Abstract
Background
Heart failure (HF) is associated with the derangement of muscle structure and metabolism, contributing to exercise intolerance, frailty, and mortality. Reduced handgrip strength is associated with increased patient frailty and higher morbidity and mortality. We evaluated handgrip strength as a marker of muscle function and frailty for prediction of clinical outcomes after ventricular assist device (VAD) implantation in patients with advanced HF.
Methods and Results
Handgrip strength was measured in 72 patients with advanced HF before VAD implantation (2.3 ± 4.9 days pre-VAD). We analyzed dynamics in handgrip strength, laboratory values, postoperative complications, and mortality. Handgrip strength correlated with serum albumin levels (r = 0.334, P = .004). Compared with baseline, handgrip strength increased post-VAD implantation by 18.2 ± 5.6% at 3 months (n = 29) and 45.5 ± 23.9% at 6 months (n = 27). Patients with a handgrip strength <25% of body weight had an increased risk of mortality, increased postoperative complications, and lower survival after VAD implantation.
Conclusion
Patients with advanced HF show impaired handgrip strength indicating a global myopathy. Handgrip strength <25% of body weight is associated with higher postoperative complication rates and increased mortality after VAD implantation. Thus, the addition of measures of skeletal muscle function underlying the frailty phenotype to traditional risk markers might have incremental prognostic value in patients undergoing evaluation for VAD placement.
Keywords: Heart failure, ventricular assist device, skeletal muscle, handgrip strength
Introduction
Heart failure (HF) is a clinical syndrome associated with progressive derangement of neurohormonal and metabolic pathways in addition to end-organ injury resulting from circulatory failure.1 Skeletal muscle oxidative capacity, capillary density, and fiber cross-sectional area are diminished in patients with advanced HF.2,3 Derangement of skeletal muscle structure and metabolic function contributes to diminished exercise tolerance and the frailty phenotype with increased morbidity and mortality.4–6 These changes are only partially explained by impaired central hemodynamics.7
Therapeutic options in patients with advanced HF are limited, and high morbidity and mortality characterize this patient population.8 The recent advent of ventricular assist devices (VADs) for mechanical support of the failing myocardium has expanded the therapeutic options for patients with advanced HF both for bridge-to-transplantation and as a destination therapy9–11 but optimal strategies for both patient selection, treatment, and monitoring are still evolving.12,13
Frailty has been identified as an important prognostic marker in various patient cohorts including the elderly,14 subclinical cardiovascular disease,15 coronary artery disease,16,17 and patients undergoing cardiac surgery.18,19 Frailty has also been proposed as an additional marker of patient outcomes in patients undergoing destination therapy VAD implantation20 but in whom definitive prospective data are lacking. Handgrip strength (HGS) is a marker of frailty and is included in several frailty scoring systems.14,21–24 HGS correlates highly with the strength of elbow flexion, knee extension, and trunk extension,25,26 and its use to approximate overall muscle function avoids many of the pitfalls of testing larger muscle groups particularly in advanced HF patients with minimal tolerance of physical exertion and in hospitalized, deconditioned patients.21 HGS has been shown to independently predict adverse health outcomes and mortality in diverse populations.22–24
No study to date has examined the relationship between baseline HGS and outcomes after VAD implantation. We therefore evaluated the association between baseline and postoperative HGS and clinical outcomes after VAD implantation.
Methods
Data Collection
We performed an observational cohort study of 72 patients with advanced HF undergoing VAD implantation between October 2010 and June 2013 at Columbia University Medical Center. HGS was measured preoperatively (2.3 ± 4.9 days before surgery) using a Jamar hand dynamometer (Sammons Preston Inc., Boiling Brook, IL). HGS was also measured at various time points after VAD implantation to assess dynamics in HGS after surgery. Subjects were asked to perform a maximal isometric contraction with each hand 3 consecutive times. Each contraction was followed by a 5-second rest period. Averages for each hand were taken. HGS was normalized for total body weight (BW).
