Abstract
Background
Endoscopic retrograde cholangiopancreatography (ERCP) has a high risk of pancreatitis although the underlying mechanisms are unclear. Transient receptor potential vanilloid 1 (TRPV1) is a cation channel expressed on C and Ad fibres of primary sensory neurons and is activated by low pH. TRPV1 activation causes release of inflammatory mediators that produce oedema and neutrophil infiltration. We previously demonstrated that neurogenic factors contribute to the pathogenesis of pancreatitis. Resiniferatoxin (RTX) is a TRPV1 agonist that, in high doses, defunctionalises C and Ad fibres. When we discovered that the pH of radio-opaque contrast solutions used for ERCP was 6.9, we hypothesised that low pH may contribute to the development of contrast-induced pancreatitis via activation of TRPV1.
Methods
Rats underwent equal pressure pancreatic ductal injection of contrast solutions at varying pH with or without RTX.
Results
Contrast solution (pH 6.9) injected into the pancreatic duct caused a significant increase in pancreatic oedema, serum amylase, neutrophil infiltration, and histological damage. Solutions of pH 7.3 injected at equal pressure caused little damage. The severity of the pancreatitis was significantly increased by injection of solutions at pH 6.0. To determine if the effects of low pH were mediated by TRPV1, RTX was added to the contrast solutions. At pH levels of 6.0 and 6.9, RTX significantly reduced the severity of pancreatitis.
Conclusions
Contrast solutions with low pH contribute to the development of pancreatitis through a TRPV1-dependent mechanism. It is possible that increasing the pH of contrast solution and/or adding an agent that inhibits primary sensory nerve activation may reduce the risk of post-ERCP pancreatitis.
Endoscopic retrograde cholangiopancreatography (ERCP) is associated with a relatively high risk of causing pancreatitis, occurring in 3–30% of cases.1 The aetiology of post-ERCP pancreatitis is not well understood. It has been postulated that increased pressure within the pancreatic duct or chemical injury from injection of contrast solution may play a role in initiating the inflammatory cascade.2
Pharmacological methods to reduce the incidence of post-ERCP pancreatitis have been studied extensively. Because the exact aetiology whereby ERCP-induced pancreatitis develops is unclear, and targets of pharmacological treatment have varied, several studies have evaluated the use of a hypoosmolar, non-ionic contrast solution compared to standard contrast media.3–6 Results did not suggest any benefit with using one agent over another. Methods to reduce the inflammatory cascade have included corticosteroids, allopurinol, octreotide, interleukin 10, heparin, non-steroidal anti-inflammatory drugs, and the protease inhibitor gabexate.7–12 However, despite some initial enthusiasm, none of these therapies has proven to be definitive.
Neurogenic inflammation has been shown to play a significant role in the inflammatory response in the pancreas.13 This type of inflammation results from the activation of specific afferent sensory neurons leading to the release of proinflammatory transmitters from nerve terminals that ultimately cause vasodilation, plasma extravasation, and inflammatory cell infiltration.14,15 These effects can lead to mast cell degranulation, neutrophil adhesion, and the production of reactive oxygen species that contribute to the inflammatory response.16–18
Transient receptor potential vanilloid 1 (TRPV1) is a pH-sensitive, non-selective cation channel expressed on C and Ad fibres of primary sensory neurons.19 TRPV1 is also known as the capsaicin receptor and is activated by capsaicin as well as a number of other diverse mediators including acid, heat, anandamide, leukotriene B4 and other icosanoids.20 TRPV1 activation causes release of inflammatory mediators, such as substance P and calcitonin gene-related peptide, which produce oedema and neutrophil infiltration. We have previously demonstrated that pharmacological blockade of TRPV1 protects animals against pancreatitis. The TRPV1 antagonist capsazepine significantly reduced the severity of pancreatitis in a caerulein model of pancreatitis.21 Moreover, neonatal rats that had undergone chemical denervation of primary sensory neurons had a significant reduction in pancreatitis severity in a caerulein model.22 In addition, rats that had local denervation via destruction of the coeliac ganglion by chemical or surgical means had less severe pancreatitis in the same model of pancreatitis.23
Genetic deletion of the substance P receptor (NK-1R) reduced the severity of acute pancreatitis produced by either high doses of caerulein or feeding a choline-deficient, ethionine-supplemented diet.13,24 Local infusion of the NK-1R antagonist CP-96345 into the pancreatic duct has also been shown to significantly reduce the severity of pressure-induced pancreatitis.25 These data demonstrate that neurogenic pathways play an important role in determining the severity of acute pancreatitis and it appears that primary sensory neurons are a common final pathway for pancreatic inflammation.
