Abstract
Background
Rifaximin therapy reduced risk of hepatic encephalopathy (HE) recurrence and HE-related hospitalisations during a 6-month, randomised, placebo-controlled trial (RCT) and a 24-month open-label maintenance (OLM) study. However, the impact of crossover from placebo to rifaximin therapy is unclear.
Aim
To study the impact of crossing over from placebo to rifaximin treatment on breakthrough HE and hospitalisation rates using a within-subjects design.
Methods
Adults with cirrhosis and history of overt HE episodes, currently in HE remission, received placebo during the RCT and crossed over to rifaximin 550 mg twice daily during the OLM study. Rate of breakthrough overt HE episodes, hospitalisations and incidence and rate of adverse events (AEs) were analysed during RCT and first 6 months of OLM.
Results
Of 82 patients randomised to placebo in the RCT who crossed over to the OLM study, 39 experienced an HE episode during the RCT compared with 14 during the OLM study (P < 0.0001). Significantly lower rates of HE events were observed with rifaximin treatment compared with placebo treatment (P < 0.0001). Rates of HE-related hospitalisation were numerically lower during rifaximin treatment compared with placebo treatment, although not significant. Rates of most common AEs, serious AEs and infection-related AEs were similar between the two treatments.
Conclusions
This analysis confirms the repeatability of results from the RCT on safety and efficacy of rifaximin 550 mg twice daily in reducing the risk of hepatic encephalopathy recurrence, and suggests these findings are translatable outside of a rigorous, controlled trial setting.
Introduction
Hepatic encephalopathy (HE) is a potentially debilitating complication of cirrhosis and is associated with neuropsychiatric symptoms and neuromuscular dysfunction of varying severity.1,2 The symptoms of HE can include confusion, disorientation and poor coordination. Overt HE imposes a substantial quality-of-life and socioeconomic burden on patients and caregivers.3–5 Prevention of HE episodes may positively impact both pre- and post-liver transplantation outcomes.6,7 The underlying pathogenesis of HE is unknown, but it is thought to be driven by cerebral oedema resulting from the combined action of accumulation of gut-derived bacterial toxins (e.g. ammonia), inflammation and oxidative stress.8–10 Most therapies for HE are, therefore, directed at removal of gut-derived bacterial toxins or modulating gut microbiota levels.11–13
Rifaximin is a minimally absorbed, gut-targeted, oral antimicrobial therapy that has been evaluated in a randomised, double-blind, placebo-controlled, 6-month trial (RCT).14 In patients with cirrhosis with a recent history of recurrent, overt HE, rifaximin significantly reduced the risk of HE recurrence and HE-related hospitalisation, and improved patient quality-of-life with an adverse event (AE) profile comparable to that of placebo.14,15 Long-term treatment with rifaximin during a ≥24-month, open-label maintenance (OLM) study provided continued protection from HE recurrence, reduced the risk of HE-related hospitalisation, and did not adversely affect patient tolerability.16 The OLM study was a controlled, yet pragmatic study comprised of different patient groups who were either continuing from the RCT or were recruited specifically for the OLM study. However, a direct comparison between rifaximin and placebo is still needed to extend the findings of the RCT into outcomes outside of the context of an RCT.
The objective of this study was to examine the repeatability of the safety and efficacy findings from the RCT by analysing data from patients initially treated with placebo who crossed over to receive rifaximin during the OLM study.
Methods
Study population
This study included adults with cirrhosis and a history of overt HE episodes treated with placebo in a 6-month RCT, who crossed over to receive rifaximin 550 mg twice daily in a ≥24-month OLM study.14,16 Patients eligible for the RCT study had a documented history of HE (Conn score ≥2; 5-point scale, where 0 = no abnormality and 4 = coma) within 6 months prior to screening, a Conn score of ≤1 at enrolment, and a Model for End-Stage Liver Disease (MELD) score ≤25.14 Eligible patients in the OLM study had a documented Conn score of ≥2 within the 12 months prior to screening and a Conn score of ≤2 at enrolment. Patients from the RCT were permitted to enrol in the OLM study and, if possible, were transitioned into the OLM study at the end of treatment visit of the RCT. Episodes of HE precipitated by gastrointestinal haemorrhage requiring ≥2 units of blood by transfusion, by medication use, by renal failure requiring dialysis or by injury to the central nervous system were not considered previous HE episodes to enable patients to meet eligibility criteria for either study.14 Each patient was required to have the support of a caregiver for the entire duration of the study. The caregiver assisted with attending scheduled and unscheduled study visits, monitored any changes in the patient's health or HE status, reminded the patient to take study medication and reminded the patient to complete daily diary entries.