Clinical and laboratory data were gathered from institutional medical records. Clinical outcomes of interest included postoperative in-hospital mortality, all-cause mortality while on VAD support, ventricular arrhythmias, right ventricular failure, bleeding, infection, renal failure, respiratory failure, and cerebrovascular accident. Approval for this study was obtained from the Institutional Review Board at Columbia University Medical Center.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation and categorical variables as number and percentages of total group number. Normality was tested using the Kolmogorov-Smirnov test. Group comparisons were made using the χ2 or Fisher’s exact test for categorical variables and Student’s t-test or 1-way analysis of variance with Tukey’s post-hoc testing for continuous variables. Comparisons of dynamics in values were analyzed using paired assessments compared with baseline values both for the entire cohort and for patients who did not drop out during the observation period. For variables that did not follow a Gaussian distribution, the Mann-Whitney U or Kruskal-Wallis analysis of variance was used. We performed receiver operating characteristic analysis to determine the optimal value of HGS that would allow discrimination of patients with higher mortality after VAD implantation and patients were subsequently stratified for subgroup analysis. Kaplan-Meier curves for survival were constructed and differences in survival rates were tested using both the log-rank and Wilcoxon tests. Data were analyzed using GraphPad Prism 5.0 (La Jolla, CA). A P value < .05 was considered statistically significant.
Results
Baseline Demographics
Demographics and clinical characteristics of all study participants are presented in Table 1. Baseline laboratory values before and dynamics after VAD implantation are shown in Tables 2 and 3. By the third month, an increase in serum albumin and decrease in total and direct bilirubin was observed that persisted at 6 months.
Table 1.
Baseline Demographics and Clinical Characteristics
| All Patients (n = 72) | HGS <25% BW (n = 16) | HGS ≥25% BW (n = 56) | P Value | |
|---|---|---|---|---|
| Male (%) | 64 (89) | 12 (75) | 52 (93) | |
| Age (y) | 59 ± 2 | 61 ± 3 | 59 ± 2 | .673 |
| BMI (kg/m2) | 28 ± 1 | 30 ± 2 | 28 ± 1 | .289 |
| Heart rate (beats/min) | 85 ± 2 | 88 ± 4 | 84 ± 2 | .401 |
| Systolic BP (mm Hg) | 104 ± 2 | 104 ± 3 | 104 ± 2 | .935 |
| Diastolic BP (mm Hg) | 65 ± 1 | 68 ± 5 | 64 ± 2 | .149 |
| New York | .061 | |||
| Heart Failure class (no. (%)) | ||||
| I | 0 (0) | 0 (0) | 0 (0) | |
| II | 1 (1) | 0 (0) | 1 (2) | |
| III | 20 (28) | 3 (19) | 17 (30) | |
| IV | 51 (71) | 13 (81) | 38 (68) | |
| Etiology of heart failure (no. (%)) | .479 | |||
| Dilated cardiomyopathy | 32 (44) | 5 (31) | 21 (38) | |
| Ischemic cardiomyopathy | 33 (46) | 9 (56) | 30 (54) | |
| Other | 7 (10) | 2 (13) | 5 (9) | |
| Ejection fraction (%) | 17.9 ± 0.6 | 20.3 ± 1.5 | 17.3 ± 0.6 | .037 |
| Years with HF (y) | 9.9 ± 1.0 | 10.8 ± 2.8 | 9.7 ± 1.1 | .662 |
| Preoperative MCS (no. (%)) | 22 (31) | 7 (44) | 15 (27) | .194 |
| Lietz-Miller score | 8.3 ± 0.5 | 8.9 ± 1.1 | 8.2 ± 0.5 | .546 |
| Medications | .503 | |||
| Diuretics | 60 (83) | 14 (88) | 46 (82) | |
| Inotropes | 60 (83) | 13 (81) | 47 (84) | |
| β-blocker | 58 (81) | 13 (81) | 45 (80) | |
| ACE/ARBs | 37 (51) | 7 (44) | 30 (54) | |
| Aspirin | 35 (49) | 9 (56) | 26 (46) | |
| Coumadin | 35 (49) | 8 (50) | 27 (48) | |
| Statins | 34 (47) | 10 (63) | 24 (43) | |
| Digoxin | 29 (40) | 6 (38) | 23 (41) | |
| Vasodilators | 5 (7) | 1 (6) | 4 (7) | |
| Functional assessment | ||||
| HGS (dominant) | 29 ± 1 | 18 ± 2 | 32 ± 1 | <.0001 |
| HGS (nondominant) | 28 ± 1 | 18 ± 2 | 31 ± 1 | <.0001 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; BTT, bridge to transplantation; BW, body weight; CAD, coronary artery disease; HGS, handgrip strength; ICM, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support.
