetracycline 500 mg qid) for 7 days. The eligible patients were randomly assigned to either group A who received lansoprazole 30 mg bid, amoxicillin 1.0 g tid, and rifabutin 150 mg bid for 1 week or group B who received lansoprazole 60 mg bid, amoxicillin 1.0 g tid, and rifabutin 150 mg bid for 7 days. After an overnight fast, endoscopy was performed and endoscopic findings recorded. H. pylori infection was defined a positivity according to at least one of the following two tests: a positive rapid urease test (CLO test Delta West, Bently, Australia) using a specimen from antrum or histologic evidence of H. pylori in any of two specimen taken from corpus by hematoxylin and eosin stain. The eradication of H. pylori was assessed with 14C-urea breath test 4 weeks after the therapy.
Exclusion criteria included the patients with the coexistence of serious concomitant illness 8 (e.g., liver cirrhosis with decompensation, and uremia), pregnant women, allergic history to the medications used and patients with previous gastric surgery antibiotics, bismuth and PPI within the previous 2 weeks and nonsteroidal anti-inflammatory drugs within the previous 4 weeks. Compliance with therapy was defined as intake of 80% more of the prescribed study medication. Intake of study medication and adverse events were interviewed after the end of treatment. In addition, the patients were instructed to contact the study site immediately in case of any severe adverse event.
14C-urea Breath Test
Patients who had not taken antibiotics or antisecretary drugs were fasted for 6 hours before performing the UB t-test (HeliprobeTM; Noster system AB, Stockholm, Sweden). The gelatin capsule containing 14C-urea was swallowed with water by the patient to avoid contamination with oral bacteria. Breath samples were taken 10–30 minutes after ingestion. Patients were instructed to exhale into the BreathCard™, which was inserted into the Heliprobe™ (Noster system AB, Stockholm, Sweden) instrument. The result was considered Δ > 50 positive is based on clinical trials using the 1μ Curie 14C-UBT.
Statistical Analysis
The analysis was conducted using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, U.S.A). The eradication rates and their 95% confidence intervals (CI) at both intention-to-treat (ITT) and per-protocol (PP) analyses were calculated for each treatment regimen. For all other variables, Fisher's exact test and t-test were used as appropriate, and p values <.05 were considered significant. The difference between the proportions eradicated was estimated. Before pooling these estimates, a Fisher's exact test was applied to investigate heterogeneity between the differences. This study was approved by Ethics Committee of our Institution (Yongin Severance Hospital, Yonsei University, Republic of Korea).
Results
Baseline Demographic and Clinical Data
From December 2007 to May 2013, a total of 59 patients were enrolled in the study (mean age, 55.3 years, range, 34–74 years). All patients had been previously treated with two courses of eradication therapy. They took as first-line therapy with PPI, clarithromycin, and amoxicillin and as second-line therapy with quadruple therapy consisting of a PPI, a bismuth salt, metronidazole, and tetracycline. Group A had 32 patients with lansoprazole 30 mg bid including rifabutin 150 mg bid daily and amoxicillin 1 g tid for 7 days. Group B had 27 patients with lansoprazole 60 mg bid including rifabutin 150 mg bid (daily) and amoxicillin 1 g tid for 7 days (Fig.1).
Figure 1.
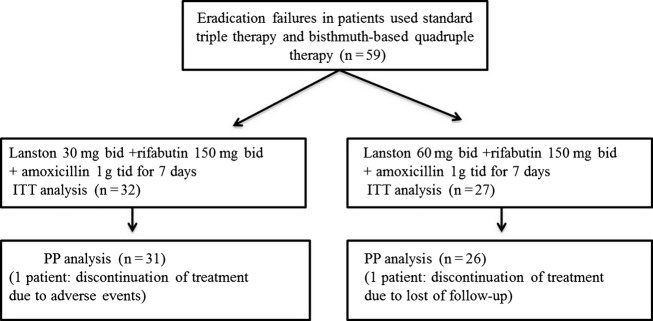
The flow diagram for eradication of Helicobacter pylori.