Resiniferatoxin (RTX) is a TRPV1 agonist that, in high doses, rapidly defunctionalises C and Ad fibres.26,27 When we discovered that the pH of three separate radio-opaque contrast solutions used for ERCP were all of pH 6.9, we hypothesised that low pH may contribute to the development of contrast-induced pancreatitis. Given that lower pH levels have been shown to decrease the temperature activation threshold for primary sensory neurons, we hypothesised that contrast solutions at higher pH levels would have a protective effect in a pressure model of pancreatitis. In addition, we hypothesised that local intraductal administration of RTX would decrease pancreatitis severity.
MATERIALS AND METHODS
Animal protocol and experimental design
Male Sprague–Dawley (CD) rats, weighing 250–300 g were purchased from Charles River Laboratories (Wilmington, Massachusetts, USA) and housed in climate-controlled rooms with a 12 h:12 h light–dark cycle. All rats were fed standard laboratory chow until an overnight fast before surgery but were given free access to food following surgery. Rats were allocated to the following groups(n=6): (1) controls; (2) contrast solutions at pH6, pH6.9 or pH 7.3; or (3) contrast solutions at pH 6 + RTX (10 mg/400 μl), pH 6.9 + RTX, or pH 7.3 + RTX. Radio-opaque contrast solution (diatrizoate meglumine injection with 29% organically bound iodine) (Bracco Diagnostics, Princeton, New Jersey, USA) had an unadjusted pH of 6.9. All solutions were adjusted with 16 mmol/l HEPES buffer to pH of 7.3, 6.9 or 6.0. On the day of the experiment, rats were anaesthetised with xylazine and ketamine in a 1:10 ratio at a dose of 0.1 ml/100 g body weight administered intramuscularly. A model of post-ERCP pancreatitis was used as described previously.25,28 At laparotomy a 25 gauge needle was used to puncture the duodenum and cannulate the biliopancreatic duct. Four hundred microlitres of solution were administered into the duct after ligation of the bile duct at the hilum and around the entry of the needle into the common duct while maintaining a pressure of 50 mm Hg. After administration of solution, ligatures were removed, the needle was withdrawn, and the incision site was closed. Rats were observed for 24 h after surgery and then euthanised in a carbon dioxide pre-charged chamber. Mixed arteriovenous blood was collected by decapitation for measurement of serum amylase activity. The pancreas was then removed, weighed and compared to body weight as a measure of oedema. Pancreas was subsequently divided for histological grading, and measurement of tissue myeloperoxidase (MPO) activity.
Serum amylase concentration
Blood was centrifuged for 10 min at 1500 g. The serum amylase concentration was measured using the procion yellow starch assay as previously described.29 A standard curve was prepared using crude type VI-B α-amylase (Sigma, St. Louis, Missouri, USA), and serum amylase was expressed as mg/ml.
Myeloperoxidase activity
Portions of pancreas were immediately frozen for assay of MPO, which was used as a marker of neutrophil infiltration as previously described.30 The assay was performed in microtitre plates using a Safire plate reader from Tecan (Männedorf, Switzerland) with measurement wavelength at 450 nm and reference wavelength at 650 nm. Human MPO (Sigma) was used as standard and results are reported as mU MPO/mg pancreas protein. Protein concentration of the pancreatic extract was determined using the micro bicinchoninic acid protein assay (Pierce, Rockford, Illinois, USA).
Histology
Portions of the pancreas were fixed overnight at room temperature in a pH neutral, phosphate-buffered, 10% formalin solution. The tissue was then embedded in paraffin, sectioned at 5 μm, and stained with haematoxylin and eosin. Histological scoring of pancreatitis severity was performed using a scale of 0–3, as previously described by an investigator blinded to the study design.21,31
Statistical analysis
Results are expressed as means with the SE. Statistical comparisons among groups were examined by one-way ANOVA with the Tukey post test, using GraphPad Prism version 4.02. Statistical significance was set at p<0.05.
