Abstract
Roux-en-Y gastric bypass is a well-accepted tool for the treatment of obesity and, compared to conventional weight loss methods (eg, diet and exercise) and other weight loss surgeries (eg, gastric banding), it results in considerable weight loss that is maintained long term. Although successful, the mechanisms for weight loss are not completely understood and it is thought that gastrointestinal hormones play a role. Several gastrointestinal hormones have been identified for their effects on appetite, including glucagon-like peptide-1 (GLP-1), peptide tyrosine-tyrosine (PYY), leptin, and ghrelin. This review encompasses a literature search that included 45 primary articles and shows that there are alterations in GLP-1, PYY, leptin, and ghrelin postoperatively. GLP-1 and PYY concentrations were usually found to be higher, whereas ghrelin levels were typically lower post- Roux-en-Y gastric bypass than in individuals with obesity, those who were overweight or of normal weight, and in those who underwent procedures other than Roux-en-Y gastric bypass or who achieved weight loss by lifestyle modification. An understanding of how gastrointestinal hormones change after Roux-en-Y gastric bypass may help dietetics practitioners optimize nutrition care for this patient population. A review of the literature also highlighted some research gaps that should be taken into consideration when designing future studies.
Roux-en-Y gastric bypass (RYGB) is a well-accepted tool for the treatment of obesity and, compared to conventional weight loss methods (eg, diet and exercise) and other weight loss surgeries (eg, gastric banding), it results in considerable weight loss that is maintained long term. Although successful, the mechanisms for weight loss are not completely understood and it is thought that surgery-induced changes in gastrointestinal hormones play a role. It is important for registered dietitians to have an understanding of how gastrointestinal hormones change after RYGB so that they can improve nutrition care in this patient population. The purpose of this review is to report the relevant literature regarding changes in gastrointestinal hormones after RYGB.
OVERVIEW OF THE RYGB PROCEDURE
Overweight and obesity are worldwide epidemics, and it is estimated that more than 1.5 billion adults are overweight (body mass index [BMI] ≥25), with 600 million being classified as obese (BMI ≥30) (1). By 2015, the World Health Organization estimates that more than 2.3 billion adults will be overweight and 700 million will be obese (1). Traditional weight loss therapies including low-energy diets, exercise, behavior therapy, and pharmacotherapy have been implemented; however, these methods have had little long-term success (2). Bariatric surgery is currently the only known method that offers both considerable and long-term weight loss (2). In fact, bariatric surgery is increasing in prevalence in the United States and worldwide due to the increasing rate of obesity; lack of effectiveness with traditional therapies; introduction of the laparoscopic method, which has made it less invasive to patients; increased media attention; and greater access to the therapy (3). There are several bariatric surgeries currently performed, including vertical banded gastroplasty, gastric banding, sleeve gastrectomy, biliopancreatic diversion, duodenal switch, and RYGB.
RYGB is the safest and most efficacious bariatric surgery and, thus, it is currently the most commonly performed operation, comprising about 70% to 75% of all bariatric procedures (2). It can be performed using either open or laparoscopic techniques, with laparoscopic being the preferred method due to its quicker recovery time and decreased postoperative complications (4,5). Weight loss is thought to be equivalent between the two methods, as the primary difference between open and laparoscopic RYGB is the method of access (2). RYGB is classically described as having both malabsorptive and restrictive components. With this procedure, the distal stomach, duodenum, and proximal jejunum are bypassed (4). The restrictive component is achieved by creating a small gastric pouch, which promotes early satiety (3,4) and thereby decreases intake (3).
During the RYGB gastric bypass surgical procedure, the distal jejunal limb is connected to the new gastric pouch, creating a Roux limb (2), also known as the alimentary limb that functions to transports nutrients (3). Roux limbs vary in length, and typically range from 75 to 150 cm (2,3). Although these physiological modifications are thought to be a primary reason for the RYGB’s success, it is also important to consider the contribution of gastrointestinal hormones and neural pathways to the process (3). Indeed, there is a growing body of evidence that favorable changes in several gastrointestinal hormones may have a substantial role in the weight loss seen after RYGB (6,7). Furthermore, Borg and colleagues (6) have suggested that the observed alterations in gastrointestinal hormones may represent an adaptive response to offset the physiologic modification created by the RYGB procedure.
GASTROINTESTINAL HORMONES AND LEPTIN IN NORMAL PHYSIOLOGY
Several gastrointestinal hormones have been identified for their effects on appetite, including glucagon-like peptide-1 (GLP-1), peptide tyrosine-tyrosine (PYY), leptin, and ghrelin (Figure 1). Although detailed reviews are available elsewhere describing the characteristics of these hormones (8–16), a brief overview on how these hormones affect appetite in normal physiological states follows here.
Figure 1.
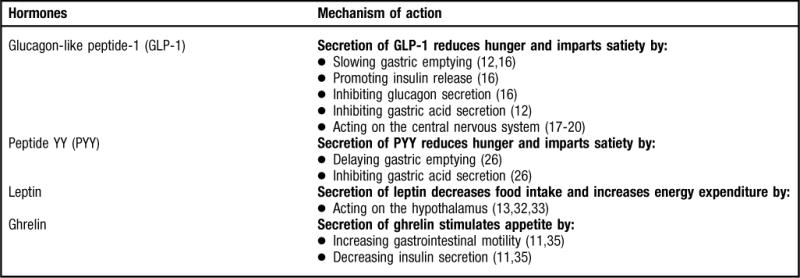
Overview of hormones. NOTE: Information from this figure is available online at www.adajournal.org as part of a PowerPoint presentation.
GLP-1
The incretin GLP-1 is secreted from the L cells in the distal ileum and colon in response to energy intake (8–11). There are two biological forms of GLP-1: GLP-17–36amide and GLP-17–37 with GLP-17–36amide being the most prevalent and active isoform (8). Consequently, future references to GLP-17–36amide in this review will be referred to as simply GLP-1.