Study design
Details on the designs of the RCT and OLM study have been previously published.14,16 Briefly, the RCT was a double-blind, placebo-controlled, multicenter trial (ClinicalTrials.gov identifier, NCT00298038), and patients were randomly assigned to receive either rifaximin (Xifaxan; Salix Pharmaceuticals, Inc., Raleigh, NC, USA) 550 mg twice daily or placebo for 6 months. The OLM study was a multicenter study (ClinicalTrials.gov identifier, NCT00686920) in which patients received rifaximin 550 mg twice daily for ≥24 months.16 Participants in the OLM study were either newly enrolled or continued from the RCT, and included patients who had received placebo during the RCT. Concomitant therapy with lactulose was optional during both studies.14 The protocols for the RCT and OLM study were approved by the institutional review boards of each center, and all patients or their legally authorised representatives provided written informed consent.14,16
Assessments
Clinic visits during the RCT occurred on days 7 and 14 and every 2 weeks thereafter through day 168 (end of treatment period), and patients were monitored by telephone during weeks without clinic visits. Clinic visits during the OLM study occurred at months 1 and 3, and then every 3 months thereafter until the end of treatment, with additional telephone contacts at week 2 and every 6 weeks after month 3. In both protocols, if a subject developed signs or symptoms of HE between clinical visits, the site conducted an unscheduled visit to evaluate the subject. Also, the investigator was required to make every effort to determine when the onset of recurrent HE symptoms first developed, which could have included, for example, a retrospective review of medical records from a treating physician and/or a discussion with the caregiver or patient regarding the symptoms experienced. Efficacy end points analysed included the rate of breakthrough episodes of HE and HE-related hospitalisations. Breakthrough overt HE was defined as an increase in Conn score to ≥2, an increase of 1 grade each for both Conn score and asterixis score for patients who had entered with a Conn score of 0, or an increase in Conn score to ≥3 for patients who had entered the OLM with a Conn score of 2. Safety assessments included monitoring of AEs, clinical laboratory tests, vital signs, and concomitant medications. Changes from baseline in clinical laboratory parameters to day 168 (or end of treatment) in the RCT and OLM study were assessed.
Statistical analysis
Efficacy and safety analyses were conducted for patients who were treated with placebo in the RCT and during the first 6 months of treatment for those who crossed over to rifaximin treatment in the OLM study. Demographical and baseline disease characteristics were summarised using descriptive statistics. Data are mean ± s.d. unless otherwise noted. The Cox proportional hazards model was used, with a two-sided test and a significance level of 0.05, to compare the time to breakthrough episode during placebo treatment in the RCT with timing during rifaximin treatment in the OLM study. Kaplan–Meier methods were used to estimate the percentage of patients maintaining HE remission over time. Person-years of exposure (PYE) for rifaximin was calculated as total exposure in days ÷ 365.25. Rate of AEs was calculated as number of patients with an event ÷ PYE, in which PYE reflected exposure up until the AE occurrence and, therefore, may have differed from the PYE for the entire patient group.
Results
Patient population
A total of 299 patients were randomised to receive rifaximin (n = 140) or placebo (n = 159) in the 6-month RCT. Of the 159 patients who received placebo in the RCT, 82 patients were enrolled in the OLM study and crossed over to rifaximin treatment (Figure1). The 82 patients in this crossover group were predominantly male (62.2%) and white (90.2%), with a mean age of 55.8 ± 9.2 years. At baseline of the RCT phase, 69.5% of patients had experienced two breakthrough HE episodes in the previous 6 months, 22% had experienced three episodes and 8.5% had experienced >3 episodes. The mean time since first diagnosis of HE was 18.8 ± 19.6 months. Comparing baseline disease characteristics during the two treatments, mean MELD scores were similar at baseline in both the RCT and OLM study (Table1). The distributions of Conn scores and asterixis grades were also similar at baseline across the two treatment periods.
Figure 1.