RESULTS
Injection of contrast solutions into the pancreatic duct under high pressure caused a significant increase in pancreatic oedema as manifested by increased pancreatic weight (fig 1). As the pH of contrast solutions was adjusted lower, the effects on pancreatic oedema were greater. The greatest amount of pancreatic oedema was observed at pH 6.0. Lower pH solutions were not tested as it seemed unlikely that these would be used in clinical situations. To determine whether the observed effects were due to pH alone, or a combination of pH and contrast solution, we tested the effects of phosphate-buffered saline (PBS) adjusted to pH 6.9 injected into the pancreatic duct. This treatment did not induce pancreatic injury. We conclude, therefore, that the lower pH buffer alone is not sufficient to cause pancreatitis.
Figure 1.
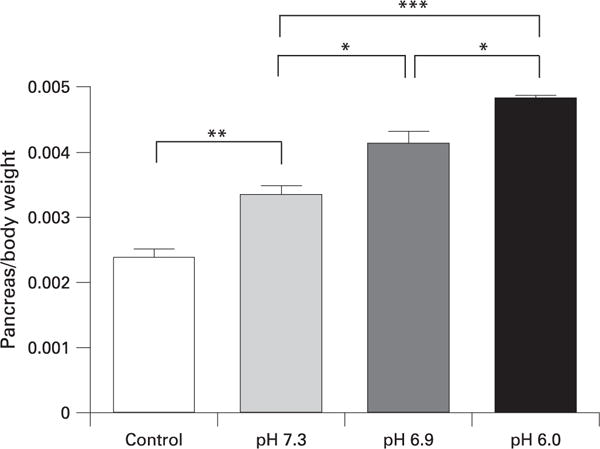
Quantification of pancreatic oedema, which was measured by comparing pancreas:body weight ratios (g/g). *p<0.05, **p<0.01, ***p<0.001.
MPO activity was similarly affected as lower pH solutions injected into the pancreatic duct caused a greater increase in MPO activity (fig 2). Injection of all solutions produced an elevation in serum amylase levels and although there was a trend for lower pH contrast solution to produce greater increases in serum amylase elevation these changes were not significantly different (fig 3). These findings support the observations made in other studies that serum amylase reflects pancreatic injury, but does not correlate with pancreatitis severity.21,32
Figure 2.
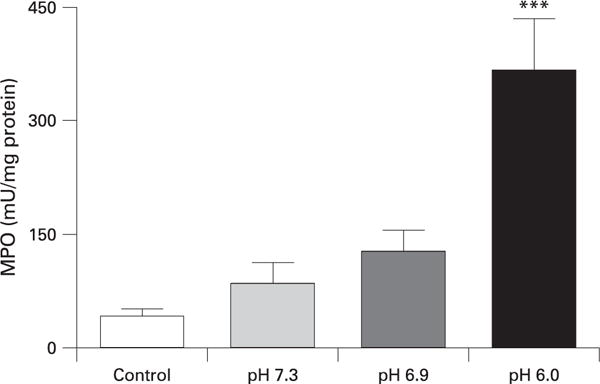
Effect of pH on pancreatic myeloperoxidase (MPO) activity. ***p<0.001 vs control.
Figure 3.
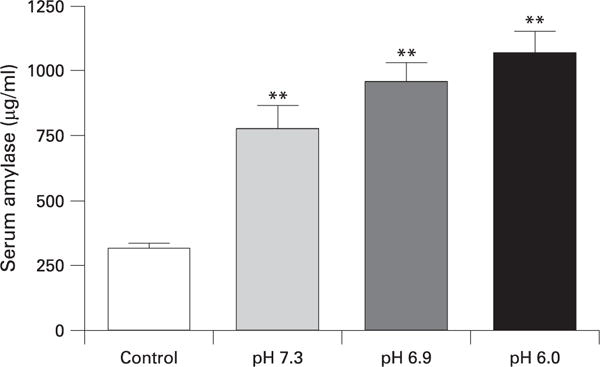
Effect of high pressure injection on serum amylase. **p<0.01 vs control.
The effects of different pH solutions on pancreatic histology are shown in fig 4. Injection of contrast material at pH 7.3 produced little detectable histological damage in the pancreas (panel B). However, solutions of pH 6.9 produced noticeable changes of pancreatic disruption (panel C). The specific parameters that contributed to the histological differences between the pH 6.9 and 7.3 groups were primarily pancreatic oedema and pyknotic nuclei indicative of early necrosis, with oedema being the most prominent feature. Slight increases in neutrophil infiltration were also observed. Oedema and acinar disruption were more marked and were accompanied by detectable neutrophil infiltration in pancreas injected with contrast solution at pH 6.0 (panel D). These changes were reflected by the pancreatitis disease severity histological score (fig 4E).
Figure 4.