GLP-1 is considered an appetite regulating hormone because secretion of it reduces hunger and imparts satiety (14). GLP-1 is secreted in response to either a mixed meal or individual nutrients such as carbohydrate, fat, protein, and fiber (10). The mechanism by which GLP-1 promotes satiety is thought to be multifaceted because it slows gastric emptying (12,16), promotes insulin release (16), inhibits glucagon secretion (16), inhibits gastric acid secretion (12), and acts on the central nervous system to induce satiety and decrease food intake (17–20). Because of these aforementioned effects, it is considered to play an important role in the ileal brake mechanism (17), which regulates the passage of nutrients through the gastrointestinal tract (21). The satiety-promoting effects of GLP-1 are evident when it is peripherally administered, as it has been found to reduce appetite and energy intake in healthy weight humans (22) as well as those with obesity (23); consequently, it has been investigated as a therapy for weight loss. Given the mechanism of action of GLP-1 it would be expected that postprandial plasma levels would be elevated in normal weight individuals and lower in those with obesity, and there are some data to support this (24); however, there are conflicting findings (25).
PYY
Similar to GLP-1, PYY is secreted from the L cells of the gut after a meal (8,11,13,16). It occurs in two forms: PYY1–36 and PYY3–36, with PYY3–36 being the major circulating form (13). Unless otherwise noted, reference to PYY3–36 in this review will be notated as PYY. PYY is also considered an appetite-regulating hormone given that secretion of it reduces hunger and imparts satiety (14). One mechanism by which PYY is thought to promote satiety is through its role in the ileal brake (17). In other words, PYY delays gastric emptying and inhibits gastric acid secretion, as discussed in a review by le Roux and Bloom (26). Intravenous PYY infusion has been found to decrease energy intake and reduce hunger in healthy individuals (27) and those with obesity (28). It has been reported that fasting and postprandial PYY levels are lower in those with obesity and higher in normal weight individuals (28,29), but not all studies have found this to be true (30,31). As a result, it has been suggested that reduced PYY release is not likely to be a mechanism involved in the etiology of obesity (8,28).
Leptin
Since the discovery of leptin in 1994, a plethora of research has been conducted to establish its role in the pathogenesis of obesity. Leptin is a product of the obesity gene (ob gene) and is understood to be involved in long-term energy balance (32). It is secreted by adipocytes and influences energy intake primarily by acting on the hypothalamus (13,32,33) to decrease food intake and increase energy expenditure (33). Leptin circulates in proportion to whole-body adipose tissue mass (32). For example, increased body fat results in increased leptin, which ultimately stimulates reduced food intake, and the converse is also true with decreased body fat (32). However, increased leptin levels do not prevent obesity (13); therefore, it has been suggested that the progression of obesity is not a result of leptin deficiency but instead leptin resistance (33).
Ghrelin
Ghrelin is another appetite regulating hormone, although, unlike GLP-1, PYY, and leptin, it is the only known orexigenic (ie, appetite stimulating) hormone (8,9,11,13). Ghrelin circulates in two forms: active (acylated) and inactive (desacyl) (34). A majority of the studies presented here measured total ghrelin (both active and inactive forms) and, consequently, unless otherwise noted, total ghrelin will be notated as simply ghrelin.
The antisatiating properties of ghrelin may be due to its biological effects to increase gastrointestinal motility and decrease insulin secretion (11,35). It is released both centrally (pituitary) and peripherally (stomach) and energy intake may be the primary regulator of plasma ghrelin levels (14). Circulating ghrelin levels are increased during states of negative energy balance (36), including diet-induced weight loss in individuals with obesity (37) and decreased during feeding and in individuals with obesity (38). This mechanism has been postulated to be a protective response to stimulate energy intake in the underweight and to suppress it in the overweight (9). However, in obesity, ghrelin is not suppressed with food intake (39); therefore, this mechanism may be an important factor in the etiology of obesity or it may actually be a consequence of obesity that results from chronic overfeeding.
METHODS
This review encompasses a literature search in the English language that yielded 100 primary articles. A comprehensive literature search was conducted using Ovid Medline with the following search query: “Roux-en-Y OR gastric bypass,” which was then run against “GI hormones OR hormones OR GLP-1 OR PYY OR leptin OR ghrelin.” Additional articles were identified from bibliographies of recent review papers. Articles were excluded if the study design included one or more of the following characteristics: use of animal models as the study population; utilization of a surgical technique that did not include RYGB; not differentiating between surgery type in analyses; failure to measure one or more of the following hormones: GLP-1, PYY, leptin, or ghrelin; case-study design; and inclusion of subjects that had previous weight loss surgeries. After applying the aforementioned exclusion criteria, 45 articles were identified and reviewed.
Each study was evaluated for its strength in research methodologies. For studies investigating GLP-1, PYY, and ghrelin, a study was considered strong if it included either a control group (eg, obese, lean, or other surgical procedure) and/or sampled for the gastrointestinal hormone at multiple time points after consumption of a meal. In terms of the latter, it is advantageous to sample at multiple time points after food intake (eg, sample at baseline and then every 30 minutes for 3 hours) to capture the gastrointestinal hormone response to a meal. However, for leptin, it is common to sample once and/or in the fasted state since it has been found to be unaffected by meal consumption as it primarily is a reflection of adipose mass (40). Therefore, in terms of the reviewed leptin literature, a study was considered to be strong if it included a control group.
GLP-1 Changes after RYGB
In the studies that utilized a control group (41–49) and/or sampled at multiple time points (6,41–47,50) (Figure 2), the data concerning GLP-1 after RYGB is consistent. In studies that compared RYGB patients to nonsurgical patients who were lean, overweight, or with obesity, all but one study (43) found that GLP-1 levels were significantly higher after RYGB (41,42,45,47,49). To control for the effects of weight loss on GLP-1 changes, the aforementioned studies weight-matched the participants to the pre-RYGB weight (eg, subjects who have obesity) (41,43,45,47) and/or the post-RYGB weight (eg, overweight subjects) (41,42,49). Even when weight-matched, RYGB patients had significantly different GLP-1 values compared to either subjects who have obesity or are overweight. Moreover, one would expect that lean individuals would have higher GLP-1 values compared to RYGB subjects; however, the one study that compared post-RYGB subjects to lean subjects did not indicate this at 6 to 36 months post-op when subjects were still considered to have obesity (45), suggesting that a component of the RYGB procedure alters the GLP-1 profile.
Figure 2.