Continuous variables are expressed as mean ± standard deviation and categorical variables as number, %.
Table 2.
Baseline Laboratory Values in Subgroups of Patients
| All Patients (n = 72) | HGS < 25% BW (n = 16) | HGS ≥ 25% BW (n = 56) | P Value | |
|---|---|---|---|---|
| WBC (×109/L) | 8.4 ± 0.4 | 8.5 ± 0.9 | 8.4 ± 0.4 | .878 |
| Hematocrit (%) | 33 ± 1 | 33 ± 2 | 32 ± 1 | .897 |
| Platelets (×109/L) | 201 ± 9 | 213 ± 19 | 198 ± 11 | .498 |
| Glucose (mg/dL) | 132 ± 6 | 152 ± 17 | 127 ± 7 | .098 |
| Sodium (mEq/L) | 135 ± 1 | 135 ± 1 | 135 ± 1 | .938 |
| Potassium (mEq/L) | 4.1 ± 0.1 | 4.0 ± 0.1 | 4.1 ± 0.1 | .706 |
| BUN (mg/dL) | 33.7 ± 17.0 | 37.6 ± 24.2 | 32.6 ± 14.5 | .434 |
| Creatinine (mg/dL) | 1.5 ± 0.1 | 1.7 ± 0.3 | 1.4 ± 0.1 | .247 |
| Albumin (g/dL) | 3.59 ± 0.06 | 3.44 ± 0.14 | 3.64 ± 0.06 | .146 |
| Total bilirubin (mg/dL) | 1.69 ± 0.17 | 1.68 ± 0.27 | 1.69 ± 0.19 | .981 |
| Direct bilirubin (mg/dL) | 0.59 ± 0.08 | 0.07 ± 0.14 | 0.57 ± 0.09 | .497 |
| AST (U/L) | 31.6 ± 2.7 | 31.1 ± 4.5 | 31.8 ± 3.2 | .914 |
| ALT (U/L) | 32.3 ± 3.7 | 32.8 ± 7.5 | 32.2 ± 4.3 | .944 |
| APT (U/L) | 93 ± 5 | 103 ± 14 | 90 ± 5 | .235 |
| MELD | 14.9 ± 1.3 | 16.0 ± 3.9 | 14.5 ± 3.1 | .626 |
| MELD-XI | 14.4 ± 0.8 | 14.1 ± 2.5 | 14.5 ± 0.7 | .866 |
| GFR (mL/min) | 57.3 ± 24.7 | 60.1 ± 28.9 | 56.5 ± 23.6 | .652 |
ALT, alanine aminotransferase; APT, alkaline phosphatase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; BW, body weight; GFR, glomerular filtration rate; HGS, handgrip strength; MELD, model for endstage liver disease; MELD-XI, model for end-stage liver disease-excluding INR; WBC, white blood count.
Table 3.