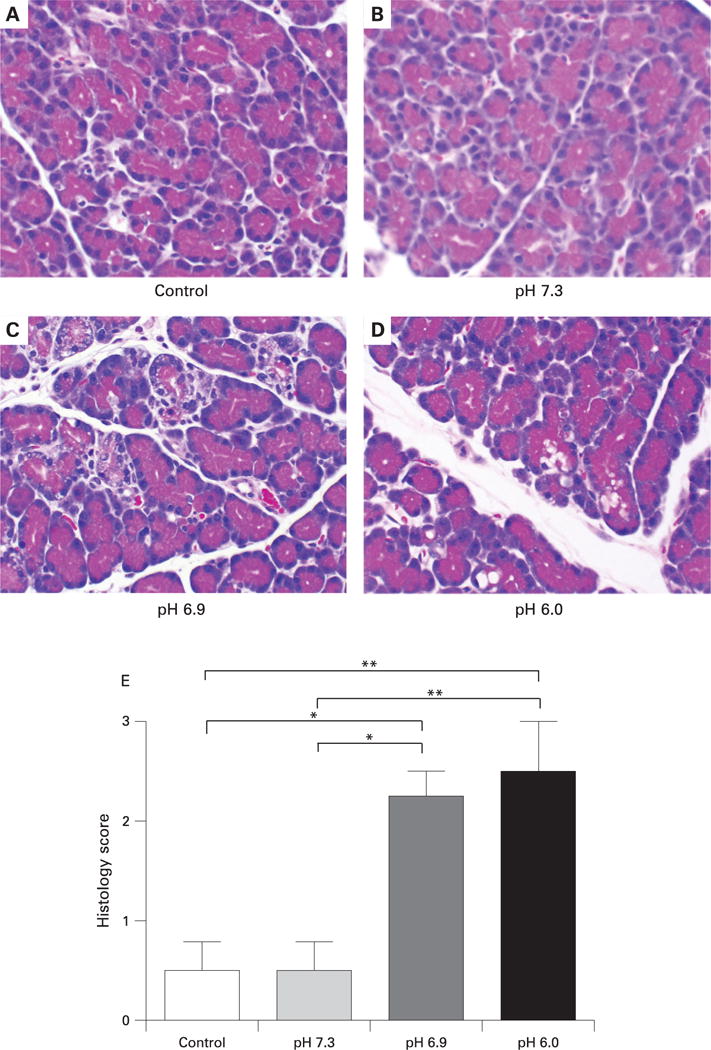
Effect of high pressure intraductal injection of contrast solutions on pancreatic histology.
(A–D) Representative photomicrographs of pancreatic tissue collected 24 h after injection of solutions of different pH into the pancreatic duct. Solutions at pH 6.9 produced histological disruption and oedema (C). These changes were more marked and accompanied by neutrophil infiltration in tissues from animals injected with solution at pH 6.0 (D). Magnification: ×6250. (E) Histological scoring of pancreatic sections was performed by an observer blinded to the study design. *p<0.05. **p<0.01.
We hypothesised that the effects of low pH on pancreatitis severity may be through activation of TRPV1. It has previously been shown that RTX, in high doses, rapidly defunctionalises primary sensory neuronal signalling by causing a prompt and sustained influx of Na+ and Ca2+.33 These changes cause immediate neuronal dysfunction but also lead to disruption of intracellular organelles and neuronal destruction.34 Therefore, we used RTX to defunctionalise TRPV1 and primary sensory nerves in the pancreas by co-injecting it with the contrast solution. As shown in fig 5, RTX co-injection completely inhibited the pancreatic oedema caused by the lower pH contrast solutions of 6.9 and 6.0. The increases in pancreatic MPO content were also reduced by addition of RTX (fig 6). Moreover, histological changes of pancreatitis due to pancreatic duct injection were essentially eliminated by RTX (fig 7). Addition of RTX to the contrast solutions at pH 6.9 and 6.0 significantly reduced the total histology scores (compare figs 4 and 7) (p<0.05). These differences were primarily attributable to reduction in oedema and necrosis. Acinar cell vacuolisation was also absent following RTX treatment.
Figure 5.