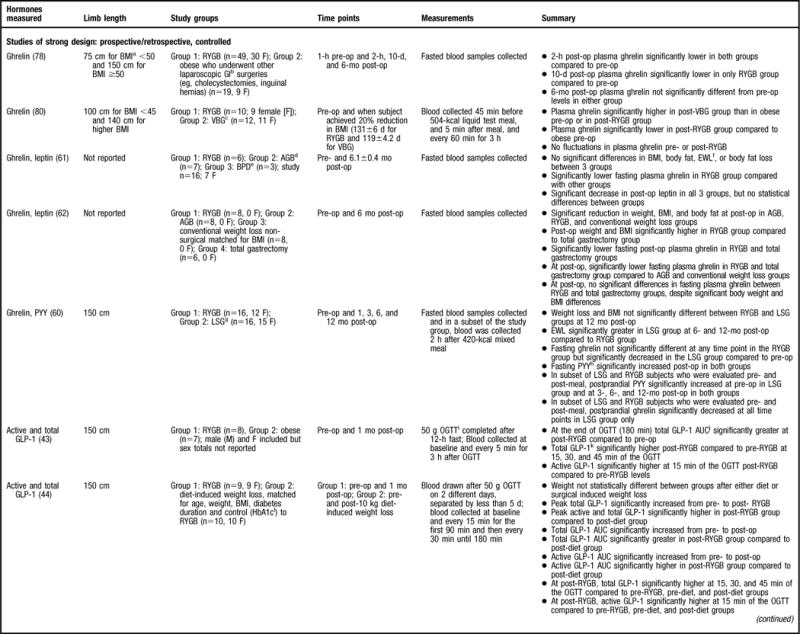
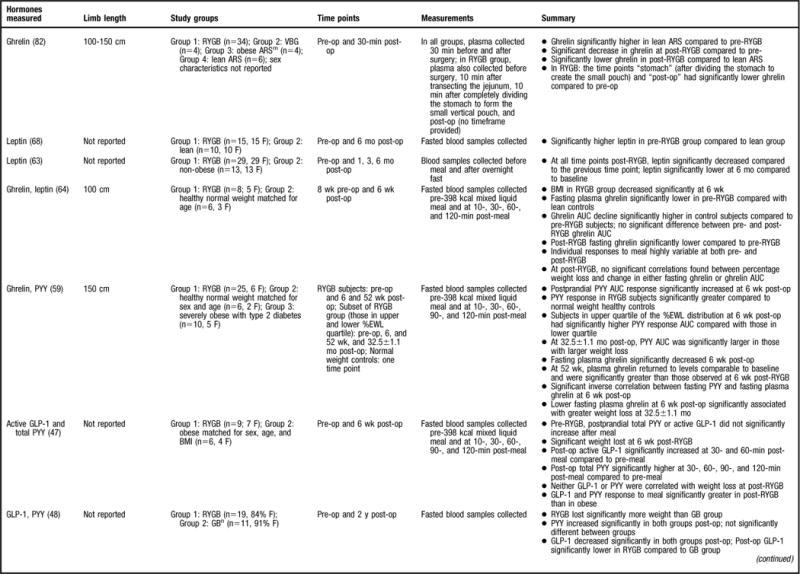
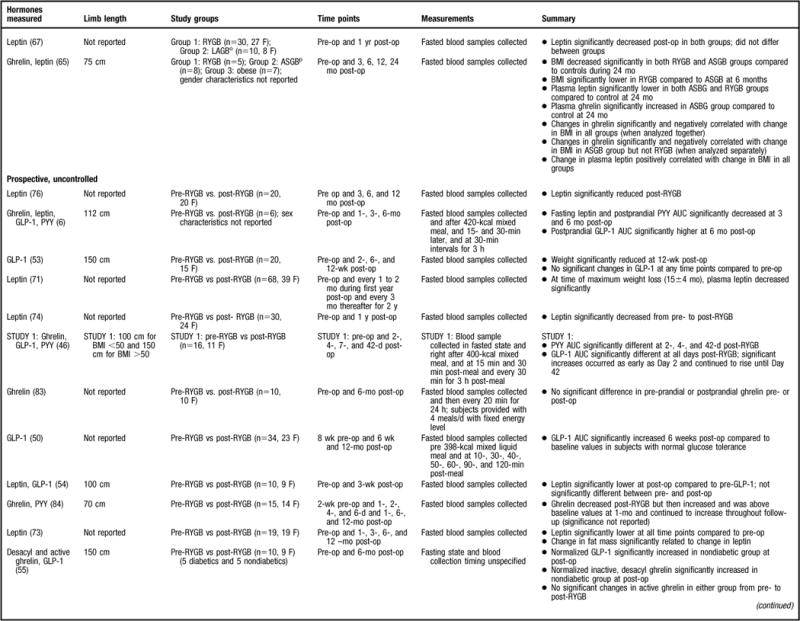
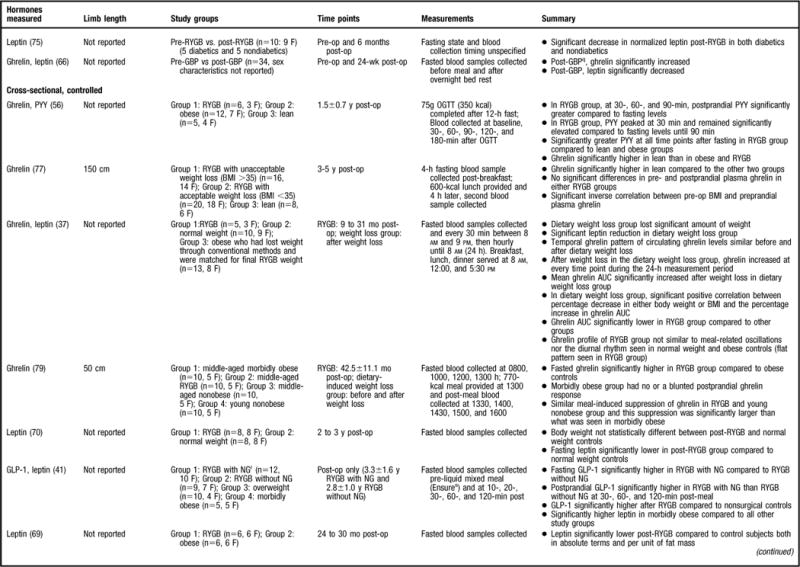
Summary of research reviewed in a study of changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass (RYGB). NOTE: Information from this figure is available online at www.adajournal.org as part of a PowerPoint presentation.