Dynamics in Laboratory Values in Patients Undergoing VAD Placement
| Pre-VAD All Patients (n = 72) |
Post-VAD
|
||||
|---|---|---|---|---|---|
| 3 Months (n = 23) |
P Value* | 6 Months (n = 20) |
P Value† | ||
| WBC (×109/L) | 8.4 ± 0.4 | 16.5 ± 8.6 | .107 | 8.0 ± 0.8 | .629 |
| Hematocrit (%) | 33 ± 1 | 35 ± 1 | .271 | 36 ± 1 | .108 |
| Platelets (×109/L) | 201 ± 9 | 240 ± 19 | .056 | 205 ± 12 | .859 |
| Glucose (mg/dL) | 132 ± 6 | 89 ± 8 | .001 | 121 ± 16 | .453 |
| Sodium (mEq/L) | 135 ± 1 | 136 ± 1 | .171 | 134 ± 1 | .397 |
| Potassium (mEq/L) | 4.1 ± 0.1 | 4.3 ± 0.1 | .055 | 4.4 ± 0.1 | .012 |
| BUN (mg/dL) | 33.7 ± 17.0 | 24.3 ± 2.0 | .019 | 28.8 ± 2.6 | .310 |
| Creatinine (mg/dL) | 1.5 ± 0.1 | 1.4 ± 1.1 | .360 | 1.3 ± 0.1 | .226 |
| Albumin (g/dL) | 3.59 ± 0.06 | 3.9 ± 0.1 | .003 | 4.2 ± 0.1 | .001 |
| Total bilirubin (mg/dL) | 1.69 ± 0.17 | 0.85 ± 0.30 | .008 | 0.87 ± 0.11 | .016 |
| Direct bilirubin (mg/dL) | 0.59 ± 0.08 | 0.24 ± 0.02 | .016 | 0.22 ± 0.4 | .016 |
| AST (U/L) | 31.6 ± 2.7 | 28.7 ± 4.2 | .586 | 26.8 ± 2.4 | .356 |
| ALT (U/L) | 32.3 ± 3.7 | 23.2 ± 3.7 | .198 | 24.3 ± 3.1 | .269 |
| APT (U/L) | 93 ± 5 | 109 ± 6 | .093 | 93 ± 7 | .991 |
ALT, alanine aminotransferase; APT, alkaline phosphatase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; VAD, ventricular assist device; WBC, white blood count.
P value for comparison of before VAD vs 3 month post-VAD.
P value for comparison of before VAD vs 6 months post-VAD.
Characterization of HGS at Baseline and After VAD Implantation
There was a blunted difference in baseline unadjusted HGS as well as HGS adjusted for BW of the dominant versus nondominant hands in patients with advanced HF (Fig. 1). Of note, HGS of both the dominant and the nondominant arm had a positive correlation with serum albumin (r = 0.334, P = .004) but not with the percentage of lymphocytes or other markers of anabolic metabolism (Fig. 2).
Fig. 1.
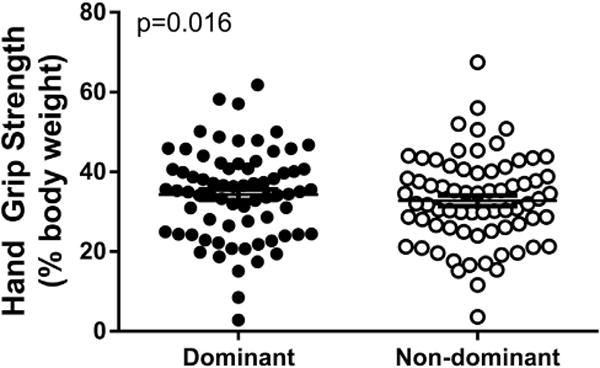
Characterization of average bilateral handgrip strength presented as a percentage of total body weight before ventricular assistive device implantation.
Fig. 2.
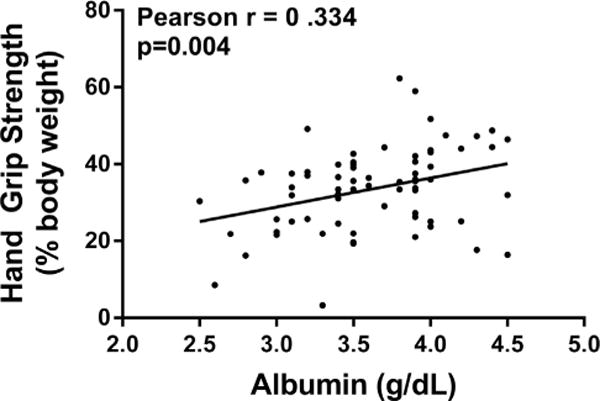
Correlation of handgrip strength and albumin. Nondominant handgrip strength (percentage of body weight) shows a positive correlation with serum albumin.
