Abstract
Objective
Transfusion-related acute lung injury is the leading cause of transfusion-related mortality. A prospective study using electronic surveillance was conducted at two academic medical centers in the United States with the objective to define the clinical course and outcomes in transfusion-related acute lung injury cases.
Design
Prospective case study with controls.
Setting
University of California, San Francisco and Mayo Clinic, Rochester.
Patients
We prospectively enrolled 89 patients with transfusion-related acute lung injury, 164 transfused controls, and 145 patients with possible transfusion-related acute lung injury.
Interventions
None.
Measurements and Main Results
Patients with transfusion-related acute lung injury had fever, tachycardia, tachypnea, hypotension, and prolonged hypoxemia compared with controls. Of the patients with transfusion-related acute lung injury, 29 of 37 patients (78%) required initiation of mechanical ventilation and 13 of 53 (25%) required initiation of vasopressors. Patients with transfusion-related acute lung injury and possible transfusion-related acute lung injury had an increased duration of mechanical ventilation and increased days in the ICU and hospital compared with controls. There were 15 of 89 patients with transfusion-related acute lung injury (17%) who died, whereas 61 of 145 patients with possible transfusion-related acute lung injury (42%) died and 7 of 164 of controls (4%) died. Patients with transfusion-related acute lung injury had evidence of more systemic inflammation with increases in circulating neutrophils and a decrease in platelets compared with controls. Patients with transfusion-related acute lung injury and possible transfusion-related acute lung injury also had a statistically significant increase in plasma interleukin-8, interleukin-10, and interleukin-1 receptor antagonist posttransfusion compared with controls.
Conclusions
In conclusion, transfusion-related acute lung injury produced a condition resembling the systemic inflammatory response syndrome and was associated with substantial in-hospital morbidity and mortality in patients with transfusion-related acute lung injury compared with transfused controls. Patients with possible transfusion-related acute lung injury had even higher in-hospital morbidity and mortality, suggesting that clinical outcomes in this group are mainly influenced by the underlying acute lung injury risk factor(s).
Keywords: acute lung injury, human leukocyte antigen, human neutrophil antigen, pulmonary edema, transfusion reaction, transfusion-related acute lung injury
Transfusion-related acute lung injury (TRALI) is defined clinically as the development of acute lung injury (ALI) during or within 6 hours after the transfusion of any blood product (1,2). It was first formally described in the early 1980s (3, 4), but it has likely been present since the advent of allogeneic blood transfusions. TRALI is an important syndrome and has been the leading cause of transfusion-related mortality for many years (5); although with the implementation of transfusion of plasma from predominately male donors, the prevalence has decreased in many countries (6–8). As described by the 2004 TRALI Consensus Conference, there are many unanswered questions about the epidemiology and clinical aspects of TRALI (2). To answer the questions on TRALI prevalence, risk factors, clinical course, and outcomes, we conducted a prospective observational study of TRALI at two academic medical centers in the United States between 2006 and 2009. This study uniquely included electronic surveillance of patients with posttransfusion hypoxemia and also included control patients who did not develop hypoxemia after transfusion. We have recently reported the results on TRALI prevalence and donor and recipient risk factors (9). We have also reported the clinical aspects of transfusion-associated circulatory overload (TACO) cases identified during the screening process of the study protocol (10). We now report prospectively collected data on the clinical course and outcomes in TRALI cases and controls. We have also included a relevant comparison group, possible TRALI, defined as new ALI developing during or within 6 hours of transfusion with a clear temporal relationship to an alternative risk factor for ALI (2), and for which data on clinical outcomes are very limited.
MATERIALS AND METHODS
Study Design
Prospective surveillance for TRALI was conducted between 2006 and 2009 at the University of California, San Francisco (San Francisco, CA) and the Mayo Clinic (Rochester, MN) using an electronic surveillance system to screen for posttransfusion hypoxemia (11), as previously described (9). Study coordinators sent case information to two critical care physicians on the four-member expert panel (O.G., R.H., M.R.L., M.A.G.). Each expert independently classified each case as TRALI, possible TRALI, TACO, TACO/TRALI, or other. The final diagnosis was that agreed upon independently by two experts. For quality assurance, all TRALI cases that had another ALI risk factor present and cases in which only one reviewer diagnosed TRALI were reviewed later at a conference call with all four members of the expert panel. The study design was approved by the institutional review board at each institution.
Definition of TRALI and Possible TRALI
TRALI was defined as new ALI that developed during or within 6 hours of transfusion, and there was no temporal relationship to an alternative risk factor for ALI (1,2). Possible TRALI was defined as new ALI that developed during or within 6 hours of transfusion, and there was a clear temporal relationship to an alternative risk factor for ALI (2).
Controls: Inclusion Criteria and Enrollment
Control patients were transfusion recipients over 6 months of age who had no pulmonary signs and symptoms during or within 12 hours after transfusion of the last unit. Their Pao2/Fio2 ratio, if measured, was required to be more than 300 and any chest roentgenogram lacked bilateral infiltrates. Control enrollment was stratified according to the number of transfused units (regardless of blood product type) given to the recipient within 6 hours of the “event,” which was defined as 2 hours after the end of the last transfusion. The study statistician obtained a random sample of controls by randomly sampling days and randomly sampling an eligible control for each day. Controls were enrolled in three strata: low (1–2U), medium (3–9U), and high volume (10 or more) transfusions. There was no matching of controls to cases for any variable.
Patient and Donor Sample Collection in Cases and Controls
Residual pre- and posttransfusion samples in the clinical laboratory for each patient were collected for measurement of cytokines and human leukocyte antigen (HLA) typing. The median time that blood was drawn before and after the onset of edema were 20 and 16 hours, respectively. Leukocyte antibody testing was performed on plasma from samples collected at the time of donation or the donor was recalled for informed consent and an additional blood draw.
Cytokine Assays
Plasma cytokines were measured using microarray kits from Bio-Rad (Hercules, CA) on the Luminex platform (Luminex Corporation, Austin, TX). Cytokines measured included granulocyte-macrophage colony-stimulating factor, interferon-γ, interleukin (IL)-1β, IL-1 receptor antagonist (IL-1RA), IL-2, IL-4, IL-6, IL-8, IL-10, and tumor necrosis factor-α.
Statistical Methods
Cytokine Analysis
Cytokine data were imported into a statistical analysis package (Stata Special Edition, Version 11.2; Stata-Corp, College Station, TX). Undetectable values were assigned a value of zero for the purposes of analysis. Because the distribution of cytokine results was skewed, the Wilcoxon test for paired samples was used to compare changes in cytokines levels of paired pre- and posttransfusion samples between groups of TRALI, possible TRALI, and control patients. A two-tailed p value of less than 0.05 was considered statistically significant.
Clinical Outcomes
Patients already intubated for more than 24 hours at the time of the first implicated unit were assumed to have the course of their mechanical ventilation influenced very little by the occurrence versus nonoccurrence of TRALI and similarly for patients intubated more than 24 hours after the first implicated unit. For analysis of duration of mechanical ventilation, we therefore restricted attention to those intubated within 24 hours before or 24 hours after transfusion of the first implicated unit. Importantly, the length of time on mechanical ventilation was calculated starting at the time of the first implicated transfusion. We did not include the amount of time that patients had been receiving mechanical ventilation prior to the implicated transfusions, because this duration of mechanical ventilation would be unrelated to transfusions.