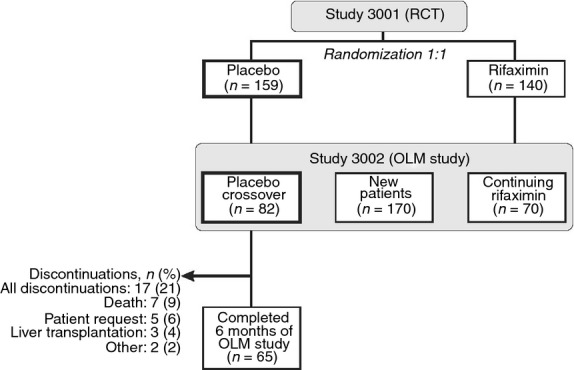
Patient disposition. OLM, open-label maintenance; RCT, randomised, controlled trial. Data from Bass et al.14
Table 1.
Baseline disease characteristics for the RCT and OLM study
| Parameter | RCT placebo treatment (n = 82) | OLM study rifaximin treatment (n = 82) |
|---|---|---|
| Duration of current remission, days, mean (s.d.) | 72.7 (50.5) | 156.6 (125.6) |
| MELD score, mean (s.d.) | 12.1 (3.8) | 12.1 (3.9) |
| MELD score, n (%) | ||
| ≤10 | 30 (36.6) | 34 (41.5) |
| 11–18 | 46 (56.1) | 44 (53.7) |
| ≥19 | 6 (7.3) | 4 (4.9) |
| Conn score, n (%) | ||
| 0 | 59 (72.0) | 56 (68.3) |
| 1 | 23 (28.0) | 19 (23.2) |
| 2* | 0 | 7 (8.5) |
| Asterixis grade, n (%) | ||
| 0 | 59 (72.0) | 60 (73.2) |
| 1 | 19 (23.2) | 15 (18.3) |
| ≥2 | 4 (4.9) | 7 (5.4) |
HE, hepatic encephalopathy; MELD, model for end-stage liver disease; OLM, open-label maintenance; RCT, randomised, controlled trial.
Baseline Conn score inclusion criteria differed between the two studies. For the RCT, patients were required to have a Conn score of 0 or 1 at enrolment. For the open-label study, patients could have a Conn score of 0, 1 or 2.
Efficacy
The comparison of time to first breakthrough overt HE episode (using Kaplan–Meier methods) between the placebo experience in the RCT and during the first 6 months of rifaximin treatment in the OLM is shown in Figure2. The ratio of the incidence of breakthrough overt HE episode for rifaximin treatment relative to placebo treatment was 0.21 (95% CI: 0.10–0.44; P < 0.0001 for between group difference in relative risk). This result represents a 79% reduction in the risk of experiencing breakthrough overt HE during rifaximin treatment in the OLM when compared with their prior placebo experience in the RCT, and corresponds to a number needed to treat of 3 to prevent a breakthrough HE episode during 6 months of treatment. Of the 82 patients, 39 patients (47.6%) experienced an episode of HE during placebo treatment in the 6-month RCT, and 14 patients (17.1%) experienced an HE episode during rifaximin treatment during the first 6 months of the OLM study. This represents a significant reduction in the rate of breakthrough HE events after switching from placebo to rifaximin treatment (P < 0.0001). Of the 39 patients who had an HE episode during placebo treatment in the RCT, only 13 (33.3%) also had an HE episode during the first 6 months of treatment with rifaximin in the OLM study.
Figure 2.
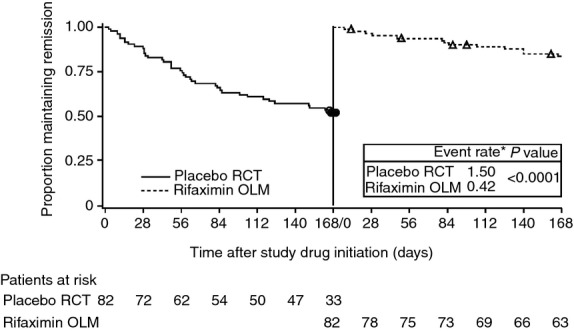
Time to first breakthrough HE event during placebo treatment in the RCT and during rifaximin treatment in OLM study. Open circles and open triangles represent censored data. *Event rate was calculated for 168 days of the RCT and the first 168 days in the OLM study. HE, hepatic encephalopathy; OLM, open-label maintenance; RCT, randomised, controlled trial.