Bilateral grip strength was assessed monthly (Fig. 3) for 6 months after VAD implantation (baseline: n = 72; 1 month: n = 31; 2 months: n = 25; 3 months: n = 29; 4 months: n = 21; 5 months: n = 22; and 6 months: n = 27). HGS progressively improved at 3 months (dominant hand: +18.2 ± 5.6% increase, P < .05; nondominant hand: +26.9 ± 11.4% increase, P < .05) and sustained at 6 months after VAD implantation (dominant hand: 45.5 ± 23.9% increase, P < .0005; nondominant hand: 38.2 ± 13.8% increase, P < .005). Data of selected patients that were followed over the entire study (excluding dropouts and transplant recipients) are shown in the Supplemental Figure.
Fig. 3.
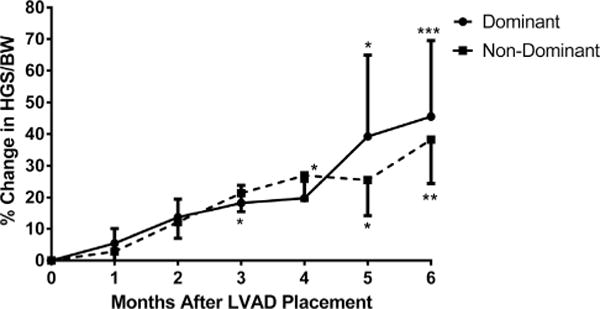
Change in handgrip strength of the dominant and nondominant hands after ventricular assistive device implantation. Handgrip strength increases significantly starting at 3 months after left ventricular assistive device implantation (*P < .05 vs baseline; **P < .005 vs baseline; ***P < .0005 vs baseline).
Clinical Outcomes and Mortality After VAD Implantation
Baseline HGS was lower in patients who died after VAD implantation compared with patients who survived. We therefore performed a receiver operating characteristic analysis to determine the optimal value of HGS that would allow discrimination of patients with higher mortality after VAD implantation and patients were subsequently stratified for subgroup analysis. This analysis revealed that HGS < 25% of total BW distinguished patients with greater likelihood of early postoperative mortality with a sensitivity of 72% and specificity of 80% (area under the curve 0.80).
There were no differences in baseline clinical or laboratory values or medical therapy between those with nondominant HGS less than 25% of BW versus those with HGS of at least 25% of BW (Table 3). Further, we found no differences in right atrial pressure (13 ± 6 in HGS < 25% BW vs 12 ± 6 in HGS ≥ 25% BW; P = .582), mean pulmonary artery pressure (36 ± 7 in HGS < 25% BW vs 36 ± 10 in HGS ≥ 25% BW; P = .77) or pulmonary capillary wedge pressure (24 ± 8 in HGS < 25% BW vs 26 ± 9 in HGS ≥ 25% BW; P = .321) between the groups.
Next, we studied the rate of postoperative clinical complications such as ventricular tachycardia, bleeding, infection, and right heart failure in those with HGS less than versus greater than 25% of BW and found no differences in incidence of adverse events except that patients with handgrip >25% had lower rates of bleeding (17 vs 54%, P = .002) and infection (54 vs 85%, P = .012).
Finally, we analyzed survival of patients after VAD implantation dichotomized by HGS adjusted for BW. Survival was lower in patients with HGS <25% of BW at 6 months after VAD implantation (75.0 vs 92.9% at 6 months, log-rank P = .0165) and persisted up to 3 years after VAD placement (Fig. 4). The cause of death at 6 months was multiorgan dysfunction in 6 patients, sepsis with stroke in 1 patient, and device malfunction and subsequent multiorgan dysfunction in 1 patient.
Fig. 4.