When comparing RYGB to other surgical procedures, both of the reviewed studies found that GLP-1 levels were significantly higher in the RYGB subjects compared to those who underwent gastric banding (42,49). In these studies, the postoperative weight was not statistically different between groups, suggesting that weight change is not a primary regulator of GLP-1. Similarly, one study also compared the gastrointestinal hormone profile of RYGB subjects to that of a diet-induced weight loss group (44). Although the mean body weight was not significantly different between the groups, the peak GLP-1 level was significantly higher after RYGB compared to diet-induced weight loss (44). As previously mentioned, GLP-1 is secreted from the distal ileum in response to nutrient intake. Increased GLP-1 levels post-RYGB have been hypothesized to occur because of the surgical component that promotes a more rapid delivery of nutrients to the distal gut (51,52).
There were eight studies of more limited design that were reviewed (6,46,48–50,53–55) (Figure 2). As would be expected, a majority of the studies comparing pre- to post-RYGB levels found a significant increase in GLP-1 levels at post-RYGB compared to pre-RYGB (6,46,50,55). In contrast, two studies did not find any differences (53,54) and one study found that GLP-1 significantly decreased 2 years post-RYGB compared to pre-RYGB levels (48). The lack of consistent findings compared to the aforementioned studies may be explained by the fact that all three studies only sampled for GLP-1 in the fasted state (Figure 2) (48,53,54). GLP-1 is secreted in response to nutrient intake and perhaps these studies would have yielded different results had the study methodology included multiple sampling for gastrointestinal hormones pre- and post-meal consumption. The balance of data coming from well-designed studies that included controls and sampled for the gastrointestinal hormone at multiple time points indicated that GLP-1 significantly increases post-RYGB.
PYY Changes after RYGB
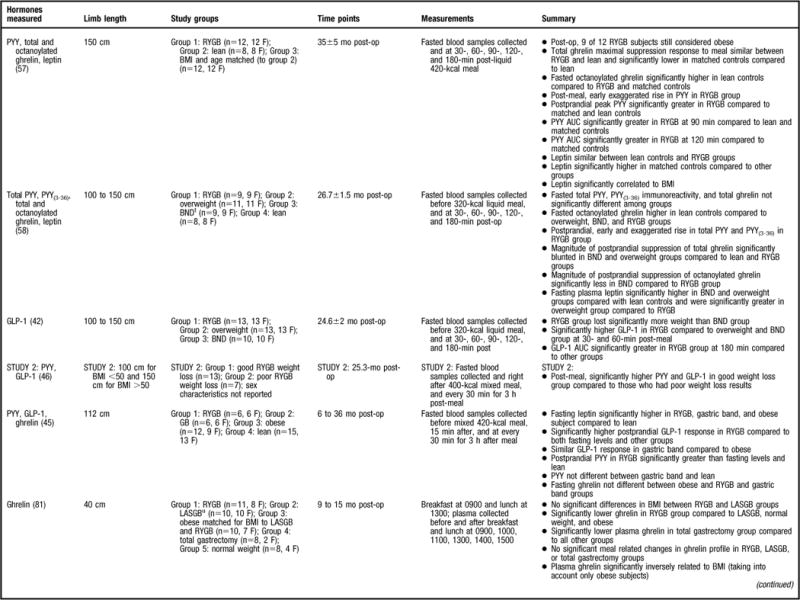
Of the strongest study designs that included control groups and/or multiple sampling points (45,47,49,56–59) (Figure 2), the general consensus is that postprandial PYY levels are higher in post-RYGB subjects compared to lean (45,56–58), normal weight (59), overweight (49, 58), obese (45,47,56,57), and/or individuals undergoing other bariatric procedures (45,49,58). More specifically, a majority of the aforementioned studies determined that the PYY response to a test meal or oral glucose tolerance test was exaggerated, occurred earlier, and remained elevated above baseline levels for the remainder of the sampling time period (45,47,49,56–58). One study evaluated total PYY and PYY3–36 and found that both forms had the same postprandial response (58).
The remainder of the reviewed studies did not include multiple PYY sampling time points or a control group (6,46,48,60) (Figure 2). In the two studies that compared pre-RYGB to post-RYGB status, both found that post-RYGB PYY levels were higher compared to pre-RYGB (6,46). Findings from le Roux and colleagues (46) suggest that this change occurs as early as 2 days post-bypass, before any weight loss, and data from Borg and colleagues (6) indicates that this effect can be seen as long as 6 months post-RYGB. In contrast, Karamanakos and colleagues (60) evaluated the gastrointestinal hormone response post-RYGB and post-laparoscopic sleeve gastrectomy and found that postprandial PYY significantly increased post-surgery; however, they did not statistically compare the PYY response between the two surgeries, and thus it is not known if there is a difference between the surgeries. In another study that did not use multiple sampling time points for gastrointestinal hormone analysis, but did have a control group (gastric banding subjects), fasting PYY increased in both groups, with no significant differences found between the two surgeries (48). PYY is released in response to a meal in proportion to the energy consumed, and perhaps different results would have been found had the authors sampled after food intake as well.
There is strong evidence that postprandial PYY levels increase post-RYGB and are higher compared to subjects who are lean, who have obesity, are overweight, and in subjects who have undergone other surgical procedures. Similar to GLP-1, the effect of RYGB on PYY levels can be seen as early as 2 days after surgery and the effect appears to be long term given that most of the studies reviewed here evaluated subjects months to years post-RYGB (6,45,46,48,49,56–60).
Leptin Changes after RYGB
More than half of the reviewed studies that measured leptin were of strong design (37,41,57,58,61–70) (Figure 2); however, some studies only compared pre-RYGB leptin levels to the post-RYGB state and did not make comparisons to the control group (eg, obese, lean) that was included in the study design (63,64,66). Similarly, others did not include a statistical analysis for leptin and only reported the values of the hormone pre- and/or post-RYGB (37,62) and, as a result, the leptin data from these two studies will not be discussed.