Duration of hospital and ICU stays were summarized using Kaplan-Meier survival curves. Lengths of stay (LOSs) were calculated from the episode of pulmonary edema (cases) or from 6 hours following the index transfusion (controls). Deaths in the ICU or in-hospital were excluded from analysis of these variables. Patients with no ICU stay were excluded from analysis of length of ICU stay. Medians were calculated and overall differences tested by generalized Wilcoxon tests. Proportional hazards models were used to obtain estimated effects of TRALI while controlling for Acute Physiology and Chronic Health Evaluation (APACHE II) score and to generate Kaplan-Meier curves adjusted to the median overall APACHE II score.
We did not apply statistical tests to characteristics that were not clinical outcomes, because there were no null hypotheses of interest about those being identical in the TRALI cases, possible TRALI cases, and controls. Their potential roles as confounders were addressed directly by evaluating them as additional predictors in multivariate models. The characteristics evaluated included APACHE II score, age, gender, study site, patient in the ICU at the time of transfusion before pulmonary edema, transfusion stratum (low = 1–2 U, medium = 3–9 U, high = 10 or more units within 6 hr before pulmonary edema), number of units of any component transfused within 6 hours before pulmonary edema, receiving mechanical ventilation before transfusion, congestive heart failure, shock before transfusion, surgery in the current hospitalization before pulmonary edema, time in hours since any surgery, liver transplantation, chronic renal failure, fluid balance per hour over the 24 hours before edema, leukemia or lymphoma, and metastatic cancer or chemotherapy in the 6 months before pulmonary edema.
As previously described (9), some patients had missing data for one or more of their transfused units. To evaluate transfusion-related factors in proportional hazards models, we therefore used multiple imputation methods with the 20 sets of data that had those missing values imputed for the previous analyses (9). Statistical analyses were performed using SAS software 9.1 (Cary, NC).
RESULTS
Table 1 is a description of the demographics and clinical profiles of the TRALI cases, controls, and possible TRALI cases. There were 89 cases of TRALI identified and 164 controls that did not develop pulmonary edema after transfusion therapy. We identified 145 cases of possible TRALI and the top ALI risk factors identified in these patients were sepsis (n = 68), shock (n = 59), pneumonia (n = 22), and aspiration (n = 16). A patient could have more than one ALI risk factor.
Table 1.
Demographic Characteristics of Controls, Transfusion-Related Acute Lung Injury, and Possible Transfusion-Related Acute Lung Injury Cases
| Variable | Controls (n = 164) | TRALI (n = 89) | Possible TRALI (n = 145) |
|---|---|---|---|
| Age (yr), mean ± sd | 56 ± 20.2 | 54 ± 20 | 58±19 |
| Age (yr), median (Q1–Q3) | 59(45–71) | 57 (47–66) | 61 (47–71) |
| Age (yr) | |||
| ≤ 19 | 9 (5.5) | 9(10.1) | 7 (4.8) |
| 20–29 | 12 (7.3) | 4 (4.5) | 3(2.1) |
| 30–39 | 14 (8.5) | 3 (3.4) | 11 (7.6) |
| 40–49 | 14 (8.5) | 12(13.5) | 18(12.4) |
| 50–59 | 37 (22.6) | 21 (23.6) | 26(17.9) |
| 60–69 | 31 (18.9) | 23 (25.8) | 38 (26.2) |
| ≥70 | 47 (28.7) | 17(19.1) | 42 (29.0) |
| Sex | |||
| Female | 73 (44.5) | 45 (50.6) | 70 (48.3) |
| Male | 91 (55.5) | 44 (49.4) | 75(51.7) |
| Race | |||
| American Indian/Alaska | 2(1.2) | 1(1.1) | 3(2.1) |
| Asian | 10(6.1) | 4 (4.5) | 6(4.1) |
| Black or African American | 6 (3.7) | 4 (4.5) | 7 (4.8) |
| Native Hawaiian/Pacific Islander | 1 (0.6) | 1(1.1) | 2(1.4) |
| Unknown or not reported | 22(13.4) | 16(18.0) | 18(12.4) |
| White | 123 (75.0) | 63 (70.8) | 109 (75.2) |
| Ethnicity | |||
| Hispanic | 11 (6.7) | 7 (7.9) | 7 (4.8) |
| Not Hispanic | 124 (75.6) | 70 (78.7) | 46(31.7) |
| Not reporting ethnicity | 29(17.7) | 12(13.4) | 92 (63.5) |
| Hospital | |||
| Mayo Clinic | 85(51.8) | 45 (50.6) | 98 (67.6) |
| University of California, San Francisco | 79 (48.2) | 44 (49.4) | 47 (32.4) |
| Transfusion strata | |||
| High (≥ 10U) | 48 (29.3) | 29 (32.6) | 20(13.8) |
| Medium (3–9U) | 63 (38.4) | 33(37.1) | 58 (40.0) |
| Low(1–2U) | 53 (32.3) | 27 (30.3) | 67 (46.2) |
| Patient location at time of transfusion | |||
| Emergency department | 0(0) | 0(0) | 1 (0.7) |
| Floor | 54 (32.9) | 14(15.7) | 11 (7.6) |
| Hematology oncology floor | 20(12.2) | 4 (4.5) | 16(11.0) |
| ICU | 29(17.7) | 27 (30.3) | 78 (53.8) |
| Operating room/postanesthesia care unrt | 53 (32.3) | 43 (48.3) | 39 (26.9) |
| Outpatient | 8 (4.9) | 1(1.1) | 0(0) |
TRALI = transfusion-related acute lung injury, Q1 = first quartile, Q3 = third quartile.
Values are numbers (%), unless otherwise specified.
Vital Signs and Supportive Care
In Table 2, we show vital signs and supportive care measures that diverged between TRALI cases and controls. TRALI patients had higher body temperatures after the onset of edema, and 35% developed new fever (≥ 1.0°C increase) during transfusion or after the onset of edema. TRALI patients had lower systolic and diastolic blood pressures, higher heart rates, and higher mean central venous pressure values. TRALI patients were more likely to require prolonged vasopressor support after the onset of edema compared with transfused controls. Among TRALI cases not on vasopressors before transfusion, vasopressors were initiated in 25% (13 of 53) during transfusion or after onset of edema. TRALI cases developed more tachypnea and hypoxemia compared with controls. In the TRALI group, the Pao2/Fio2 ratios were improved at 24 and 48 hours compared with the onset of edema but remained lower than the controls. Importantly, among TRALI cases and controls that were not mechanically ventilated before the onset of edema, 78% of TRALI patients (29 of 37) required institution of positive pressure mechanical ventilation after the onset of edema compared with 10% of controls (8 of 79). For either control or TRALI cases that were already mechanically ventilated, TRALI patients had higher peak airway pressures, likely indicating reduced respiratory compliance. TRALI patients were also more likely to receive noninvasive ventilatory support (continuous positive airway pressure or bilevel positive airway pressure) compared with controls (32% vs 2%, respectively).
Table 2.