There were 25 males (mean age 58.8 years) and 34 females (mean age 52.9 years). Indications for eradication therapy mainly included peptic ulcer disease (32/58) and nonulcer disease (27/58). A history of smoking and alcohol use was present in 9 (15.5%) and 12 (20.7%) patients, respectively. Demographic and clinical data are summarized in Table1. There were no statistically significant differences between the two groups in terms of demographic characteristics, history of smoking and alcohol, and indication for eradication therapy. One patient in group A was not enrolled for PP analysis due to adverse events such as abdominal pain and red-colored urine and one patient in group B due to loss of follow-up; 56 patients were fully compliant with the treatment taking more than 80% of the prescribed tablets. In group A, H. pylori eradication was achieved in 25 (78.1%) of the 32 patients in the ITT analysis and in 25 (80.6%) of the 31 patients in the PP analysis. In group B, H. pylori eradication was achieved in 26 (96.3%) of the 27 patients in the ITT analysis and in 27 (100%) of the 27 patients in the PP analysis. There was statistically significant difference between the two groups in terms of the eradication rates in PP analysis (p = .047), whereas a marginally statistical significance was found in terms of the eradication rates in ITT analysis (p = .051; Table2).
Table 1.
Clinical characteristics of patients
| Lansoprazole 60 mg (n = 32) | Lansoprazole 120 mg (n = 27) | p-Value | |
|---|---|---|---|
| Age (years, mean) | 54.6 | 56.2 | .595 |
| Sex (M : F) | 12 : 20 | 13/14 | .444 |
| Smoking | 4 (12.5%) | 5 (18.5%) | .983 |
| Alcohol | 7 (21.9%) | 5 (18.5%) | .775 |
| Disease | |||
| Gastric ulcer | 18 (56.3%) | 8 (29.6%) | .132 |
| Duodenal ulcer | 5 (15.6%) | 1 (3.7%) | |
| Functional dyspepsia | 9 (28.1%) | 18 (66.7) | |
Table 2.
Eradication rates of Helicobacter pylori
| Lansoprazole 60 mg (n = 32) | Lansoprazole 120 mg (n = 27) | p-Value | |
|---|---|---|---|
| ITT (intention to treat) | 25/32 (78.1%, 60.0–90.7) | 26/27 (96.3%, 81.0–99.9) | .051 |
| PP (per protocol) | 25/31 (80.7%, 62.5–92.5) | 26/27 (100%, 86.8–100) | .047 |
The most common adverse events were epigastric pain in the three patients (9.3%) versus one patient (3.7%), epigastric discomfort in two patients (6.2%) versus, one patient (3.7%), and nausea in one patient (3.1%) versus one patient (3.7%), in the groups A and B. There was no statistically significant difference between the two groups in terms of adverse events (Table3). Reported side effects were mild, and treatment was well tolerated. No major changes in physical examination or in standard laboratory parameters were observed after treatment. As drug compliance and tolerability were optimal in all patients, we searched for other factors possibly associated with treatment failure (Table4). Neither demographic data nor endoscopic lesions were predictive of treatment failure.
Table 3.
Adverse effects
| Lansoprazole 60 mg (n = 32, %) | Lansoprazole 120 mg (n = 27, %) | |
|---|---|---|
| Epigastric pain | 3 (9.3) | 1 (3.7) |
| Epigastric discomfort | 2 (6.2) | 1 (3.7) |
| General weakness | 1 (3.1) | |
| Nausea | 1 (3.1) | 1 (3.7) |
| Urine color change | 1 (3.1) | |
| Sleepy | 1 (3.1) | |
| Lip discomfort | 1 (3.1) |
Table 4.