The leptin data are not as consistent as those reported for the gastrointestinal hormones. Some studies have demonstrated significantly lower fasting leptin concentrations in the post-RYGB patient population compared to pre-RYGB (67,68), normal weight (70), overweight (58), and obese (41,65,69) subjects, whereas others did not find a statistically significant difference between study populations (57,61,67). For example, Korner and colleagues (57) found similar leptin concentrations between lean and post-RYGB subjects that were significantly lower than the BMI-matched controls. This is an interesting finding, as the post-RYGB subjects were still considered to be overweight or have obesity and it would be expected that the lean individuals would have significantly lower leptin compared to both groups. Alternatively, Molina and colleagues (63) reported that leptin levels were significantly higher in a pre-RYGB compared to overweight subjects, most likely because their BMI was substantially higher than the overweight group (49±6 vs 26.8±2.2, respectively) (63) and leptin is secreted in proportion to adipose mass. Molina and colleagues (63) did not evaluate post-RYGB leptin levels compared to the control group. Furthermore, of the studies reviewed, only two studies examined the correlation between anthropometric measurements (eg, BMI) and leptin levels (57,65). Stoeckli and colleagues (65) determined that the change in leptin concentration was significantly correlated with change in BMI, with similar findings reported by Korner and colleagues (57). Due to control group variability, and differences in follow-up intervals, it is difficult to make definitive conclusions regarding the effect of RYGB on leptin concentrations.
Eleven additional studies that lacked a leptin control group were reviewed (6,54,63,64,66, 71–76) (Figure 2). In all of these studies, post-RYGB leptin concentrations were found to be significantly decreased compared to pre-RYGB (6,54,63,64,66,71–76). Furthermore, five of the studies conducted a correlation analysis between anthropometric measurements and leptin levels (63,66,71–73) with similar results reported between studies. Most determined that changes in weight (73), fat mass (73), and BMI (63,66) were significantly associated with changes in leptin post-RYGB. However, one study did not find a correlation between BMI and leptin concentrations after RYGB surgery (72), and another only evaluated preprocedure leptin concentrations with both baseline body weight and absolute weight reduction; no significant correlations were found between leptin and either variable (71). Taken together, the data strongly suggest that leptin decreases after RYGB and is associated with anthropometric measurements. These findings are congruent with the notion that leptin is secreted in proportion to body fat mass.
Ghrelin Changes after RYGB
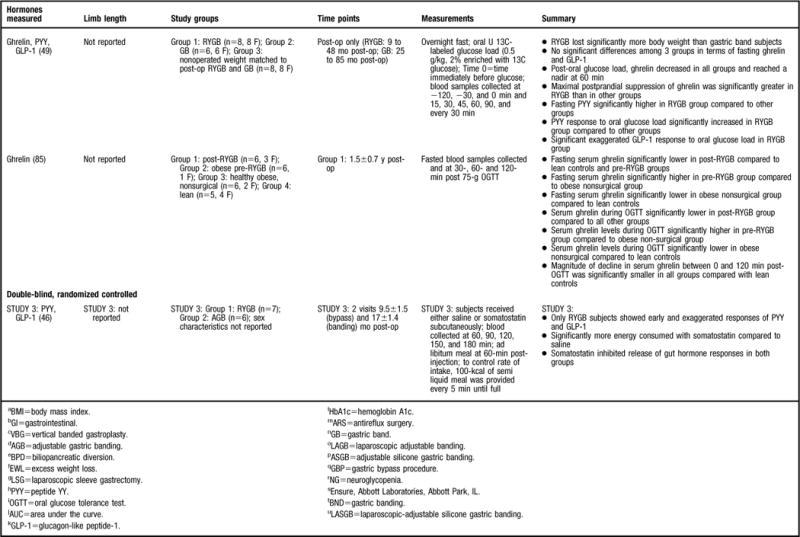
A considerable amount of research has been devoted to ghrelin changes in RYGB patient populations and 25 studies were found that fit the previously described inclusion criteria (6,37,45,46,49,55–62,64–66,77–85) (Figure 2). Ghrelin was first described to be reduced in response to the RYGB procedure by Cummings and colleagues (37) in 2002. This finding was received with much curiosity because Cummings and colleagues (37) also found that ghrelin levels significantly increased after weight loss achieved by energy restriction, suggesting that the RYGB procedure had a positive effect on the “hunger hormone,” whereas other methods of weight loss did not. Because of that landmark study, studies of stronger design (37,45,46,49,56–58,64,77,79–82, 85) (Figure 2) have generally shown that the postprandial and/or fasted ghrelin concentration is significantly lower post-RYGB compared to pre-RYGB (64,82,85), lean (45,56,77,85), normal-weight (37,64,81), overweight (49,58), obese (37,56,79,81,85), and/or compared to other bariatric surgical procedures (49,58,81), with two studies not finding such an effect (46,57). Three studies also evaluated active ghrelin (57,58,80) and found significantly lower fasted ghrelin in post-RYGB subjects compared to those who have obesity (80) with similar postprandial findings in gastric banding (58) and in lean (57) subjects. The consistent finding of decreased ghrelin levels after RYGB procedure offers a partial explanation for the success of the procedure, since low ghrelin levels would not contribute to feelings of hunger. Decreased ghrelin occurs immediately post-op, as Lin and colleagues (82) evaluated subjects 30 minutes post-RYGB and found significantly decreased ghrelin levels compared to before RYGB. Studies indicate that this ghrelin effect is maintained for more than 1 year (37,45,49,56,58,77,79,81,85).
Researchers have also evaluated the fluctuations in the postprandial ghrelin profile after RYGB by sampling at multiple time points after meal consumption. Similar secretion patterns have been reported across studies. For example, after RYGB it has been observed that the ghrelin profile is flat and does not have the meal-related fluctuations that have been observed in normal-weight individuals (37,81), those who have obesity (37,81), or subjects who underwent other types of weight loss surgeries (80). In addition, Cummings and colleagues (37) sampled for a 24-hour period and determined that the post-RYGB subjects did not have the same ghrelin diurnal rhythm found in normal weight individuals, those who have obesity, or in subjects who had lost weight by dietary restriction.