Vital Signs and Supportive Care in Transfusion-Related Acute Lung Injury Cases and Controls
| Variable | Controls (n = 164) | Transfusion-Related Acute Lung Injury (n = 89) |
p |
|---|---|---|---|
| Temperature (°C) | |||
| Highest before edema (T1) | 37.0 ± 0.6 (n = 157) | 37.1 ± 1.0 (n = 87) | 0.79 |
| Highest after edema (T2) | 372 ± 0.7 (n = 153) | 37.7 ± 1.3 (n = 88) | 0.0006 |
| Δ(T2–T1) | 0.2 ± 0.8 (n = 151) | 0.6 ± 1.2 (n = 86) | 0.0025 |
| New fever (%) | |||
| Increase to ≥ 38°C at T1 or T2 | 13/144(9.0) | 25/77 (32.5) | < 0.0001 |
| Increase by ≥ 1 °C at T1 or T2 | 24/151 (15.9) | 30/87 (34.5) | 0.001 |
| Blood pressure (mm Hg) | |||
| Average systolic blood pressure (T1) | 118±16.9 (n = 160) | 112 ± 20.4 (n = 89) | 0.0031 |
| Average systolic blood pressure (T2) | 123±18.7 (n = 153) | 117±21.6 (n = 88) | 0.035 |
| Δ(T2–T1) | 6.1 ±14.7 (n = 153) | 5.4 ± 20.8 (n = 88) | 0.80 |
| Average diastolic blood pressure (TO) | 64±11.3 (n = 160) | 59 ± 13.1 (n = 89) | 0.0002 |
| Average diastolic blood pressure (T2) | 66±11.7 (n = 153) | 60±13.4(n = 88) | < 0.0001 |
| Δ(T2–T1) | 1.8±10.4(n = 153) | 1.0 ± 11.4 (n = 88) | 0.54 |
| Heart rate | |||
| Average heart rate (T1) | 84±15.3 (n = 160) | 90±18.8(n = 89) | 0.03 |
| Average heart rate (T2) | 86±16.3 (n = 153) | 101±23.6(n = 88) | < 0.0001 |
| Δ(T2–T1) | 1.9±10.3 (n = 153) | 10.7±16.8(n = 88) | < 0.0001 |
| Central venous pressure (mm Hg) | |||
| Average central venous pressure (T1) | 9.7 ± 4.6 (n = 38) | 9.2 ± 3.1 (n = 33) | 0.94 |
| Average central venous pressure (T2) | 8.3 ± 3.9 (n = 33) | 10.3±3.6(n = 48) | 0.015 |
| Δ(T2–T1) | −1.2 ± 4.3 (n = 31) | 1.1 ±3.6 (n = 31) | 0.056 |
| Respiratory rate | |||
| Average respiratory rate (TO) | 16 ± 4.5 (n = 157) | 16±5.4(n = 88) | 0.51 |
| Average respiratory rate (T2) | 17±4.4(n = 155) | 22±9.7(n = 89) | 0.0002 |
| Δ (T2 – TO) | 1.2 ± 4.4 (n = 154) | 6.7±9.5(n = 88) | < 0.0001 |
| Pao2/Fio2 | |||
| Average Pao2/Fio2 (TO) | 386±107 (n = 46) | 329±112(n = 49) | 0.011 |
| Average Pao2/Fio2 (T2) | 413±73 (n = 34) | 154±74(n = 83) | < 0.0001 |
| Average Pao2/Fio2 (T3) | 364 ± 115 (n = 20) | 193 ± 91 (n = 70) | < 0.0001 |
| Average Pao2/Fio2 (T4) | 349 ± 117 (n = 9) | 211±120(n = 55) | 0.0032 |
| Δ (T2 – T0) | 8 ± 95 (n = 28) | −166±128(n = 49) | < 0.0001 |
| Δ (T3 – T2) | −53 ± 85 (n = 16) | 36±115 (n = 69) | 0.0039 |
| Δ (T4 – T2) | −93±121 (n = 8) | 45±129 (n = 53) | 0.0094 |
| Mechanical ventilation (%) | |||
| New mechanical ventilation after edema | 8/79(10) | 29/37 (78) | < 0.0001 |
| On mechanical ventilation before edema | 85/164(52) | 52/89 (58) | 0.32 |
| Never on mechanical ventilation | 71/164(43) | 8/89 (9) | < 0.0001 |
| Peak airway pressure (cm H2O) | |||
| Average peak airway pressurea | 25 ± 6(n = 81) | 30±10(n = 50) | 0.0007 |
| Noninvasive ventilation (%)b | |||
| Yes | 3(2) | 28 (32) | < 0.0001 |
| No | 161 (98) | 61 (68) | |
| Vasopressors (%) | |||
| Yes (TO) | 29(18) | 36 (40) | < 0.0001 |
| Yes (T2) | 14(9) | 39 (44) | < 0.0001 |
| Yes (T3) | 12(7) | 37 (42) | < 0.0001 |
| Yes (T4) | 4(2) | 21 (24) | < 0.0001 |
| New vasopressors (T1 or T2) | 0/135(0) | 13/53 (25) | < 0.0001 |
T0 = 0–6 hr before transfusion, T1 = during and < 6hr after transfusion, T2 = 0 to < 1 2 hr after onset of pulmonary edema, T3 = data closest to 24 hr (range >, 1 2 to < 36 hr) after onset of pulmonary edema, T4 = data closest to 48 hr (range > 36 to < 60 hr) after onset of pulmonary edema.
Highest value within ± 12 hr of intubation.
Noninvasive ventilation = continuous positive airway pressure or bilevel positive airway pressure.
Values are mean ± sd or number (%).
Laboratory Values
In this observational study, we could not mandate that routine laboratory tests be done, but in most cases, results from these tests were available for review. There were no significant differences in mean WBCs or neutrophils before or within 12 hours after the onset of edema. However, neutrophils statistically significantly increased in the TRALI group at 24 and 48 hours after edema onset versus controls (Table 3). Platelet counts after the onset of edema decreased in the TRALI group versus controls compared with baseline platelet counts (Table 3). There were two TRALI cases that developed dynamic leukopenia. Brain natriuretic peptide measurements were made in very few TRALI cases or controls.
Table 3.