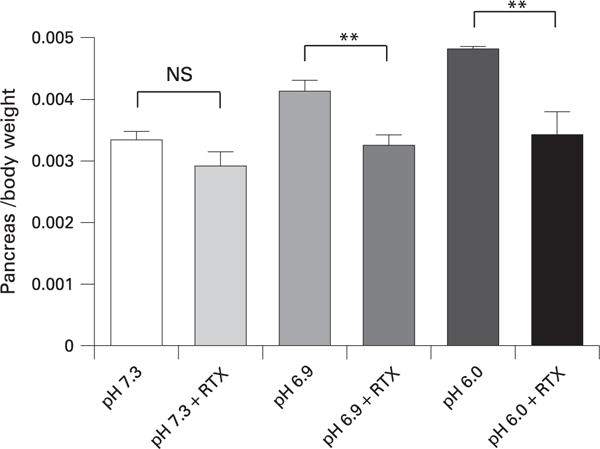
Effects of resiniferatoxin (RTX) on pancreatic oedema when co-injected into the pancreatic duct with contrast solutions of different pH. **p<0.01.
Figure 6.
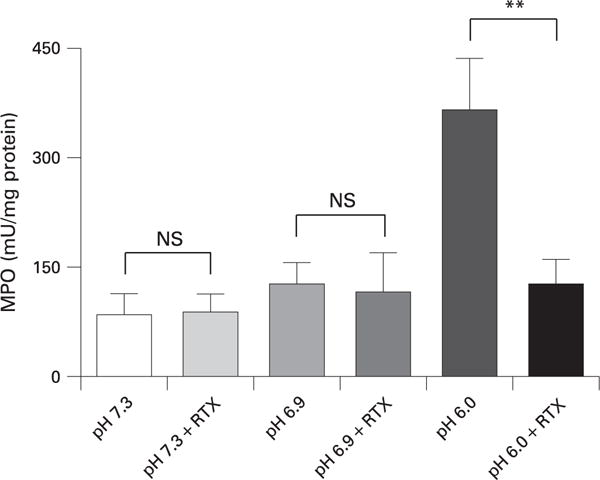
Effects of resiniferatoxin (RTX) on pancreatic myeloperoxidase (MPO) activity when co-injected into the pancreatic duct with contrast solutions of different pH. **p<0.01.
Figure 7.
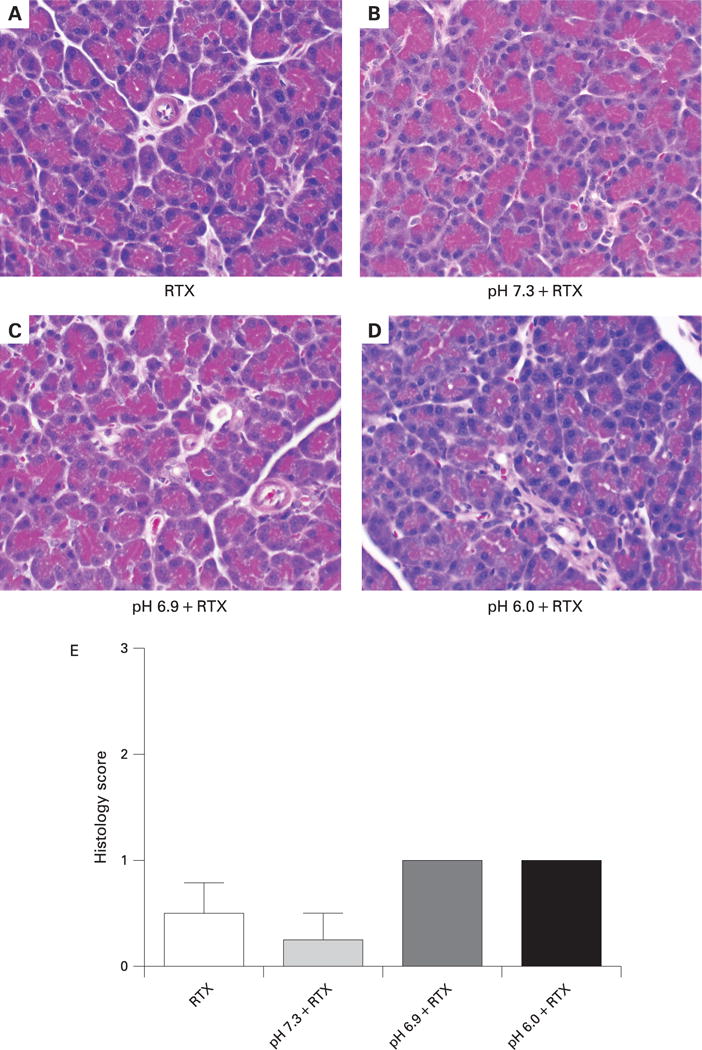
Effects of resiniferatoxin (RTX) on pancreatic histology when co-injected into the pancreatic duct with contrast solutions of different pH.