Although numerous studies have determined that ghrelin declines after RYGB more so compared to other surgical procedures (49,58,81), it is unclear what component of the surgery promotes this alteration. It has been suggested that because the distal stomach, the site of ghrelin release, is bypassed in the RYGB, ghrelin secretion is suppressed, thereby reducing hunger. To test the hypothesis that the RYGB procedure’s success is primarily due to exclusion of the stomach and consequently ghrelin suppression, two studies have compared the RYGB procedure to that of other gastric surgeries that do not involve complete division of the stomach: gastrectomy and antireflux surgery (conducted in both lean and overweight subjects) (82). In terms of the gastrectomy, Lin and colleagues did not find a significant decrease in ghrelin levels post-gastrectomy like they had when comparing pre- to post-RYGB ghrelin levels (82). Similar results were evident in the antireflux surgery group; they did not see a significant decrease in ghrelin post–antireflux surgery surgery in either the lean subjects or in subjects with obesity (82). Ghrelin levels in the post–antireflux surgery subjects were also significantly greater compared to those of post-RYGB subjects (82). This group was also interested in establishing what component of the RYGB procedure is responsible for the greatest decline in ghrelin levels (82). They collected plasma before surgery, 10 minutes after transecting the jejunum to form the Roux limb, 10 minutes after dividing the stomach to form the small gastric pouch, and immediately after completion of the surgery (82). As would be expected, they found that ghrelin levels significantly declined after dividing the stomach to form the small gastric pouch (82). Overall, authors (82) concluded that the RYGB’s complete division of the stomach promotes the reduced ghrelin levels. In contrast, a second study evaluated another gastric surgery, a total gastrectomy, which also involves a complete division of the stomach (81). Compared to normal weight individuals, those with obesity, RYGB patients, and individuals who underwent gastric banding, those with a total gastrectomy had significantly lower ghrelin levels, further implicating the importance of the stomach for ghrelin release (81). This has clinical importance because weight loss surgeries that do not involve a bypass of the stomach may not sufficiently lower ghrelin levels to reduce hunger and promote weight loss and/or maintenance.
Eleven other studies were reviewed that were of weaker design (6,55,59–62,65,66,78,83, 84) (Figure 2). Some of the studies did compare ghrelin levels to a control group; however, they only sampled for ghrelin in the fasted state (61,62,65) and these studies will be discussed first. Similar to the stronger studies that included multiple sampling time points following meal consumption, ghrelin was found to be significantly lower in the post-RYGB group, compared to pre-RYGB (62), subjects who lost weight using conventional methods (62), and/or in those who underwent other weight loss surgeries (61,62). Alternatively, Stoeckli and colleagues (65) determined that ghrelin was lower in post-RYGB subjects compared to both individuals who have obesity and subjects who underwent gastric banding; however, it did not reach statistical significance. It is unclear whether the authors conducted a statistical test to compare the gastric banding subjects to that of RYGB because there was almost a 49% difference between post-gastric banding and post-RYGB ghrelin levels, but no statistics were reported regarding this difference (65). Overall, the data from these studies provides information regarding ghelin changes post-RYGB compared to other subject groups; however, because these studies did not sample at multiple time points, it is impossible to determine the ghrelin secretion profile in response to a meal.
Studies that compared ghrelin levels between the pre- and post-RYGB state obtained unexpected results. For example, a majority of the reviewed studies did not find a difference in either fasted (6,60,83) or postprandial (83) ghrelin levels between pre- and 6 (6,78,83) to 12 months post-RYGB (60), whereas only two studies found that ghrelin increased during this same time period (66,84). Morinigo and others (59) also found that at 6 weeks post-RYGB fasted ghrelin levels had significantly decreased compared to pre-RYGB; however, at 52 weeks there was a significant increase in the hormone compared to the 6-week measurement and levels were comparable to that of baseline. It is unclear why the weaker studies did not find a significant decrease in ghrelin post-RYGB, as would be expected based on the data from the better designed studies discussed previously. Sample size may be an issue; Couce and colleagues (78) initially found a significant decline in ghrelin from pre- to both 2 hours and 10 days post-RYGB when the sample size was 49 and 18, respectively. However, at 6 months post-op, the study sample had decreased to 11 and the finding was no longer statistically significant (78). However, this cannot be the primary reason for inconsistent results because with the exception of Borg and colleagues (6), who did not report attrition, the remainder of the studies reported 100% follow-up rates from pre- to post-RYGB. An alternative explanation is that perhaps the shorter follow-up time (≤1 year) failed to capture the true time course given that a majority of the stronger studies evaluated subjects for years post-RYGB.
Overall, data from the stronger studies indicates that ghrelin is greatly reduced in the post-RYGB state compared to those who are lean, normal weight, overweight, or have obesity, or who have had other weight loss surgeries. The mechanism by which this occurs is likely related to the surgical component of the RYGB that bypasses the stomach, from which ghrelin is primarily secreted. Data from the weaker studies are not as conclusive, with some studies finding no change from pre- to post-RYGB and others actually reporting an increase. Differing methodologies might partially explain this discrepancy.
SYNTHESIS AND TRANSLATION
RYGB is an effective weight loss treatment option for those in which traditional therapies have failed. It offers long-term weight loss that may be the result of the physiological and gastrointestinal hormone changes associated with the procedure. This review looked at the current evidence showing that alterations in GLP-1, PYY, leptin, and ghrelin do occur postoperatively and generally do so in a favorable direction. In the majority of studies, post-RYGB GLP-1 and PYY concentrations were usually found to be higher, whereas ghrelin levels were typically lower compared to the concentrations of these hormones in individuals undergoing other surgical procedures, individuals who were normal and overweight, and those who lost weight by diet alone.
There are notable gaps in the literature. For instance, several of the reviewed studies did not include an appropriate control group and only evaluated changes pre- and post-RYGB. Therefore, it is not always easy to ascertain whether it was a component of the RYGB that caused the improvement in gastrointestinal hormones. Although it would be best to have a double-blind, randomized control study design, in studies involving RYGB and other surgical procedures this is not always possible due to logistical and ethical issues. In addition, follow-up times varied substantially between studies, and in some studies only one time point was measured (ie, cross-sectional studies); therefore, it was difficult to make comparisons between studies. Moreover, some of the reviewed studies only included one sampling time point for gastrointestinal hormones, usually in the fasted state. It is of interest to determine how these appetitive hormones are affected in the postprandial state. Therefore, future studies should sample before and after a meal to capture the gastrointestinal hormone response profile. Furthermore, studies that vary the macronutrient composition need to be performed to shed light on how gastrointestinal hormones contribute to satiety in post-RYGB patients, since secretion of gut hormones is often related to protein, carbohydrate, or fat ingestion. Future research in these areas is warranted.