Eradication rates of Helicobacter pylori according to various clinical factors
| Eradication rates (%) | p-Value | |
|---|---|---|
| Age | ||
| <60 | 28/33 (84.8, 68.1–94.8) | .957 |
| >60 | 23/26 (92.0, 69.8–97.5) | |
| Sex | ||
| Male | 22/24 (91.7, 73.0–99.0) | .686 |
| Female | 28/34 (82.4, 65.3–93.2) | |
| Smoking | ||
| Smoker | 8/9 (88.9, 73.2–94.1) | .983 |
| Nonsmoker | 42/49 (85.7, 51.7–99.7) | |
| Alcohol drinking | ||
| Drinker | 11/12 (91.7, 71.6–93.8) | .775 |
| Nondrinker | 39/46 (84.18, 61.5–99.7) | |
| Diseases | ||
| Gastric ulcer | 23/25 (92.0, 65.1–95.6) | .883 |
| Duodenal ulcer | 5/6 (83.3, 35.8–99.6) | |
| Gastritis | 16/18 (88.9, 70.8–97.6) | |
| Side effects | ||
| Positive | 7/8 (87.5, 47.3–99.6) | .983 |
| Negative | 42/49 (85.7, 73.7–94.3) | |
Discussion
Our study shows that as empirical third-line therapy, rifabutin-based high-dose PPI and amoxicillin combined therapy is more effective in patients with refractory H. pylori infection after two eradication failures with key antibiotics such as clarithromycin, metronidazole, and tetracycline previously prescribed. High-dose PPI and amoxicillin are recommended as one of several options for empirical rescue therapy for H. pylori infection in the absence of antimicrobial susceptibility testing. The dosing schedule of antibiotics is also important for the treatment of infectious diseases. Antibiotics with the beta-lactam ring, such as amoxicillin, have little postantibiotic effects on the gram-negative rods 22. The frequent dosing of amoxicillin is required to sustain the levels of amoxicillin higher than the MIC level for a long time, which makes the bioavailability of amoxicillin enhanced in comparison with the twice-daily dosing from the point of view of pharmacology of antibiotics 23. Miehlke et al. 24 reported in PP analysis, eradication rate of high-dose rabeprazole (40 mg trice) and amoxicillin 1.0 g trice for 14 days was 75%. Four studies from Japan reported the efficacy of a second-line treatment with a PPI plus amoxicillin after the failure of the standard triple therapy 23–25. Surprisingly, three of these studies (106 patients) reported optimal results (eradication rates of 87, 91 and 100%), dosing 10 mg of rabeprazole and 500 mg of amoxicillin four times daily, for 14 days. The fourth study (63 patients), with a 58% eradication, administered 20 mg of rabeprazole plus 1 g of amoxicillin twice a day, for 14 days. The differences between these studies may be explained by the different dosage scheme, especially on the pharmacokinetics of these drugs. An oral dose of 500 mg of amoxicillin is rapidly absorbed, with a peak in plasma concentrations between 1 and 2 hours, and approximately a 75% is excreted between 6 and 8 hours after its administration. A dosage of 500 mg every 6 hours can probably maintain higher plasma dosages of amoxicillin than a dosage of 1000 mg twice daily 26.
The large multicenter surveillance studies have confirmed that resistance of H. pylori to amoxicillin is in the order of 1% 27,28. In Korea, primary amoxicillin resistance was seen in 2.2-5.6% (mean 2.3%, 8/350) 29,30. Other clinical trials did not detect any pre- or post-treatment resistance to amoxicillin 31,32. Also, it is known that the genotype of CYP2C19 is associated with the metabolism of the PPIs. Not knowing whether the patients are extensive or poor metabolizers, or their CYP2C19 polymorphisms, higher PPI doses or newer PPI types might be more effective 33–35. Potent acid inhibition is important for the eradication of H. pylori. Potent inhibition increases the stability and antibiotics in gastric mucosa 34. Furthermore, acid inhibition allows H. pylori to reach its growth phase, rendering the bacteria more sensitive to antibiotics. The distribution frequencies of people with the homEM genotype are 30–40% in Asian countries 36. The usual doses PPIs used in the standard regimen are insufficient for patients with the homEM genotype, as well as that a higher dose of a PPI causes few adverse effects and is considered to be sufficiently safe 36. The rifabutin-based high-dose PPI and amoxicillin combined therapy can resolve the problems of clarithromycin and metronidazole resistance and homozygous extensive metabolizers of CYP2C19 gene polymorphisms, the main reasons for eradication failure 37.