Laboratory Values in Transfusion-Related Acute Lung Injury Cases and Controls
| Variable | Controls (n = 164) | Transfusion-Related Acute Lung Injury (n = 89) |
p |
|---|---|---|---|
| WBCs(× 109/L) | |||
| Average WBCs (TO) | 8.6 ± 9.2 (n = 153) | 8.4 ± 5.1 (n = 83) | 0.27 |
| Average WBCs (T2) | 9.2 ± 8.1 (n = 135) | 8.5±5.4(n = 86) | 0.98 |
| Average WBCs (T3) | 10.2 ± 9.6 (n = 132) | 9.5±5.4(n = 88) | 0.68 |
| Average WBCs (T4) | 10.4±19.8(n = 125) | 9.6 ± 5.1 (n = 83) | 0.52 |
| Neutrophils (× 109/L) | |||
| Average neutrophils (TO) | 5.1 ± 4.4 (n = 87) | 5.4 ± 4.6 (n = 43) | 0.73 |
| Average neutrophils (T2) | 4.9 ± 4.4 (n = 45) | 6.3 ± 4.5 (n = 35) | 0.081 |
| Average neutrophils (T3) | 6.9±11.1 (n = 47) | 8.3 ± 5.7 (n = 40) | 0.016 |
| Average neutrophils (T4) | 5.4 ± 5.2 (n = 48) | 8.6 ± 5.6 (n = 39) | 0.0023 |
| Platelets (× 1 09/L) | |||
| Average platelets (TO) | 158 ± 147 (n = 143) | 162 ± 98 (n = 82) | 0.10 |
| Average platelets (T2) | 145±104(n = 135) | 129 ± 81 (n = 86) | 0.53 |
| Average platelets (T3) | 146±118 (n = 133) | 121±80(n = 88) | 0.44 |
| Average platelets (T4) | 151 ±138 (n = 124) | 115±81 (n = 83) | 0.31 |
| Δ (T2 – T0) | −18.7±75.5 (n = 115) | −31.5 ± 61.2 (n = 80) | 0.018 |
| BNP(pg/mL) | |||
| Average BNP (TO and T1) | 581±512(n = 8) | 217 ± 300 (n = 5) | 0.12 |
| Average BNP(T2) | 323±17 (n = 2) | 597±813(n = 21) | 0.78 |
T0 = 0–6 hr before transfusion, T1 = during and < 6hr after transfusion, T2 = 0 to < 1 2 hr after onset of pulmonary edema, T3 = data closest to 24 hr (range > 1 2 to < 36 hr) after onset of pulmonary edema, T4 = data closest to 48 hr (range > 36 to < 60 hr) after onset of pulmonary edema, BN P = brain natriuretic peptide.
Mean ± sd. p value based on Mann-Whitney test.
Plasma Cytokines
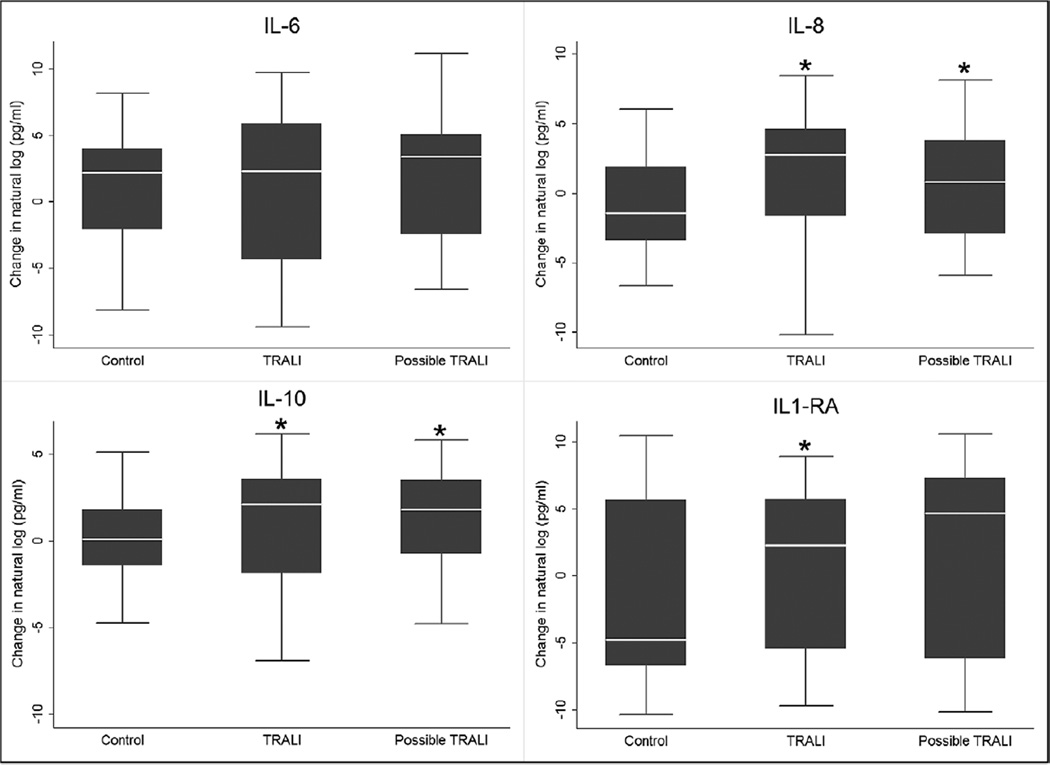
We measured cytokine values in plasma collected from TRALI and possible TRALI cases and controls. We restricted our analysis to patients with paired pre- and postevent samples. The availability of paired samples limited our cytokine analyses to 109 control patients, 57 TRALI patients, and 38 possible TRALI patients. IL-6 plasma levels increased (pre- to postevent) in all three groups, but there were no statistically significant differences between the groups (Fig. 1). Pre- to postevent changes in IL-8 and IL-10 levels were higher in the TRALI and possible TRALI groups compared with controls (Fig. 1). Changes in IL-1RA (pre- to postevent) plasma levels were higher in the TRALI and possible TRALI groups compared with controls, although the possible TRALI comparison was not statistically significant (Fig. 1).
Figure 1.
Systemic inflammatory response in patients with transfusion-related acute lung injury (TRALI) (n = 38) and possible TRALI (n = 57) compared with transfused controls (n = 109). Natural log transformed levels of interleukin (IL)-6, IL-8, IL-10, and IL-1 receptor antagonist (IL-1RA) from paired samples prior to and following transfusion are presented as box-and-whisker plots. The box plot shows the median (horizontal line) and interquartile range (IQR) (25th–75th percentile) (box). The whiskers show the lowest data within 1.5 IQR of the lower quartile and highest data within 1.5 IQR of the upper quartile. Extreme outliers are not shown. Asterisks indicate statistically significant values in TRALI and possible TRALI patients compared with control patients (p < 0.05).