(A–D) Photomicrographs of representative pancreatic tissue collected 24 h after injection of solutions of different pH into the pancreatic duct. Magnification: ×6250. (E) Histological scoring of pancreatic sections was performed by an observer blinded to the study design. *p<0.05, **p<0.01.
DISCUSSION
This study is based on the initial observation that radiocontrast solutions typically used for ERCP have a pH of ~6.9. This pH is lower than that of normal pancreatic juice which is alkaline due to high levels of bicarbonate secretion. Previous studies by our group and others on TRPV1, a pH-sensitive cation channel, in pancreatitis pointed to a possibility that pH may contribute to the propensity for pancreatitis following intrapancreatic duct injection of contrast solutions. In the current study, we observed that low pH solutions (6.9 and 6.0) produced pancreatitis under the same high pressure conditions that did not cause pancreatitis when the pH was higher (7.3). This finding suggested that pH is critical in this model of pancreatitis. To investigate the role of the pH-sensitive cation channel, TRPV1, we used a high dose of RTX to block TRPV1 signalling. Importantly, RTX prevented the deleterious effects of low pH contrast solutions on pancreatitis. These findings implicate TRPV1 and primary sensory nerves in the pathogenesis of contrast-induced pancreatitis.
Pancreatitis is one of the most frequent complications of ERCP but the exact aetiology as to why pancreatitis develops in some patients is unknown. Increased pressure within the pancreatic duct has been indirectly implicated as a cause of post-ERCP pancreatitis as multiple studies have shown that placement of a pancreatic stent following ERCP in high-risk patients reduced the incidence of pancreatitis.2 He et al25 described a model of inducing pancreatitis based on the theory that high pressure intraductal injection causes pancreatitis perhaps through disruption of the ductal integrity. In that study, solution was injected into the pancreatic duct under high pressure thus inducing local pancreatic damage and subsequent inflammation. However, the importance of pressure alone has been debated as Freeman1 found that in humans, acinarisation (overfilling of the main pancreatic duct causing pancreatic parenchyma to be visualised on fluoroscopy) was not an independent risk factor for developing post-procedure pancreatitis. Thus it seemed possible that something inherent to the contrast itself may be playing a role in the development of pancreatitis. To determine whether the lower pH by itself incited the pancreatic damage, or whether pH-dependent changes in the conformation or charge of the contrast solution was responsible for these observations, in our study, we examined the effects of PBS. Unlike contrast solution of pH 6.9, PBS at this same pH did not cause pancreatitis. These findings suggest that lower pH together with contrast solution is more damaging to the pancreas than buffer alone. It remains to be determined what biochemical properties of contrast media may be affected by pH.
Neurogenic inflammation is mediated through the release of neuropeptides such as substance P from C and Ad primary afferent sensory neurons via activation of the vanilloid capsaicin receptor TRPV1.35 Substance P in turn binds to the neurokinin-1 receptor (NK-1R) and causes extravasation of plasma from postcapillary venules in the pancreas as well as other gastrointestinal organs. Activation of NK-1R results in the release of various proinflammatory agents.21 These receptors are located on a variety of cell types including inflammatory cells, smooth muscle cells, vascular endothelial cells, surface epithelium, and importantly, pancreatic acinar cells.21,36,37 In support of this pathogenic mechanism, it has been shown that intrapancreatic duct injection of a NK-1R antagonist attenuated pancreatic inflammation.25
Realising that sensory nerves of the pancreas are responsible for the neurogenic contribution to pancreatitis, we previously evaluated the effect of direct disruption of pancreatic nerves as they course through the coeliac ganglion.23 We observed that local denervation of primary sensory nerves going to the pancreas reduced the severity of pancreatitis. We postulated, therefore, that because TRPV1 is sensitive to local pH, that any disruption to the pancreatic duct that accompanies high pressure retrograde duct injection together with low pH contrast solution, may be sufficient to activate TRPV1 and induce pancreatitis through a neurogenic mechanism.
RTX is a member of the vanilloid family but is much more potent than capsaicin.38 When RTX is applied to TRPV1-expressing cells a significant and prolonged increase in free intracellular calcium occurs.33 The resultant calcium toxicity destroys TRPV1-expressing cells by the disrupting mitochondrial structure, endoplasmic reticulum and nuclear membrane.39 In the present study, 10 mg of RTX was combined with contrast agent and injected into the pancreatic duct under high pressure to try and eliminate TRPV1-expressing primary sensory neurons. As was seen with local denervation of primary sensory neurons within the coeliac ganglion, RTX administered within the pancreatic duct reduced the severity of pancreatitis when the injectate solution was acidic.