Rifabutin-based triple therapy was found to be highly effective and reliable as an alternative rescue therapy for the treatment of H. pylori infection after two failed eradication treatments. Borody et al. showed a 12 days of half the dose of rifabutin (150 mg daily) combined with a high dose of pantoprazole (80 mg thrice daily) and amoxicillin (1 g or 1.5 g thrice daily) produced higher eradication rates of 91 and 97%, respectively, as a rescue therapy, suggesting that the treatment regimen is considered highly effective as a rescue therapy. In the presence of low-dose rifabutin (150 mg), combined frequent high dosing of PPI and amoxicillin for 12 days is effective for rescue therapy 38. Our present open single-center study showed that high dose of PPI (lansoprazole 60 mg twice daily) and amoxicillin (1 g trice daily) combined rifabutin therapy achieved significant better eradication rate (100%) than standard dose of PPI (lansoprazole 30 mg twice daily) 80.6% in PP analysis (p = .047) after failures in two courses of previous H. pylori eradication therapy. Primary rifabutin resistance in H. pylori isolates is low, ranging from 1.3% to 2.4%. Unlike other antibiotics, rifabutin is chemically stable at a wide pH range and is not likely affected by inadequate acid suppression 39. The combination of rifabutin with either metronidazole or amoxicillin shows additive effects 40.
For treating H. pylori infection, the length of treatment for the rifabutin regimen has controversies, as does the influence this has on the treatment outcome. A mean H. pylori eradication rate of 75% (95% CI from 68 to 83%) for the 7-day regimen was calculated, while the 10- to 14-day regimen was similar or even slightly lower (71%; 95% CI, 63–79%) 21. However, as previously reviewed, when a subanalysis was performed depending on the duration of the second-line rifabutin therapy, better results were observed with 10–12 days (92%) than with 7 days (69%). Finally, therapies between 12 and 14 days have yielded results similar to the 10-day course and are likely to increase the incidence of adverse event 21.
Our data suggest that in the presence of 7 days relatively short duration, frequent dosing of amoxicillin and a high-dose PPI can improve eradication rate and reducing side effects. It has been suggested that rifabutin efficacy decreases with increasing number of failed previous therapies, perhaps due to patients who had failed at least two courses of eradication therapy and may have harbored H. pylori strains that were more difficult to eradicate 41. Overall, mean H. pylori eradication rate (intention-to-treat analysis) with rifabutin-containing regimens (1008 patients) was 73% (67–79%). Respective cure rates for second-line (223 patients), third-line (342 patients), and fourth-/fifth-line (95 patients) rifabutin therapies were 79% (67–92%), 66% (55–77%), and 70% (60–79%), respectively 41. Antimicrobial susceptibility testing of H. pyloriis was desirable before initiation of third-line therapy, although the culture-based antibiotic susceptibility testing for H. pylori is expensive, time-consuming, and not always available on a routine basis 42. The sensitivity of a bacterial culture is not 100%, and therefore, the antimicrobial susceptibility cannot be obtained in all cases. Therefore, rifabutin (together with a PPI and amoxicillin) can be administered as a rescue treatment without the need for a prior antibiogram 13.
The mean rate of adverse effects to rifabutin treatment in H. pylori studies has been approximately 22% 21. In our study, this incidence was reported to be somewhat lower (15.5%), but in most cases, symptoms were well tolerated and no side effects were considered serious. In our study, most frequent side effects were epigatric pain (7.0%), epigastric discomfort (5.2%), and nausea (3.5%). Myelotoxicity is the most significant adverse event of rifabutin. Overall, this complication is rare and is far more likely when high dose (600 mg/day) and prolonged duration therapy is used. However, myelotoxicity was not reported in most of the studies evaluating rifabutin for H. pylori infection. Until now, all patients have recovered from leucopenia uneventful in few days 43,44. Several concerns remained regarding rifabutin treatment. First, this drug is extremely expansive; second, severe leucopenia and thrombocytopenia can occur to a patient under treatment with rifabutin. Finally, there is some concern about widespread use of rifabutin, a member of class of established antimycobacterial drugs in patients with H. pylori infection 45. Because multiresistant strains of Mycobacterium tuberculosis have increased in number, indications for these drugs should be chosen very carefully to avoid further acceleration of development of resistance. Rifabutin should be used only as rescue therapy after amoxicillin, clarithromycin, metronidazole, tetracycline, and levofloxacin have failed to eradicate to patients who have experienced failure of H. pylori 42. The main limitation of our study is that it was a single-centered study and that no antibiotic testing has been performed.
In conclusion, rifabutin-based high-dose PPI-combined therapy as empirical rescue treatment is more effective than standard dose PPI-combined rifabutin-based therapy, safe and best tolerable in third-line therapy in the Korean population. The key to successful rescue therapy with rifabutin–amoxicillin–PPI regimen may be to increase doses of PPI and amoxicillin. Further randomized clinical trials with antimicrobial susceptibility tests are needed.