Duration of Mechanical Ventilation
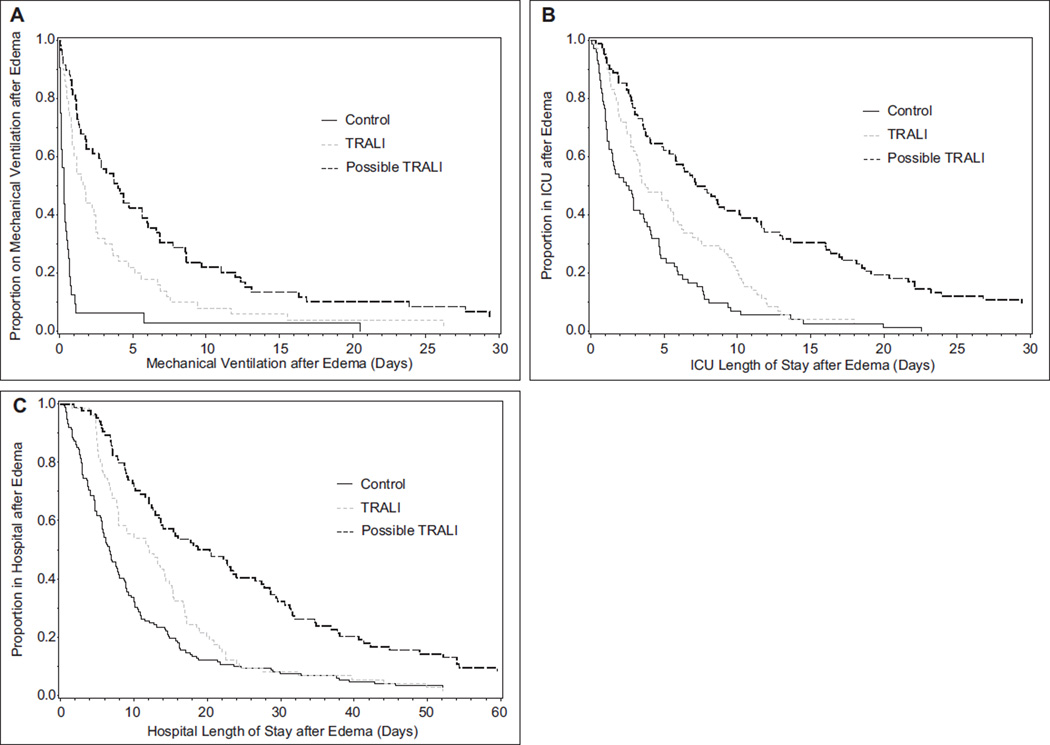
We next tested the effect of TRALI on the length of time of mechanical ventilation in those patients who required this support. The duration of mechanical ventilation was increased in both the TRALI (n = 50) and possible TRALI (n = 59) groups compared with control group (n = 32) (Fig. 2A). Median duration of mechanical ventilation was 0.3 days in the control group (n = 32), 1.7 days in the TRALI group (n = 50), and 4.0 days in the possible TRALI group (n = 59; generalized Wilcoxon overall p < 0.0001). When compared with the TRALI group alone, possible TRALI patients had statistically significantly longer duration of mechanical ventilation (generalized Wilcoxon p = 0.0063). To estimate the independent effect of TRALI on duration of mechanical ventilation versus controls, we performed proportional hazards regression analyses controlling for possible confounders, with the APACHE II score forced into all models to control for disease severity. Controlled only for APACHE II score, earlier discontinuation of mechanical ventilation was associated inversely with TRALI (hazard ratio [HR] = 0.32; 95% CI, 0.20–0.51). Also controlling for anyone of the other possible confounders resulted in little change to these results (HR for discontinuation of mechanical ventilation ranging from 0.28 to 0.36). Simultaneously controlling for metastatic cancer or chemotherapy in the past 6 months before pulmonary edema, shock before transfusion, and liver transplantation (which were chosen by forward stepwise selection based on all having p< 0.05) strengthened the TRALI effect somewhat (HR = 0.21; 95% CI, 0.12–0.36).
Figure 2.
A, Duration of mechanical ventilation in transfusion-related acute lung injury (TRALI), possible TRALI, and controls. Overall test for some difference among the groups had p < 0.0001 by generalized Wilcoxon test (see text for pairwise comparisons). B, ICU length of stay (LOS) in TRALI, possible TRALI, and controls. Overall test for some difference among the groups had p < 0.0001 by generalized Wilcoxon test (see text for pairwise comparisons). C, Hospital LOS in TRALI, possible TRALI, and controls. Overall test for some difference among the groups had p < 0.0001 by generalized Wilcoxon test (see text for pairwise comparisons).
ICU and Hospital LOS
These analyses counted LOS from the time of the development of pulmonary edema (cases) or 6 hours after transfusion (controls) until discharge from the ICU and hospital, respectively. Both ICU (Fig. 2B) and hospital LOS (Fig. 2C) were increased in the TRALI and possible TRALI groups compared with control group. Median ICU LOS was 2.5 days in the control group (n = 72), 3.8 days in the TRALI group (n = 71), and 7.5 days in the possible TRALI group (n = 82; generalized Wilcoxon overall p< 0.0001). Median hospital LOS was 6.7 days in the control group (n = 149), 12.1 days in the TRALI group (n = 74), and 19.5 days in the possible TRALI group (n = 84; generalized Wilcoxon overall p< 0.0001). When compared with the TRALI group alone, possible TRALI patients had statistically significantly longer ICU LOS (generalized Wilcoxon p = 0.0013) and hospital LOS (generalized Wilcoxon p = 0.0001). To estimate the independent effect of TRALI on LOSs versus controls, we performed proportional hazards regression analyses controlling for possible confounders, with the APACHE II score forced into all models to control for disease severity. Controlled only for APACHE II score, earlier discharge from the ICU was associated inversely with TRALI (HR = 0.64; 95% CI, 0.46–0.90) as was earlier discharge from the hospital (HR = 0.73; 95% CI, 0.55–0.97). Also controlling for any one of the other possible confounders resulted in little change to these results (HR for ICU discharge ranging from 0.59 to 0.68; HR for hospital discharge ranging from 0.70 to 0.78). Forward stepwise selection did not find more than one potential confounder with p value of less than 0.05 for these outcomes.
Mortality and Discharge Status
Among the TRALI cases, 10 patients (11%) died in the ICU, whereas there was just one death (0.6%) in the ICU in the control group (p< 0.0001) (Table 4). There were 15 in-hospital deaths (17%) in the TRALI group, whereas there were seven deaths within the control group (4%; p = 0.007) (Table 4). More control patients were discharged home (n = 127, 77%) versus TRALI patients (n = 59, 66%) or to a nursing home (controls, n = 26 [16%] versus TRALI, n = 9 [10%]; p = 0.0014 overall). Additionally, we observed 42% in-hospital mortality in the possible TRALI group (61 of 145 patients) compared with 17% in TRALI patients and 4% in controls (p< 0.0001). Odds ratios for risk of in-hospital mortality from a logistic regression model controlling for APACHE II score were 3.4 (95% CI, 1.30–9.0; p = 0.013) for TRALI group versus control group and 13.3 (95% CI, 5.8–30.8; p< 0.0001) for possible TRALI group versus control group. Also, controlling for any one of the other possible confounders resulted in odds ratios ranging from 3.2 to 4.7 for TRALI group versus control group and 11.4–15.9 for possible TRALI group versus control group. Simultaneously, controlling for metastatic cancer or chemotherapy in the past 6 months before pulmonary edema, surgery before pulmonary edema, and receiving mechanical ventilation before transfusion (which were chosen by forward stepwise selection based on all having p< 0.05) strengthened the TRALI effect somewhat (odds ratio = 4.7; 95% CI, 1.69– 12.8) and also the possible TRALI effect (odds ratio = 15.1; 95% CI, 6.3–36.2).
Table 4.
Clinical Outcomes in Transfusion-Related Acute Lung Injury Cases and Controls
| Variable | Controls (n = 164) | Transfusion-Related Acute Lung Injury (n = 89) |
p |
|---|---|---|---|
| ICU discharge status (%) | |||
| Alive | 88 (53.7) | 76 (85.4) | < 0.0001 |
| Deceased | 1 (0.6) | 10(11.2) | |
| Not applicable | 75 (45.7) | 3 (3.4) | |
| Hospital discharge status (%) | |||
| Deceased | 7 (4.3) | 15(16.9) | 0.0014 |
| Discharged home | 127 (77.4) | 59 (66.3) | |
| Nursing home | 26(15.9) | 9(10.1) | |
| Other hospital | 4 (2.4) | 6 (6.7) | |
“Not applicable” is patients not in an ICU.