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST: No potential conflict of interest was reported by the authors.
For additional information on this topic, visit ADA’s Evidence Analysis Library at www.adaevidencelibrary.com
References
- 1.Obesity and overweight. World Health Organization Web site. http://www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed November 25, 2008.
- 2.Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: Bariatric surgery. Endocrinol Metab Clin North Am. 2008;37:943–964. doi: 10.1016/j.ecl.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Elder KA, Wolfe BM. Bariatric surgery: A review of procedures and outcomes. Gastroenterology. 2007;132:2253–2271. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Consensus Conference Panel Consensus conference statement bariatric surgery for morbid obesity: Health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005;1:371–381. doi: 10.1016/j.soard.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 7.Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 2008;32:1640–1646. doi: 10.1038/ijo.2008.157. [DOI] [PubMed] [Google Scholar]
- 8.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 9.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 10.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frezza EE, Wachtel MS, Chiriva-Internati M. The multiple faces of glucagon-like peptide-1—Obesity, appetite, and stress: What is next? A review. Dig Dis Sci. 2007;52:643–649. doi: 10.1007/s10620-006-9096-2. [DOI] [PubMed] [Google Scholar]
- 13.Dhillo WS. Appetite regulation: An overview. Thyroid. 2007;17:433–445. doi: 10.1089/thy.2007.0018. [DOI] [PubMed] [Google Scholar]
- 14.Vincent RP, Ashrafian H, le Roux CW. Mechanisms of disease: The role of gastrointestinal hormones in appetite and obesity. Nat Clin Pract Gastroenterol Hepatol. 2008;5:268–277. doi: 10.1038/ncpgasthep1118. [DOI] [PubMed] [Google Scholar]
- 15.Vincent RP, le Roux CW. The satiety hormone peptide YY as a regulator of appetite. J Clin Pathol. 2008;61:548–552. doi: 10.1136/jcp.2007.048488. [DOI] [PubMed] [Google Scholar]
- 16.Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf) 2008;69:173–179. doi: 10.1111/j.1365-2265.2007.03164.x. [DOI] [PubMed] [Google Scholar]
- 17.Jayasena CN, Bloom SR. Role of gut hormones in obesity. Endocrinol Metab Clin North Am. 2008;37:769–87. xi. doi: 10.1016/j.ecl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005;21:91–117. doi: 10.1002/dmrr.538. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci. 2007;27:7125–7129. doi: 10.1523/JNEUROSCI.1025-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Pannacciulli N, Le DS, Salbe AD, Chen K, Reiman EM, Tataranni PA, Krakoff J. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage. 2007;35:511–517. doi: 10.1016/j.neuroimage.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Citters GW, Lin HC. The ileal brake: A 15-year progress report. Curr Gastroenterol Rep. 1999;1:404–409. doi: 10.1007/s11894-999-0022-6. [DOI] [PubMed] [Google Scholar]
- 22.Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: A potent regulator of food intake in humans. Gut. 1999;44:81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rossner S, Hellstrom PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–311. doi: 10.1038/sj.ijo.0800818. [DOI] [PubMed] [Google Scholar]
- 24.Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—Effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 25.Feinle C, Chapman IM, Wishart J, Horowitz M. Plasma glucagon-like peptide-1 (GLP-1) responses to duodenal fat and glucose infusions in lean and obese men. Peptides. 2002;23:1491–1495. doi: 10.1016/s0196-9781(02)00087-6. [DOI] [PubMed] [Google Scholar]
- 26.le Roux CW, Bloom SR. Peptide YY, appetite and food intake. Proc Nutr Soc. 2005;64:213–216. doi: 10.1079/pns2005427. [DOI] [PubMed] [Google Scholar]
- 27.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 28.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 29.le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 30.Kim BJ, Carlson OD, Jang HJ, Elahi D, Berry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J Clin Endocrinol Metab. 2005;90:6665–6671. doi: 10.1210/jc.2005-0409. [DOI] [PubMed] [Google Scholar]
- 31.Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR, Chanoine JP. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 32.Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- 33.Bloomgarden ZT. Gut and adipocyte peptides. Diabetes Care. 2006;29:450–456. doi: 10.2337/diacare.29.02.06.dc06-0006. [DOI] [PubMed] [Google Scholar]
- 34.Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Nakai Y, Kangawa K. Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab. 2005;90:6–9. doi: 10.1210/jc.2004-1640. [DOI] [PubMed] [Google Scholar]
- 35.Zwirska-Korczala K, Konturek SJ, Sodowski M, Wylezol M, Kuka D, Sowa P, Adamczyk-Sowa M, Kukla M, Berdowska A, Rehfeld JF, Bielanski W, Brzozowski T. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol. 2007;58(suppl 1):13–35. [PubMed] [Google Scholar]
- 36.Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jorgensen JO. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002;56:203–206. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 37.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 38.Cummings DE, Foster-Schubert KE, Overduin J. Ghrelin and energy balance: Focus on current controversies. Curr Drug Targets. 2005;6:153–169. doi: 10.2174/1389450053174569. [DOI] [PubMed] [Google Scholar]
- 39.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87:2984. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 40.Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab. 1997;82:561–565. doi: 10.1210/jcem.82.2.3757. [DOI] [PubMed] [Google Scholar]
- 41.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 42.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery vs hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 47.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 48.Reinehr T, Roth CL, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg. 2007;17:1571–1577. doi: 10.1007/s11695-007-9323-8. [DOI] [PubMed] [Google Scholar]
- 49.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 50.Morinigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 51.Naslund E, Kral J. Impact of gastric bypass surgery on gut hormones and glucose homeostasis in type 2 diabetes. Diabetes. 2006;55(suppl):S92–S97. [Google Scholar]
- 52.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: Mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 53.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. [PubMed] [Google Scholar]