In conclusion, in a model of post-ERCP pancreatitis, there is an inverse relationship between the severity of pancreatitis and the pH of contrast solution. Administration of RTX within the pancreatic duct significantly ameliorated the pancreatic inflammation. These findings support the concept that pH and activation of primary sensory neurons within the pancreas play a role in mediating pancreatitis. It is possible that increasing the pH of contrast media and/or adding an agent that selectively inhibits primary sensory neurons may reduce the incidence of post-ERCP pancreatitis.
Acknowledgments
Funding: This work was supported by NIH grants DK-073908, DK-064213, and the Department of Veterans Affairs.
Footnotes
Competing interests: None.
Ethics approval: All animal experiments were performed with approval of the Duke University Institutional Animal Care and Use Committee, on 24 April 2004.
References
- 1.Freeman ML. Post-ERCP pancreatitis: patient and technique-related risk factors. J Pancreas. 2002;3:169–76. [PubMed] [Google Scholar]
- 2.Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845–64. doi: 10.1016/s0016-5107(04)00353-0. [DOI] [PubMed] [Google Scholar]
- 3.Hannigan BF, Keeling PW, Slavin B, et al. Hyperamylasemia after ERCP with ionic and non-ionic contrast media. Gastrointest Endosc. 1985;31:109–10. doi: 10.1016/s0016-5107(85)72018-4. [DOI] [PubMed] [Google Scholar]
- 4.Johnson GK, Geenen JE, Bedford RA, et al. A comparison of nonionic versus ionic contrast media: results of a prospective, multicenter study. Midwest Pancreaticobiliary Study Group. Gastrointest Endosc. 1995;42:312–6. doi: 10.1016/s0016-5107(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 5.Sherman S, Hawes RH, Rathgaber SW, et al. Post-ERCP pancreatitis: randomized, prospective study comparing a low- and high-osmolality contrast agent. Gastrointest Endosc. 1994;40:422–7. doi: 10.1016/s0016-5107(94)70204-7. [DOI] [PubMed] [Google Scholar]
- 6.Cunliffe WJ, Cobden I, Lavelle MI, et al. A randomised, prospective study comparing two contrast media in ERCP. Endoscopy. 1987;19:201–2. doi: 10.1055/s-2007-1013018. [DOI] [PubMed] [Google Scholar]
- 7.Manolakopoulos S, Avgerinos A, Vlachogiannakos J, et al. Octreotide versus hydrocortisone versus placebo in the prevention of post-ERCP pancreatitis: a multicenter randomized controlled trial. Gastrointest Endosc. 2002;55:470–5. doi: 10.1067/mge.2002.122614. [DOI] [PubMed] [Google Scholar]
- 8.Budzynska A, Marek T, Nowak A, et al. A prospective, randomized, placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy. 2001;33:766–72. doi: 10.1055/s-2001-16520. [DOI] [PubMed] [Google Scholar]
- 9.Cavallini G, Tittobello A, Frulloni L, et al. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. Gabexate in digestive endoscopy – Italian Group. N Engl J Med. 1996;335:919–23. doi: 10.1056/NEJM199609263351302. [DOI] [PubMed] [Google Scholar]
- 10.Dumot JA, Conwell DL, Zuccaro G, Jr, et al. A randomized, double blind study of interleukin 10 for the prevention of ERCP-induced pancreatitis. Am J Gastroenterol. 2001;96:2098–102. doi: 10.1111/j.1572-0241.2001.04092.x. [DOI] [PubMed] [Google Scholar]
- 11.Murray B, Carter R, Imrie C, et al. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786–91. doi: 10.1016/s0016-5085(03)00384-6. [DOI] [PubMed] [Google Scholar]
- 12.Toulon P, Chadeuf G, Bouillot JL, et al. Involvement of heparin cofactor II in chymotrypsin neutralization and in the pancreatic proteinase–antiproteinase interaction during acute pancreatitis in man. Eur J Clin Invest. 1991;21:303–9. doi: 10.1111/j.1365-2362.1991.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia M, Saluja AK, Hofbauer B, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A. 1998;95:4760–5. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–68. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 15.Kenins P. Identification of the unmyelinated sensory nerves which evoke plasma extravasation in response to antidromic stimulation. Neurosci Lett. 1981;25:137–41. doi: 10.1016/0304-3940(81)90321-9. [DOI] [PubMed] [Google Scholar]
- 16.Baluk P, Bertrand C, Geppetti P, et al. NK1 receptors mediate leukocyte adhesion in neurogenic inflammation in the rat trachea. Am J Physiol. 1995;268(2 Pt 1):L263–9. doi: 10.1152/ajplung.1995.268.2.L263. [DOI] [PubMed] [Google Scholar]