Values are number (%). p value based on chi-square test.
Predictors of Decreased Duration of Mechanical Ventilation and ICU or Hospital LOS in TRALI Patients
We tested all of the recipient and transfusion risk factors that were identified in our previous study to be independent risk factors for the development of TRALI (9). Of the recipient risk factors tested, only liver surgery (transplantation) and fluid balance before transfusion were statistically significantly associated with these clinical outcomes. Liver surgery (transplantation) was associated with a shorter ICU LOS in TRALI patients (Table 5). Higher fluid balance before transfusion was associated with a shorter duration of mechanical ventilation in TRALI patients and a trend toward short ICU LOS (Table 5).
Table 5.
Predictors of Decreased Duration of Mechanical Ventilation and ICU or Hospital Length of Stay in Transfusion-Related Acute Lung Injury Cases
| Variable | Mechanical Ventilationa | ICU Length of Staya | Hospital Length of Staya |
|---|---|---|---|
| Recipient risk factors | |||
| Liver surgery (transplantation) | 1.55 (0.70–3.5), p = 0.28 |
1.98(1.01–3.9), p = 0.045 |
1.34 (0.70–2.6), p = 0.38 |
| Fluid balance before transfusion (increment per liter) |
1.03(1.001–1.06) p = 0.041 |
1.02(0.995–1.05), p = 0.12 |
1.01 (0.98–1.04), p = 0.54 |
| Transfusion risk factors | |||
| Plasma from female donor | 1.05(0.57–1.94), p = 0.86 |
1.29 (0.80–2.09), p = 0.30 |
1.16(0.72–1.86), p = 0.54 |
| Total quantity of cognate anti-HLA-class II (MFI> 1,500) (per 10-fold increase) |
1.31 (0.93–1.83), p = 0.12 |
1.36(1.03–1.80) p = 0.03 |
1.23(0.93–1.61), p = 0.14 |
| Total volume of anti-human neutrophil antigen positive by granulocyte immunofluorescence test among all units (per 1 00-mL increase) |
1.04(0.90–1.21), p = 0.58 |
1.03(0.90–1.18), p = 0.67 |
1.06(0.92–1.23), p = 0.41 |
| Total quantity of cognate anti-HLA-class I (MFI > 2,500) (per 1 0-fold increase) |
1.08(0.87–1.35), p = 0.48 |
1.04(0.86–1.25), p = 0.68 |
0.98(0.82–1.17), p = 0.84 |
HLA = human leukocyte antigen, MFI = mean fluorescent intensity.
All values from two-predictor models, adjusted for Acute Physiology and Chronic Health Evaluation II score.
Estimated hazard ratios (HRs) and 95% CI from proportional hazards models. For example, the HR of 1.03 for fluid balance in the Mechanical Ventilation column ndicates that the chance of extubation at any given time increases by 3% for each 1 L increase in fluid balance.
We have previously reported that the volume of strong, cognate HLA class II antibody and the amount of human neutrophil antigen (HNA) antibody transfused were significant, independent risk factors for TRALI (9). We tested if these two strong predictors, and also the transfusion of plasma from female donors, impacted clinical outcomes in TRALI patients. Transfusion of plasma from female donors or HNA antibody did not appear to substantially impact length of time of mechanical ventilation or the number of days in the ICU or hospital (Table 5). On the other hand, the quantity of strong, cognate HLA class II antibody transfused was initially associated with a shorter ICU LOS after adjusting for APACHE II score and trends toward shorter duration of mechanical ventilation and shorter hospital LOS (Table 5). To assess possible confounding with clinical factors, we additionally controlled for liver surgery and fluid balance, which weakened the effects of the amount of strong, cognate HLA class II antibody to HRs of 1.23 (95% CI, 0.86–1.75; p = 0.25) for duration of mechanical ventilation, 1.29 (95% CI, 0.96–1.73; p = 0.09) for ICU LOS, and 1.15 (95% CI, 0.86–1.54; p = 0.33) for hospital LOS.
DISCUSSION
The principal findings of this study of TRALI in general, transfused patients can be summarized as follows: 1) TRALI produced a signature of vital sign abnormalities that mirrors the systemic inflammatory response, with elevated body temperatures, tachycardia, tachypnea, lower blood pressure, and in many cases, the need for new mechanical ventilation (78%) and vasopressor therapy (25%); 2) TRALI patients had a decrease in platelet counts and an increase in neutrophils and proinflammatory and anti-inflammatory cytokines in the peripheral blood; 3) TRALI patients had substantial in-hospital morbidity with a longer duration of mechanical ventilation and longer ICU and hospital stays; and 4) possible TRALI cases had even higher morbidity and mortality compared with TRALI cases.
The diagnosis of TRALI is made using clinical criteria and clinical judgment, which can be challenging in cases in which other ALI risk factors are present or when it is difficult to discern the volume status of the patient to exclude TACO The finding of systemic inflammatory response syndrome (SIRS)-like physiology and hemodynamic instability in TRALI cases should help clinicians in the determination of TRALI probability, although it is imperative to consider the many causes of ALI that could also produce these characteristic features, including bacterial contamination of transfused blood products (12). Leukocytosis or leukopenia is a SIRS criterion and others have reported dynamic leukopenia in TRALI cases (13–17) and in TRALI animal models (18). However, mean changes in leukocytes among TRALI cases within 12 hours after the onset of edema were similar to those of controls in our study, and the development of severe leukopenia was observed in very few cases.
It has been presumed that TRALI would produce characteristic inflammatory changes that have been observed in patients with ALI from varied etiologies. Indeed, in many of our TRALI cases, we were able to compare the inflammatory profile in the plasma before and soon after the development of pulmonary edema. We also identified cases of possible TRALI in whom the etiology of ALI was assessed by expert opinion to not be transfusion related. The plasma cytokine profile in TRALI cases mirrored closely the cytokine profile in possible TRALI cases with a balance of proinflammatory and anti-inflammatory mediators. The increase in neutrophil counts 24–48 hours after onset of edema in the TRALI group is consistent with a compensatory inflammatory response, and the decrease in platelet counts has been observed in experimental TRALI (19), TRALI case reports (13–15), and in patients with acute respiratory distress syndrome (ARDS) (20).
The requirement for mechanical ventilation (4) and for longer duration in TRALI patients compared with transfused controls (21) is consistent with previous reports. A limitation of the mechanical ventilation analysis is that we restricted our analysis to patients intubated within 24 hours before or 24 hours after transfusion, which limits the generalizability of our findings to all transfused, critically ill patients. Reports of TRALI mortality rates have varied from 5% to 35% (22) with larger cases series on the lower end of this range (4). We observed an in-hospital mortality rate of 17% in our TRALI patients, which is likely influenced both by the development of TRALI and also underlying clinical factors that prompted transfusion therapy.