Acknowledgments
The authors would like to thank Yong Chan Lee, MD, PhD (leeyc@yuhs.ac, Yonsei University, College of Medicine, Internal Medicine) who helped in designing the study protocol and reviewing the study results.
Competing interests: the authors have no competing interests.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Qua CS, Manikam J, Goh KL. Efficacy of 1-week proton pump inhibitor triple therapy as first-line Helicobacter pylori eradication regime in Asian patients: is it still effective 10 years on? J Dig Dis. 2010;11:244–8. doi: 10.1111/j.1751-2980.2010.00445.x. [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki M, Ogasawara N, Utsumi K, et al. Changes in 12-year first-line eradication rate of Helicobacter pylori based on triple therapy with proton pump inhibitor, amoxicillin and clarithromycin. J Clin Biochem Nutr. 2010;47:53–8. doi: 10.3164/jcbn.10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connor A, Gisbert JP, McNamara D, O'Morain C. Treatment of Helicobacter pylori infection 2010. Helicobacter. 2010;15:46–52. doi: 10.1111/j.1523-5378.2010.00774.x. [DOI] [PubMed] [Google Scholar]
- 6.Selgrad M, Malfertheiner P. Treatment of Helicobacter pylori. Curr Opin Gastroenterol. 2011;27:565–70. doi: 10.1097/MOG.0b013e32834bb818. [DOI] [PubMed] [Google Scholar]
- 7.Park HG, Jung MK, Jung JT, et al. Randomized clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naive patients. Aliment Pharmacol Ther. 2012;35:56–65. doi: 10.1111/j.1365-2036.2011.04902.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor A, Gisbert JP, McNamara D, O'Morain C. Treatment of Helicobacter pylori infection 2011. Helicobacter. 2011;16:53–8. doi: 10.1111/j.1523-5378.2011.00881.x. [DOI] [PubMed] [Google Scholar]
- 9.Gumurdulu Y, Serin E, Özer B, Kayaselcuk F, Ozsahin K, Cosar AM, Gursoy M, Gur G, Yilmaz U, Boyacioglu S. Low eradication rate of Helicobacter pylori with triple 7–14 days and quadruple therapy in Turkey. World J Gastroenterol. 2004;10:668–71. doi: 10.3748/wjg.v10.i5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bigard MA, Delchier JC, Riachi G, Thibault P, Barthelemy P. One-week triple therapy using omeprazole, amoxicillin and clarithromycin for the eradication of Helicobacter pylori in patients with non-ulcer dyspepsia: influence of dosage of omeprazole and clarithromycin. Aliment Pharmacol Ther. 1998;12:383–8. doi: 10.1046/j.1365-2036.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- 11.Chuah SK, Tsay FW. Hsu PI, Wu DC. A new look at anti- Helicobacterpylori therapy. World J Gastroenterol. 2011;17:3971–5. doi: 10.3748/wjg.v17.i35.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gisbert JP, Gisbert JL, Marcos S, Moreno-Oteero R, Pajares JM. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment Pharmacol Ther. 2006;24:167–80. doi: 10.1111/j.1365-2036.2006.03149.x. [DOI] [PubMed] [Google Scholar]
- 13.Gisbert JP, Pajares JM. Helicobacter pylori “rescue” therapy after failure of two eradication treatments. Helicobacter. 2005;10:363–72. doi: 10.1111/j.1523-5378.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 14.Cianci R, Montalto M, Pandolfi F, Gasbarini GB, Cammarota G. Third-line rescue therapy for Helicobacter pylori infection. World J Gastroenterol. 2006;12:2313–9. doi: 10.3748/wjg.v12.i15.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong WM, Gu Q, Lam SK, et al. Randomized controlled study of rabeprazole, levofloxacin and rifabutin triple therapy vs. quadruple therapy as second-line treatment for Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:553–60. doi: 10.1046/j.1365-2036.2003.01459.x. [DOI] [PubMed] [Google Scholar]