- 17.Saban MR, Saban R, Bjorling D, et al. Involvement of leukotrienes, TNF-alpha, and the LFA-1/ICAM-1 interaction in substance P-induced granulocyte infiltration. J Leukoc Biol. 1997;61:445–51. doi: 10.1002/jlb.61.4.445. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe T, Otani H, Bao L, et al. Intracellular signaling pathway of substance P-induced superoxide production in human neutrophils. Eur J Pharmacol. 1996;299:187–95. doi: 10.1016/0014-2999(95)00816-0. [DOI] [PubMed] [Google Scholar]
- 19.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 20.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 21.Nathan JD, Patel AA, McVey DC, et al. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1322–8. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- 22.Nathan JD, Peng RY, Wang Y, et al. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G938–46. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 23.Noble MD, Romac J, Wang Y, et al. Local disruption of the celiac ganglion inhibits substance P release and ameliorates caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G128–34. doi: 10.1152/ajpgi.00442.2005. [DOI] [PubMed] [Google Scholar]
- 24.Maa J, Grady EF, Yoshimi SK, et al. Genetic deletion of the neurokinin-1 receptor improves survival in diet-induced hemorrhagic pancreatitis in mice. Surg Forum. 1999;50:34–5. [Google Scholar]
- 25.He ZJ, Winston JH, Yusuf TE, et al. Intraductal administration of an NK1 receptor antagonist attenuates the inflammatory response to retrograde infusion of radiological contrast in rats: implications for the pathogenesis and prevention of ERCP-induced pancreatitis. Pancreas. 2003;27:e13–7. doi: 10.1097/00006676-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Dray A. Neuropharmacological mechanisms of capsaicin and related substances. Biochem Pharmacol. 1992;44:611–5. doi: 10.1016/0006-2952(92)90393-w. [DOI] [PubMed] [Google Scholar]
- 27.Szallasi A. Vanilloid (capsaicin) receptors in health and disease. Am J Clin Pathol. 2002;118:110–21. doi: 10.1309/7AYY-VVH1-GQT5-J4R2. [DOI] [PubMed] [Google Scholar]
- 28.Kivisaari L. Contrast absorption and pancreatic inflammation following experimental ERCP. Invest Radiol. 1979;14:493–7. doi: 10.1097/00004424-197911000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Jung DH. Preparation and application of procion yellow starch for amylase assay. Clin Chim Acta. 1980;100:7–11. doi: 10.1016/0009-8981(80)90179-5. [DOI] [PubMed] [Google Scholar]
- 30.Bradley PP, Priebat DA, Christensen RD, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 31.Spormann H, Sokolowski A, Letko G. Effect of temporary ischemia upon development and histological patterns of acute pancreatitis in the rat. Path Res Pract. 1989;184:507–513. doi: 10.1016/S0344-0338(89)80143-8. [DOI] [PubMed] [Google Scholar]
- 32.Nathan JD, Romac J, Peng RY, et al. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–27. doi: 10.1053/j.gastro.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 33.Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–20. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 34.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 35.Figini M, Emanueli C, Grady EF, et al. Substance P and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am J Physiol. 1997;272(4 Pt 1):G785–93. doi: 10.1152/ajpgi.1997.272.4.G785. [DOI] [PubMed] [Google Scholar]
- 36.Bowden JJ, Garland AM, Baluk P, et al. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A. 1994;91:8964–8. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renzi D, Pellegrini B, Tonelli F, et al. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol. 2000;157:1511–22. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralevic V, Jerman JC, Brough SJ, et al. Pharmacology of vanilloids at recombinant and endogenous rat vanilloid receptors. Biochem Pharmacol. 2003;65:143–51. doi: 10.1016/s0006-2952(02)01451-x. [DOI] [PubMed] [Google Scholar]
- 39.Olah Z, Szabo T, Karai L, et al. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem. 2001;276:11021–30. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]