- 54.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, Ikramuddin S. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: A preliminary study. J Surg Res. 2007;141:31–39. doi: 10.1016/j.jss.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 56.Chan JL, Mun EC, Stoyneva V, Mantzoros CS, Goldfine AB. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring) 2006;14:194–198. doi: 10.1038/oby.2006.25. [DOI] [PubMed] [Google Scholar]
- 57.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 58.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 59.Morinigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–275. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 60.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: A prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 61.Fruhbeck G, Diez-Caballero A, Gil MJ, Montero I, Gomez-Ambrosi J, Salvador J, Cienfuegos JA. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg. 2004;14:606–612. doi: 10.1381/096089204323093363. [DOI] [PubMed] [Google Scholar]
- 62.Fruhbeck G, Rotellar F, Hernandez-Lizoain JL, Gil MJ, Gomez-Ambrosi J, Salvador J, Cienfuegos JA. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208–1215. doi: 10.1381/0960892042386904. [DOI] [PubMed] [Google Scholar]
- 63.Molina A, Vendrell J, Gutierrez C, Simon I, Masdevall C, Soler J, Gomez JM. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes Surg. 2003;13:615–621. doi: 10.1381/096089203322190844. [DOI] [PubMed] [Google Scholar]
- 64.Morinigo R, Casamitjana R, Moize V, Lacy AM, Delgado S, Gomis R, Vidal J. Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res. 2004;12:1108–1116. doi: 10.1038/oby.2004.139. [DOI] [PubMed] [Google Scholar]
- 65.Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–350. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- 66.Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, Simon I, Soler J, Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity. Obes Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 67.Riedl M, Vila G, Maier C, Handisurya A, Shakeri-Manesch S, Prager G, Wagner O, Kautzky-Willer A, Ludvik B, Clodi M, Luger A. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008;93:2307–2312. doi: 10.1210/jc.2007-2383. [DOI] [PubMed] [Google Scholar]
- 68.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: A possible link to the resistance to leptin? J Clin Endocrinol Metab. 2002;87:1184–1188. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- 69.Hickey MS, Pories WJ, MacDonald KG, Jr, Cory KA, Dohm GL, Swanson MS, Israel RG, Barakat HA, Considine RV, Caro JF, Houmard JA. A new paradigm for type 2 diabetes mellitus: Could it be a disease of the foregut? Ann Surg. 1998;227:637–643. doi: 10.1097/00000658-199805000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faraj M, Jones P, Sniderman AD, Cianflone K. Enhanced dietary fat clearance in postobese women. J Lipid Res. 2001;42:571–580. [PubMed] [Google Scholar]
- 71.Czupryniak L, Pawlowski M, Kumor A, Szymanski D, Loba J, Strzelczyk J. Predicting maximum roux-en-Y gastric bypass-induced weight reduction—Preoperative plasma leptin or body weight? Obes Surg. 2007;17:162–167. doi: 10.1007/s11695-007-9042-1. [DOI] [PubMed] [Google Scholar]
- 72.Ramos AP, de Abreu MR, Vendramini RC, Brunetti IL, Pepato MT. Decrease in circulating glucose, insulin and leptin levels and improvement in insulin resistance at 1 and 3 months after gastric bypass. Obes Surg. 2006;16:1359–1364. doi: 10.1381/096089206778663706. [DOI] [PubMed] [Google Scholar]
- 73.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, Van Loan MD, Ali MR, Wolfe BM, Havel PJ. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–1911. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, Dallal GE, Saltzman E. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 75.Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, Ikramuddin S. Adipokine response in diabetics and nondiabetics following the roux-en-Y gastric bypass: A preliminary study. J Surg Res. 2007;142:295–300. doi: 10.1016/j.jss.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 76.Bobbioni-Harsch E, Morel P, Huber O, Assimacopoulos-Jeannet F, Chassot G, Lehmann T, Volery M, Golay A. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–4700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 77.Christou NV, Look D, McLean AP. Pre- and post-prandial plasma ghrelin levels do not correlate with satiety or failure to achieve a successful outcome after Roux-en-Y gastric bypass. Obes Surg. 2005;15:1017–1023. doi: 10.1381/0960892054621071. [DOI] [PubMed] [Google Scholar]
- 78.Couce ME, Cottam D, Esplen J, Schauer P, Burguera B. Is ghrelin the culprit for weight loss after gastric bypass surgery? A negative answer. Obes Surg. 2006;16:870–878. doi: 10.1381/096089206777822151. [DOI] [PubMed] [Google Scholar]
- 79.Engstrom BE, Ohrvall M, Sundbom M, Lind L, Karlsson FA. Meal suppression of circulating ghrelin is normalized in obese individuals following gastric bypass surgery. Int J Obes (Lond) 2007;31:476–480. doi: 10.1038/sj.ijo.0803440. [DOI] [PubMed] [Google Scholar]
- 80.Foschi D, Corsi F, Colombo F, Vago T, Bevilaqua M, Rizzi A, Trabucchi E. Different effects of vertical banded gastroplasty and roux-en-Y gastric bypass on meal inhibition of ghrelin secretion in morbidly obese patients. J Invest Surg. 2008;21:77–81. doi: 10.1080/08941930701883624. [DOI] [PubMed] [Google Scholar]
- 81.Leonetti F, Silecchia G, Iacobellis G, Ribaudo MC, Zappaterreno A, Tiberti C, Iannucci CV, Perrotta N, Bacci V, Basso MS, Basso N, Di Mario U. Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J Clin Endocrinol Metab. 2003;88:4227–4231. doi: 10.1210/jc.2003-030133. [DOI] [PubMed] [Google Scholar]
- 82.Lin E, Gletsu N, Fugate K, McClusky D, Gu LH, Zhu JL, Ramshaw BJ, Papanicolaou DA, Ziegler TR, Smith CD. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch Surg. 2004;139:780–784. doi: 10.1001/archsurg.139.7.780. [DOI] [PubMed] [Google Scholar]
- 83.Mancini MC, Costa AP, de Melo ME, Cercato C, Giannella-Neto D, Garrido AB, Jr, Rosberg S, Albertsson-Wikland K, Villares SM, Halpern A. Effect of gastric bypass on spontaneous growth hormone and ghrelin release profiles. Obesity (Silver Spring) 2006;14:383–387. doi: 10.1038/oby.2006.51. [DOI] [PubMed] [Google Scholar]
- 84.Sundbom M, Holdstock C, Engstrom BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: Influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 85.Tritos NA, Mun E, Bertkau A, Grayson R, Maratos-Flier E, Goldfine A. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res. 2003;11:919–924. doi: 10.1038/oby.2003.126. [DOI] [PubMed] [Google Scholar]