- 16.Toracchio S, Capodicasa S, Soraja DB, Cellini L, Marzio L. Rifabutin based triple therapy for eradication of H. pylori primary and secondary resistant to tinidazole and clarithromycin. Dig Liver Dis. 2005;37:33–8. doi: 10.1016/j.dld.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Heep M, Beck D, Bayerdorffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother. 1999;43:1497–9. doi: 10.1128/aac.43.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canducci F, Ojetti V, Pola P, Gasbarrini G, Gasbarrini A. Rifabutin-based Helicobacter pylori eradication rescue therapy. Aliment Pharmacol Ther. 2001;15:143. doi: 10.1046/j.1365-2036.2001.00909.x. [DOI] [PubMed] [Google Scholar]
- 19.Perri F, Festa V, Clemente R, Quitadamo M, Andriulli A. Rifabutin-based rescue therapy for Helicobacter pylori infected patients after failure of standard regimens. Aliment Pharmacol Ther. 2000;14:311–6. doi: 10.1046/j.1365-2036.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 20.Qasim A, Sebastian S, Thornton O, Dobson M, McLoughlin R, Buckley M, O'Connor H, Morain C. Rifabutin- and furazolidone-based Helicobacter pylori eradication therapies after failure of standard first- and second-line eradication attempts in dyspepsia patients. Aliment Pharmacol Ther. 2005;21:91–6. doi: 10.1111/j.1365-2036.2004.02210.x. [DOI] [PubMed] [Google Scholar]
- 21.Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35:209–21. doi: 10.1111/j.1365-2036.2011.04937.x. [DOI] [PubMed] [Google Scholar]
- 22.Athamna A, Athamna M, Medlej B, Bast DJ, Rubinstein E. In vitro post-antibiotic effect of fluroquinolones, macrolides, beta-lactams, tetracyclines, vancomycin, clindamycin, linezolid, chloroamphenicol, quinupristin/dafopristin and rifampicin on Bacillus anthraces. J Antimicrob Chemother. 2004;53:1469–74. doi: 10.1093/jac/dkh130. [DOI] [PubMed] [Google Scholar]
- 23.Shirai N, Sugimoto M, Kodaira C, Nishino M, Ikuma M, Kajimura M, Ohashi K, Takashi I, Hishida A, Furuta T. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007;63:743–9. doi: 10.1007/s00228-007-0302-8. [DOI] [PubMed] [Google Scholar]
- 24.Miehlke S, Hansky K, Schneider-Brachert W, et al. Randomized trial of rifabutin-based triple therapy and high –dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment Pharmacol Ther. 2006;24:395–403. doi: 10.1111/j.1365-2036.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- 25.Furuta T, Shirai N, Xiao F, Takashita M, Sugimoto M, Kajimura M, Ohashi K, Ishizaki T. High-dose rebeprazole/amoxicillin therapy as the second-line regimen after failure to eradicate therapy H. pylori by triple therapy with the usual doses of a proton pump inhibitor, clarithromycin and Amoxicillin. Heaptogastreoenterology. 2003;50:2274–8. [PubMed] [Google Scholar]
- 26.Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy(for Helicobacter pylori eradication) Expert Opin Pharmacother. 2003;14:843–61. doi: 10.1517/14656566.2013.782286. [DOI] [PubMed] [Google Scholar]
- 27.Meyer JM, Sailinan NP, Qang W, Siepman NY, Sugg JE, Morris D, Zhang J, Bhattacharyya H, King EC, Hopkins RJ. Risk factors for Helicobacter pylori resistance in the United States: the surveillance of H. pylori antimicrobial resistance partnership(SHARP) study. 1993–1999. Ann Intern Med. 2002;136:13–24. doi: 10.7326/0003-4819-136-1-200201010-00008. [DOI] [PubMed] [Google Scholar]
- 28.Glupezynski Y, Megaud F, Lopez-brea M, Andersen LP. European multicenter survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 2001;20:820–3. doi: 10.1007/s100960100611. [DOI] [PubMed] [Google Scholar]
- 29.Chung JW, Lee GH, Jeong JY, Lee SM, Jung JH, Choi KD, Song HJ, Jung HY, Kim JH. Resistance of Helicobacter pylori restrains to antibiotics in Korea with a focus on fluoroquinolone resistance. J Gastroenterol Hepatol. 2012;27:493–7. doi: 10.1111/j.1440-1746.2011.06874.x. [DOI] [PubMed] [Google Scholar]