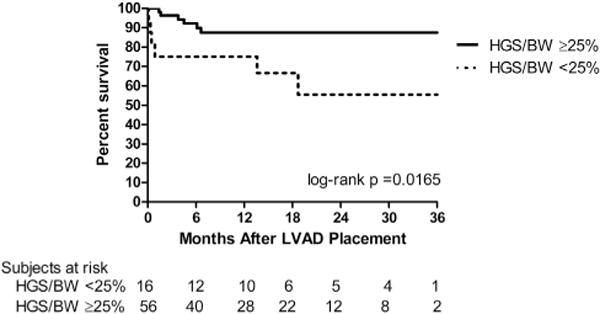
Survival after VAD implantation. Mortality after VAD implantation is higher in those with handgrip strength <25% of total body weight (solid line, handgrip strength >25% body weight, n = 56; dotted line, handgrip strength <25% body weight, n = 16). BW, body weight; HGS, handgrip strength; LVAD, left ventricular assistive device; VAD, ventricular assistive device.
Discussion
Our study demonstrates that patients with advanced HF experience a global myopathy that decreases the physiologic difference in HGS between the dominant and nondominant hands. VAD placement results in a significant improvement in skeletal muscle function reflected by an increase in HGS of the dominant hand by 4 months after surgery. The physiologic difference in HGS between the dominant and nondominant hand returns at 6 months after VAD implantation. Stratification of all patients based on baseline HGS (<25% of BW vs at least 25% of BW) was not associated with differences in baseline clinical characteristics or laboratory values, but was associated with decreased survival after VAD implantation. These data suggest that HGS provides data not available from routine clinical and laboratory evaluation that may provide additional insight for risk stratification during the evaluation of patients with advanced HF for VAD implantation. The cutoff value of HGS <25% of total BW, which corresponded to an absolute strength value of around 15 kg in our cohort, is lower than previously reported values of HGS associated with increased risk of adverse outcomes and implies that our patient population is more compromised than those previously studied.16 This indicates that the definition of optimal cut-points for risk prediction need to be tailored to the population of interest.
Although frailty measures have not yet been formally evaluated in patients undergoing VAD therapy, there has been recent discussion of its application to the patient selection process for destination therapy candidates.20 The frailty phenotype has been established by Fried et al as the presence of 3 or more of the following characteristics: unintentional weight loss of ≥10 pounds or ≥5% of BW in the previous year, grip strength in the lowest quintile, self-reported exhaustion, slowness in walking speed, and low physical activity level.14 The authors found that frailty was predictive of mechanical falls, worsening mobility, hospitalizations, and death in a large cohort of community-dwelling adults 65 years of age and older. Despite variation in the criteria used, frailty is now well-established as a predictor of adverse outcomes and mortality in patients with heart disease and those undergoing cardiovascular surgery.16,17,19 Because implantation of a VAD has the potential to ameliorate cardiac contributions to frailty, Flint et al argue for a distinction between “VAD-responsive” and “VAD-independent” components and the need to establish measures of frailty that reflect the capacity for reversal in its contributing factors.20
Several components of the frailty score such as slowness, self-reported exhaustion, and low physical activity are nearly ubiquitous findings in patients with advanced HF, which suggests that both frailty and HF share common biological mechanisms. For example, unintentional weight loss is a sign of cardiac cachexia and the progressive catabolic state that is part of the advanced HF syndrome, whereas it is also a cardinal feature of the frailty phenotype. Therefore, this measure might largely be determined by underlying comorbidities rather than representing a specific manifestation of frailty. Among the components of the Fried frailty score, HGS has the highest potential to be a specific measure of frailty that can be readily assessed in a physically compromised or even bed-bound patient population. It is, therefore, also applicable to patients with highly compromised hemodynamic state (Interagency Registry for Mechanically Assisted Circulatory Support 1–3) in addition to patients who are not hospitalized but show signs of worsening HF state (Interagency Registry for Mechanically Assisted Circulatory Support 4–7). Measuring HGS requires minimal equipment and time, places low demand on performing staff and is well-tolerated even by wheelchair-and bed-bound patients. Furthermore, our current study demonstrates that HGS improves after VAD implantation, and as a VAD-responsive element of frailty, shows potential to be particularly relevant in the clinical assessment of patients with advanced HF considered for mechanical circulatory support. Of note, in a prior study, when HGS alone was compared with other individual or composite measures of frailty, it was found to be a stronger predictor of 6-month mortality after adjustment for confounders.16 Further, it correlates well with other markers of total body as well as single extremity muscle function and strength.21 HGS, therefore, offers the potential as a single-item measure of skeletal muscle function and frailty and could enhance the characterization of patients with advanced HF to improve patient selection for VAD implantation.