The temporal profile of HE breakthrough events across the 82 patients could be described with four categories: (i) HE breakthrough episode occurrence in the RCT only: n = 26; (ii) occurrence in both the RCT and OLM study: n = 13; (iii) occurrence in the OLM study only: n = 1; and (iv) no occurrences in the RCT or OLM study: n = 42. Patients with no occurrence of HE in the RCT or OLM study had a slightly lower mean (±s.d.) MELD score (11.1 ± 3.7) at baseline compared with patients who experienced an HE occurrence during the RCT (13.1 ± 4.0) or during the RCT and OLM study (13.2 ± 3.5).
There was a trend towards reduction in HE-related hospitalisations during the first 6 months of rifaximin treatment in the OLM study (12 events; rate = 0.36 events/PYE) vs. placebo treatment in the RCT (15 events; rate = 0.57 events/PYE; P = 0.365; Figure3). However, there was no reduction in all-cause hospitalisations during the first 6 months of open-label rifaximin treatment.
Figure 3.
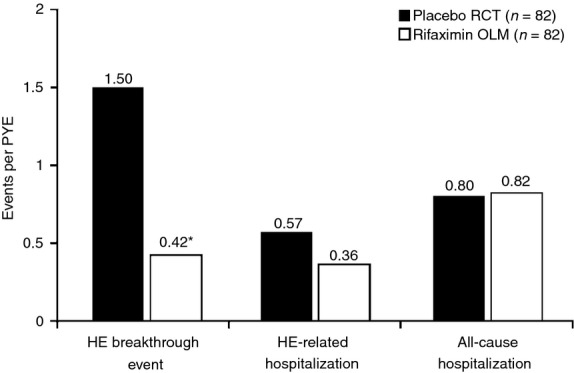
Rate of HE breakthrough episodes and HE-related and all-cause hospitalisations. The event rate was calculated for the first 6 months of rifaximin exposure during the OLM study. *P < 0.0001 vs. placebo administration during the RCT. HE, hepatic encephalopathy; OLM, open-label maintenance; PYE, person-years of exposure; RCT, randomised, controlled trial.
Safety
The rate of the most commonly reported AEs (ascites, headache, nausea and peripheral oedema) in the 82 patients during first 6 months of the OLM study was similar to that observed for the same patients in the RCT (Table2). The most common serious AEs (occurring in ≥2 patients) during 6 months of rifaximin treatment were anaemia, ascites, cellulitis and hyponatremia in three patients each, and acute renal failure, chest pain, hepatic cirrhosis, hypoglycaemia, hyperkalemia, pneumonia and urinary tract infection in two patients each. The most common serious AEs during the RCT in this group were atrial fibrillation, bacterial peritonitis and cellulitis in two patients each.
Table 2.
Summary of adverse events
| Adverse events | RCT placebo treatment (n = 82; PYE = 26.6) | OLM study 6-month rifaximin treatment (n = 82; PYE = 36.4) |
|---|---|---|
| Most common AEs*, n (rate†) | ||
| Ascites | 5 (0.19) | 9 (0.27) |
| Headache | 9 (0.38) | 0 (0.0) |
| Nausea | 11 (0.47) | 9 (0.26) |
| Peripheral oedema | 9 (0.36) | 10 (0.29) |
| Infection-related AEs‡, n (rate†) | ||
| Cellulitis | 2 (0.08) | 6 (0.17) |
| Peritonitis | 3 (0.11) | 2 (0.06) |
| Pneumonia | 0 (0.0) | 3 (0.08) |
| Sepsis/septic shock | 2 (0.08) | 3 (0.08) |
| Urinary tract/kidney infection | 7 (0.29) | 5 (0.14) |
AE, adverse event; PYE, person-years of exposure; RCT, randomised, controlled trial.
Reported in ≥10% of patients during therapy with either treatment.
AE rates were calculated as number of patients with an event ÷ PYE, in which PYE reflected exposure up until the AE occurrence and, therefore, may have differed from the PYE for the entire patient group.
Reported in ≥2 patients during therapy with either treatment. Peritonitis included bacterial peritonitis. Pneumonia included lobar pneumonia. Sepsis/septic shock included bacteremia, Escherichia bacteremia, fungemia, Klebsiella bacteremia, Staphylococcus bacteremia and urosepsis.