- 30.An B, Moon BS, Kim HJ, Lim HC, Lee YC, Lee GS, Kim SH, Park M, Kim JB. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in single center in Korea. Ann Lab Med. 2013;13:1–5. doi: 10.3343/alm.2013.33.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heep M, Kist M, Strobel S, Beck D, Lehn N. Secondary resistance among 554 isolates of Helicobacter pylori after failure of therapy. Eur J Clin Microbiol Infect Dis. 2000;19:538–41. doi: 10.1007/s100960000288. [DOI] [PubMed] [Google Scholar]
- 32.Gomollon F, Sicilia B, Ducons JA, Sierra E, Revillo M. Third-line treatment for Helicobacter pylori: a prospective, culture- guided study in peptic ulcer patients. Aliment Pharmacol Ther. 2000;14:1335–8. doi: 10.1046/j.1365-2036.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, Ishizaki T, Hishida A. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12:317–23. doi: 10.1111/j.1523-5378.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 34.Grayson ML, Eliopoulos GM, Ferraro MJ, Moellering RC., Jr Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1989;8:888–9. doi: 10.1007/BF01963775. [DOI] [PubMed] [Google Scholar]
- 35.McNicholl Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36:414–25. doi: 10.1111/j.1365-2036.2012.05211.x. [DOI] [PubMed] [Google Scholar]
- 36.Furuta T, Shirai N, Misako T, Xiao F, Hanai H, Sugimura H, Ohashi K, Ishizaki T, Kaneko E. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001;69:158–68. doi: 10.1067/mcp.2001.113959. [DOI] [PubMed] [Google Scholar]
- 37.Miura S, Hokari R. Seeking an optimal eradication therapy for Helicobacter pylori. Gut. 1998;43:S56–60. doi: 10.1111/j.1440-1746.2011.06953.x. [DOI] [PubMed] [Google Scholar]
- 38.Borody TJ, Pang G, Wettstein AR, Clancy R, Herdman R, Sugare R. Efficacy and safety of rifabutin containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;23:481–8. doi: 10.1111/j.1365-2036.2006.02793.x. [DOI] [PubMed] [Google Scholar]
- 39.Song M, Ang TL. Second and third line treatment options for Helicobacter pylori eradication. World J Gastroenterol. 2014;20:1517–27. doi: 10.3748/wjg.v20.i6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bock H, Koop H, Lehn N, Heep M. Rifabutin-based triple therapy after failure of Helicobacter pylori eradication treatment: a preliminary experience. J Clin Gastroenterol. 2000;31:222–5. doi: 10.1097/00004836-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Gisbert J, Pajares J. Helicobacter pylori rescue’ regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol Ther. 2002;6:1047–57. doi: 10.1046/j.1365-2036.2002.01276.x. [DOI] [PubMed] [Google Scholar]
- 42.Peitz U, Sulliga M, Wolle M, et al. High rate of post-therapeutic resistance after failure of macrolide-nitroimidazole triple therapy to cue Helicobacter pylori infection: impact of two-second-line therapies in a randomised study. Aliment Pharmacol Ther. 2002;16:315–24. doi: 10.1046/j.1365-2036.2002.01173.x. [DOI] [PubMed] [Google Scholar]
- 43.Breuer T, Graham DY. Cost of diagnosis and treatment of Helicobacter pylori infection: when does choosing the treatment regimen based on susceptibility testing become cost effective? Am J Gastroenterol. 1999;94:725–9. doi: 10.1111/j.1572-0241.1999.00943.x. [DOI] [PubMed] [Google Scholar]
- 44.Griffith DE, Brown BA, Girard WM, Wallace RJ., Jr Adverse events associated with high-dose rifabutin in macrolide containing regimens for the treatment of Mycobacterium avium complex lung disease. Clin Infect Dis. 1995;21:594–8. doi: 10.1093/clinids/21.3.594. [DOI] [PubMed] [Google Scholar]
- 45.Apseloff G, Fluids G, LaBoy-Goral L, Kraut E, Vincent J. Severe neutropenia caused by recommended prophylactic doses of rifabutin. Lancet. 1996;348:685. doi: 10.1016/s0140-6736(05)65109-4. [DOI] [PubMed] [Google Scholar]