A novel finding of this study is that possible TRALI patients had worse outcomes including a higher mortality compared with TRALI patients. The likely explanation for the worse clinical outcomes is that by definition, there was a temporal relationship to an alternative ALI risk factor in possible TRALI cases (2), and per the clinical judgment of the expert panel, the underlying ALI risk factor in these cases was the major factor causing ALI. Indeed, the mortality of ALI/ARDS is 35–40% (23), which is nearly identical to the observed mortality of possible TRALI in our study. The Leukocyte Antibody Prevalence Study-II also showed that the occurrence of possible TRALI was unrelated to HLA antibody status of the blood product donor, and therefore, possible TRALI was likely not a result of the blood transfusion (24). There are two implications of this finding. First, it provides an explanation for why in this same study population, we found that TRALI prevalence decreased but possible TRALI prevalence did not decrease with implementation of plasma from predominately male donors (9). This implies different etiologies for TRALI and possible TRALI and suggests that TRALI is indeed related in part to transfusion factors, whereas possible TRALI is related to ALI risk factors and not to transfusion factors. Second, possible TRALI cases should perhaps be separated from TRALI cases in studies and reports, including Food and Drug Administration incidence reports since the likely etiologies, outcomes, and effective mitigation strategies are different. Perhaps, “transfused ALI” or “transfused ARDS” (25) may be a more appropriate designation than “possible TRALI” for transfused patients who develop new ALI during or within 6 hours of transfusion, when there is a clear temporal relationship to an alternate risk factor for ALI.
We did not identify any donor or recipient risk factors that were associated with prolonged mechanical ventilation or ICU and hospital LOS in TRALI cases. We did identify that liver transplantation was associated with a shorter ICU LOS, which is not surprising because these patients generally have uneventful postoperative courses. TRALI patients with a higher positive fluid balance before transfusion had shorter duration of mechanical ventilation. This finding may be explained by these patients being more diuretic-responsive posttransfusion and thus similar to TACO patients, although we do not have any evidence for the effect of diuretics in these patients.
The only transfusion risk factor that was initially statistically significantly associated with TRALI outcomes was the amount of strong, cognate HLA class II antibody, which is an independent risk factor for the development of TRALI (9). This risk factor was associated with less in-hospital morbidity, although the associations were small and not statistically significant when controlled for liver surgery and fluid balance. Of note, in our previous study, we did not find that the accumulation of lysophosphatidylcholines in stored blood products was associated with the development of TRALI (9). However, we did not test for alternative lipid mediators that accumulate during the storage of leukoreduced blood products and that maybe associated with the development of TRALI and TRALI clinical outcomes, because this new information was published after our study ended (26).
Our study has some limitations. First, this study was necessarily observational, because TRALI could not be randomly assigned. Although we evaluated many possible confounders, it is possible that unmeasured or unknown confounders could have biased our results since our control patients were not matched for baseline characteristics and comorbidities. Matching for baseline characteristics and comorbidities during study enrollment would have been a better approach to studying the clinical course and outcomes of TRALI cases and controls but would not have provided the flexibility to investigate TRALI prevalence and risk factors. Second, to minimize selection bias, we did not use a design that would have required written informed consent, which would have prevented enrollment of many TRALI cases, and consequently, we did not mandate the collection of blood or laboratory tests in TRALI cases or controls. Therefore, we have some missing data, especially for the plasma cytokine studies, which combined with varying collection intervals for cytokine measurements relative to transfusion limit our power to detect differences between groups. Third, our study spanned a period of pre- and post-TRALI mitigation, but our analysis comprised the entire study period, so we are unable to comment separately on the clinical course and outcomes of TRALI in the pre- versus postmitigation era. Finally, our study was limited to two academic medical centers in the United States and may not be representative of the experience at all medical centers.
CONCLUSIONS
In this large, controlled, prospective study of TRALI, we found that TRALI was associated with SIRS-like physiology, which makes it difficult to separate clinically from acute infections and ALI. TRALI produced substantial in-hospital morbidity, with many patients requiring vasopressor and mechanical ventilation support leading to prolonged stays in the ICU and hospital and a mortality rate of 17%. Patients with possible TRALI had greater morbidity and mortality, suggesting that the underlying ALI risk factor in these cases is the major factor causing ALI and possibly not the transfusion event. TRALI remains a serious complication of transfusion therapy and efforts should continue to identify high-risk donors and mitigate exposure to susceptible recipients.
ACKNOWLEDGMENTS
Transfusion-Related Acute Lung Injury Study Group: Steering Committee. Pearl Toy, MD (Principal Investigator), Ognjen Gajic, MD, Mark R. Looney, MD, Rolf Hubmayr, MD, Michael A. Gropper, MD, PhD, Michael A. Matthay, MD, Richard B. Weis-kopf, MD, Edward L. Murphy, MD, and Clifford A. Lowell, MD, PhD. Advisory Committee: Steve Kleinman, MD, David Stroncek, MD, Ram Kakaiya, MD, Thomas H. Price, MD, Michael Busch, MD, PhD, Dean Sheppard, MD. Statistics: Peter Bacchetti, PhD, Erin Madden, Barbara Grimes, PhD. Clinical site coordinators and research assistants: University of California, San Francisco: Monique Koenigsberg, RN, Kelly Lang, RN, Christopher Chin, Deanna Lee, PhD, Lynda Bartek, RN; Mayo Clinic: Gregory Wilson, CCRC, Tami Krpata, Deborah Rasmussen, Cindy Medcalfe. Blood banks and investigators: Blood Centers of the Pacific: Nora Hirschler, MD; Blood Services Research Institute: Rosa Sanchez Rosen, MD, Philip Norris, MD, Dan Hindes; University of California, San Francisco: Pearl Toy, MD; Mayo Clinic: Breanndan Moore, MD (deceased), leffrey Winters, MD, Manish Gandhi, MD. American Red Cross National Neutrophil Reference Laboratory: David Mair, MD, Randy Schuller. HLA antibody testing and analysis (Mayo Clinic): Breanndan Moore, MD, Manish Gandhi, MD, Steven DeGoey, Nancy Ploeger, Philip Norris, MD. Neutrophil priming and cytokine laboratory (American Red Cross): Clifford Lowell, MD, PhD, Yong Mei Hu, Ping Wu. Lysophosphatidylcholine laboratory (Mayo Clinic): loseph McConnell, PhD. Administrator: Charlene Anderson.