Rates of the most commonly reported infections during 6 months of rifaximin treatment in the OLM study were generally comparable with those observed during placebo treatment. No clinically significant changes in laboratory values from baseline to month 6 of rifaximin in the OLM study were observed. In addition, no significant differences in mean change from baseline were noted for coagulation test results when comparing placebo treatment during the RCT to 6 months of rifaximin treatment in the OLM study (prothrombin time; −0.04 s vs. 0.42 s, respectively) and international normalised ratio (−0.04 vs. −0.01, respectively).
Discussion
Rifaximin 550 mg administered twice daily for 6 months has been shown in an RCT to maintain HE remission vs. placebo in patients with cirrhosis and a recent history of overt HE.14 In addition, the durability of HE remission with daily rifaximin therapy was demonstrated during long-term administration of rifaximin 550 mg twice daily for at least 24 months.16 The current post hoc analysis examined the repeatability of these findings for rifaximin by evaluating the response of patients who were initially treated with placebo during the RCT and crossed over to treatment with rifaximin for 6 months in the OLM study. Evaluating patients who crossed over from placebo to rifaximin therapy minimised patient heterogeneity during analyses, as each patient acted as his or her own control.
The current analysis confirmed and expanded upon results from the RCT and OLM study by demonstrating that patients who switched from placebo to rifaximin treatment in the OLM study experienced a significant protective effect against HE recurrence. More than 65% of the patients who experienced an overt HE episode during placebo treatment in the RCT were protected from a recurrent episode during the first 6 months of rifaximin therapy in the OLM study. It is intriguing that the NNT of 3 observed in this analysis is similar to what was reported in the RCT (NNT of 4).14 This is encouraging, because it indicates that the efficacy of rifaximin to prevent HE was maintained and is translatable to conditions outside of a rigorous, randomised and controlled trial. Prospective studies in independent populations during routine clinical practice are, nevertheless, important for confirming these findings. Also noteworthy is that 16% of patients had a recurrence of HE during both placebo and rifaximin treatment. It is not clear from the data why these particular patients were pre-disposed to breakthrough HE episodes, but it shows that patients with HE, despite treatment, may still need continued care to receive prompt medical treatment if HE episodes recur.
Similarly, there was a trend towards a lower rate of HE-related hospitalisations in these patients during the first 6 months of the OLM study compared with placebo administration during the RCT. While not reaching statistical significance in this analysis, it should be noted that Mullen et al. reported a marked reduction in the rate of HE-related hospitalisations, relative to historical placebo controls, when investigating long-term (≥2 year) outcomes in the entire population (N = 392) exposed to rifaximin.16
This study design and findings are analogous to a retrospective study that compared incidence of HE-related hospitalisations in patients with HE who were treated with lactulose for ≥6 months followed by rifaximin for ≥6 months, and demonstrated that rifaximin treatment was associated with a lower frequency and shorter duration of hospitalisations.17 Consistent with these findings, a case–control study in a group of patients with alcoholic cirrhosis demonstrated an independent association between long-term administration (up to 5 years) of rifaximin and a lower risk of developing HE, variceal bleeding, spontaneous bacterial peritonitis and hepatorenal syndrome.18
The safety profile and rates of AEs observed in this patient population were similar to what was previously reported for patients in the RCT and long-term OLM study,14,16 and rifaximin appears to be suitable for maintenance therapy in patients with cirrhosis and a history of HE. The favourable safety profile of rifaximin may be attributed to its minimal systemic absorption, gut-targeted local action and low risk for development of bacterial antibiotic resistance.19,20
The ability of the patients to serve as their own controls to compare rates of HE breakthrough events, HE-related hospitalisations and AEs before and after initiation of rifaximin therapy is a strength of this study. The trend towards reduction in hospitalisations is a critical issue in patients with cirrhosis, given the high costs as well as risk for nosocomial infections.3,21
The post hoc nature of the analyses, as well as the open-label and, thus, unblinded administration of rifaximin in the OLM, are study limitations. In addition, the less frequently scheduled contacts with study personnel in the OLM relative to the RCT may have compromised the ability to detect breakthrough HE episodes in the OLM period. It should be emphasised, however, that as a part of the inclusion criteria, caregivers were required to be available and to provide ongoing patient support during the entire duration of the study. Any changes in mental status prompted an unscheduled visit to the study site. Importantly, the majority of breakthrough HE episodes in both treatment periods (59% in the RCT vs. 86% in the OLM, P = 0.10 by Fisher's exact test) were diagnosed by retrospective review rather than in-person assessment of the patient. Finally, the Kaplan–Meier curve describing the time to first breakthrough HE episode for rifaximin-treated patients in the OLM was nearly superimposable to that obtained for the rifaximin-treated patients in the original RCT.14 Taken together, these findings suggest that the less frequently scheduled visits in the OLM did not contribute appreciably to the present findings.
In conclusion, findings from this study lend further support to the repeatability and durability of rifaximin treatment effects. This analysis, although limited in certain regards, reinforces the potential benefits of daily rifaximin therapy in maintaining HE remission for patients with cirrhosis and a history of recurrent HE.
Authorship
Guarantor of the article: WP Forbes.
Author contributions: JS Bajaj conceptualised the sub-analysis and critically reviewed and edited the manuscript. AC Barrett analysed data and critically reviewed and edited the manuscript. E Bortey contributed to clinical trial study design, analysed data and critically reviewed and edited the manuscript. C Paterson analysed data and critically reviewed and edited the manuscript. WP Forbes contributed to clinical trial study design, analysed data, and critically reviewed and edited the manuscript. All authors approved the final version of the article for submission, including the authorship list.
Acknowledgments
Technical editorial and medical writing assistance was provided, under the direction of the authors, by Pratibha Hebbar, PhD, for Synchrony Medical Communications, LLC, West Chester, PA.
Declaration of personal interests: JS Bajaj's institution has received grant funding from Salix within the last 2 years. He did not receive any funding for this current study. AC Barrett, E Bortey and C Paterson are employees of Salix and own stock in Salix. WP Forbes is an officer and employee of Salix and owns stock in Salix.
Declaration of funding interests: Study and writing support were funded by Salix Pharmaceuticals, Inc., Raleigh, NC.
References
- Ferenci P, Lockwood A, Mullen K. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Cordoba J, Mullen KD. Review article: the design of clinical trials in hepatic encephalopathy–an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739–47. doi: 10.1111/j.1365-2036.2011.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034–41. doi: 10.1016/j.cgh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci. 2003;48:1622–6. doi: 10.1023/a:1024784327783. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Wade JB, Gibson DP. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646–53. doi: 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J, Rimola A, Ventura PJ. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890–5. doi: 10.1016/s0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl. 2007;13:1366–71. doi: 10.1002/lt.21129. [DOI] [PubMed] [Google Scholar]
- Mullen KD, Ferenci P, Bass NM, Leevy CB, Keefe EB. An algorithm for the management of hepatic encephalopathy. Semin Liver Dis. 2007;27:32–48. [Google Scholar]
- Riordan SM, Williams R. Gut flora and hepatic encephalopathy in patients with cirrhosis. N Engl J Med. 2010;362:1140–2. doi: 10.1056/NEJMe1000850. [DOI] [PubMed] [Google Scholar]
- Shawcross D, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62:2295–304. doi: 10.1007/s00018-005-5089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth M, Funakoshi N, Cadranel JF, Blanc P. Hepatic encephalopathy: from pathophysiology to therapeutic management. Eur J Gastroenterol Hepatol. 2011;23:8–22. doi: 10.1097/MEG.0b013e3283417567. [DOI] [PubMed] [Google Scholar]
- Khungar V, Poordad F. Hepatic encephalopathy. Clin Liver Dis. 2012;16:301–20. doi: 10.1016/j.cld.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–47. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- Bass NM, Mullen KD, Sanyal A. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–81. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- Sanyal A, Younossi ZM, Bass NM. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy – a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:853–61. doi: 10.1111/j.1365-2036.2011.04808.x. [DOI] [PubMed] [Google Scholar]
- Mullen KD, Sanyal AJ, Bass NM. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12:1390–7.e2. doi: 10.1016/j.cgh.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52:737–41. doi: 10.1007/s10620-006-9442-4. [DOI] [PubMed] [Google Scholar]
- Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–5. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- Gillis JC, Brogden RN. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs. 1995;49:467–84. doi: 10.2165/00003495-199549030-00009. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Mensa L, Pons MJ, Vila J, Gascon J. Development of Escherichia coli rifaximin-resistant mutants: frequency of selection and stability. J Antimicrob Chemother. 2008;61:1016–9. doi: 10.1093/jac/dkn078. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Arroyo V. Bacterial infections in cirrhosis: a growing problem with significant implications. Clin Liver Dis. 2013;2:102–5. doi: 10.1002/cld.169. [DOI] [PMC free article] [PubMed] [Google Scholar]