Dr. Looney received support for article research from the National Institutes of Health (NIH). He was supported by the National Heart, Lung, and Blood Institute Transfusion Medicine (NHLBI) (HL107386). His institution received grant support, support for travel, support for writing/reviewing the manuscript, and provision of writing assistance from the NIH. Dr. Roubinian received support for article research from the NIH. His institution received grant support from the NIH. Dr. Gajic received support for article research from the NIH. Dr. Gropper received support for article research from the NIH. His institution received grant support from the NHLBI. Dr. Hubmayr consulted for Philips Medical, is employed by Mayo Clinic, has stock options with Respithera (for scientific board membership), served as board member for Respithera (has stock options), and received support for article research from the NIH. His institution received grant support from the NIH. Dr. Lowell received support for article research from the NIH. His institution received grant support from the NIH. Dr. Bacchetti received support for article research from the NIH and was supported by UCSF-CTSI (grant numbers UL1 RR024131 and UL1 TR000004). His institution received grant support from the NIH. Mr. Wilson received support for article research from the NIH. His institution received grant support from the NIH. Ms. Koenigsberg received support for article research from the NIH. Her institution received grant support and support for writing/reviewing the manuscript from the NIH. Dr. Lee is employed by the University of California, San Francisco and received support for article research from the NIH. Her institution received grant support from the NIH and from the University of California, San Francisco. Dr. Wu is employed by Blood Centers of the Pacific, San Francisco, and has past employment with Immunogenetics, Foster City, and University of California, San Francisco, and he received support for article research from the NIH. His institution received grant support and support for writing/reviewing the manuscript from the NIH. Dr. Grimes is employed by University of California, San Francisco and received support for article research from the NIH. Her institution received grant support and support for writing/ reviewing the manuscript from the NIH. Dr. Norris served as a board member for Charisela; provided expert testimony for Wlliams & Connolly, Covington & Burling, and Supple & Canvel; and received support for article research from the NIH. Dr. Gandhi received support for article research from the NIH. His institution received grant support from the NIH. Dr. Wnters consulted for Grifoils (consultation on plasma exchange clinical trial design) and TerumoBCT (member of committee responsible for selection on plasma exchange grant award recipients) and received support for article research from the NIH. His institution received grant support from the NIH (transfusion-related acute lung injury URALI] Specialized Centers of Clinically Oriented Research [SCCOR]), Fenwal, Asahi Kasei Kuraray, Food and Drug Administration, and National Health Service and received support for travel from the NIH (TRALI SCCOR) and the American Society for Hematology. Dr. Mair received support for article research from the NIH. His institution received grant support from the NIH. Dr. Schuller received support for article research from the NIH and is employed by the American Red Cross. His institution received grant support from the NIH. Dr. Hirschler is employed by Blood Centers of the Pacific and received support for article research from the NIH. Her institution received grant support from University of California, San Francisco. Dr. Matthay consulted for Cerus (acute lung injury), Roche-Genetec (Chair, DSMB for clinical trial of asthma, DSMB for ARDS trial-trauma), and Biogen (consulting for acute respiratory distress syndrome, ARDS); received support for travel from the Intensive Care Society of France (Societe Reanimation Francaise); received support for article research from the NIH; and was supported by the NHLBI (HL51856). He and his institution received grant support from GSK (to study biomarkers in ARDS and sepsis). His institution received grant support from the N HLBI. Dr. Toy was employed by the University of California, San Francisco and received support for article research from the NIH. Her institution received grant support, and she received support for travel, support for writing/reviewing the manuscript, and support for manuscript preparation from the NIH and was supported by the NHLBI (SCCOR P50HL081027). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Toy P, Popovsky MA, Abraham E, et al. National Heart, Lung and Blood Institute Working Group on TRALI: Transfusion-related acute lung injury: Definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: Statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 3.Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. Am Rev Respir Dis. 1983;128:185–189. doi: 10.1164/arrd.1983.128.1.185. [DOI] [PubMed] [Google Scholar]
- 4.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 5.Fatalities reported to FDA following blood collection and transfusion. [Accessed August 7, 2013];Annual summary for fiscal year 2012. Available at: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm346639.htm.
- 6.Eder AF, Herron RM, Jr, Strupp A, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006–2008) Transfusion. 2010;50:1732–1742. doi: 10.1111/j.1537-2995.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Saw CL, Hannach B, et al. Transfusion-related acute lung injury prevention measures and their impact at Canadian Blood Services. Transfusion. 2012;52:567–574. doi: 10.1111/j.1537-2995.2011.03330.x. [DOI] [PubMed] [Google Scholar]
- 8.Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: Before-and-after comparative cohort study. Transfusion. 2011;51:1278–1283. doi: 10.1111/j.1537-2995.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 9.Toy P, Gajic O, Bacchetti P, et al. TRALI Study Group: Transfusion-related acute lung injury: Incidence and risk factors. Blood. 2012;119:1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy EL, Kwaan N, Looney MR, et al. TRALI Study Group: Risk factors and outcomes in transfusion-associated circulatory overload. Am J Med. 2013;126:357.e29–357.e38. doi: 10.1016/j.amjmed.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay HE, Cassorla L, Feiner J, et al. Designing and testing a computer-based screening system for transfusion-related acute lung injury. Am J Clin Pathol. 2005;124:601–609. doi: 10.1309/1XKQKFF83CBU4D6H. [DOI] [PubMed] [Google Scholar]
- 12.Rollins MD, Molofsky AB, Nambiar A, et al. Two septic transfusion reactions presenting as transfusion-related acute lung injury from a split plateletpheresis unit. Crit Care Med. 2012;40:2488–2491. doi: 10.1097/CCM.0b013e3182544f85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ausley MB., Jr Fatal transfusion reactions caused by donor antibodies to recipient leukocytes. Am J Forensic Med Pathol. 1987;8:287–290. doi: 10.1097/00000433-198712000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Leger R, Palm S, Wulf H, et al. Transfusion-related lung injury with leukopenic reaction caused by fresh frozen plasma containing anti-NB1. Anesthesiology. 1999;91:1529–1532. doi: 10.1097/00000542-199911000-00048. [DOI] [PubMed] [Google Scholar]
- 15.Yomtovian R, Kline W, Press C, et al. Severe pulmonary hypersensitivity associated with passive transfusion of a neutrophil-specific antibody. Lancet. 1984;1:244–246. doi: 10.1016/s0140-6736(84)90124-7. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa M, Toy P. Acute and transient decrease in neutrophil count in transfusion-related acute lung injury: Cases at one hospital. Transfusion. 2004;44:1689–1694. doi: 10.1111/j.0041-1132.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 17.Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: A review. Chest. 2004;126:249–258. doi: 10.1378/chest.126.1.249. [DOI] [PubMed] [Google Scholar]
- 18.Looney MR, Su X, Van Ziffle JA, et al. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest. 2006;116:1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looney MR, Nguyen JX, Hu Y, et al. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider RC, Zapol WM, Carvalho AC. Platelet consumption and sequestration in severe acute respiratory failure. Am Rev Respir Dis. 1980;122:445–451. doi: 10.1164/arrd.1980.122.3.445. [DOI] [PubMed] [Google Scholar]
- 21.Vlaar AP, Binnekade JM, Prins D, et al. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: A nested case-control study. Crit Care Med. 2010;38:771–778. doi: 10.1097/CCM.0b013e3181cc4d4b. [DOI] [PubMed] [Google Scholar]
- 22.Silliman CC, Fung YL, Ball JB, et al. Transfusion-related acute lung injury (TRALI): Current concepts and misconceptions. Blood Rev. 2009;23:245–255. doi: 10.1016/j.blre.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 24.Kleinman SH, Triulzi DJ, Murphy EL, et al. National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-ll: The Leukocyte Antibody Prevalence Study-ll (LAPS-II): A retrospective cohort study of transfusion-related acute lung injury in recipients of high-plasma-volume human leukocyte antigen antibody-positive or -negative components. Transfusion. 2011;51:2078–2091. doi: 10.1111/j.1537-2995.2011.03120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 26.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–2554. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]