This study has several limitations, largely owing to its single-center, observational design and the relatively small study cohort. We did not have repeat measures of HGS for all patients at each time point after VAD implantation. It is difficult to determine if patients without available follow-up data were systematically different in some way from those who contributed to the follow-up analyses. This study would have been strengthened by the availability of all components of the frailty phenotype that would have enabled comparison of the predictive power of HGS with that of, for example, gait speed or a composite measure of frailty in this cohort. The majority of our patient population, however, had severe physical impairment or was bed-bound before VAD implantation, which would have precluded them from undertaking a walk test. Because VADs are applied to patients at earlier stages of advanced HF, the evaluation of the frailty phenotype warrants systematic study in other cohorts such as younger patients and selected bridge to transplantation or destination therapy populations. Because the cohort studied was small, our findings require validation in a larger, more diverse population of patients undergoing VAD implantation.
In conclusion, HGS is a predictor of mortality in patients undergoing VAD implantation and its incorporation in patient selection criteria might improve outcomes after VAD implantation.
Supplementary Material
Acknowledgments
Disclosures
This work was supported by grants from the National Heart, Lung, and Blood Institute (K23 HL095742-01, P30 HL101272-01, UL1 RR 024156, HL073029) and the Herbert and Florence Irving Scholar Award to Dr. Schulze and from the Doris Duke Charitable Foundation to Columbia University to fund Clinical Research Fellow Christine J. Chung.
Footnotes
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cardfail.2014.02.008.
References
- 1.Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115:836–47. doi: 10.1378/chest.115.3.836. [DOI] [PubMed] [Google Scholar]
- 2.Mancini DM, Coyle E, Coggan A, et al. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989;80:1338–46. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 3.Duscha BD, Kraus WE, Keteyian SJ, et al. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II–III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33:1956–63. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 4.Vescovo G, Volterrani M, Zennaro R, et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart. 2000;84:431–7. doi: 10.1136/heart.84.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–34. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 6.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–8. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis GS, Greenberg BH, Hsu DT, et al. ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant: a report of the ACCF/AHA/ACP Task Force on Clinical Competence and Training. Circulation. 2010;122:644–72. doi: 10.1161/CIR.0b013e3181ecbd97. [DOI] [PubMed] [Google Scholar]
- 9.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 10.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transpl. 2011;30:115–23. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Lietz K. Destination therapy: patient selection and current outcomes. J Card Surg. 2010;25:462–71. doi: 10.1111/j.1540-8191.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transpl. 2010;29:S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 16.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriat Soc. 2006;54:1674–81. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 17.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–21. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 18.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–76. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–8. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 20.Flint KM, Matlock DD, Lindenfeld J, Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail. 2012;5:286–93. doi: 10.1161/CIRCHEARTFAILURE.111.963215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriat Soc. 2003;51:636–41. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120:337–42. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Izawa KP, Watanabe S, Osada N, et al. Handgrip strength as a predictor of prognosis in Japanese patients with congestive heart failure. Eur J Cardiovasc Prev Rehab. 2009;16:21–7. doi: 10.1097/HJR.0b013e32831269a3. [DOI] [PubMed] [Google Scholar]
- 24.Chang YT, Wu HL, Guo HR, et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrology. 2011;26:3588–95. doi: 10.1093/ndt/gfr013. [DOI] [PubMed] [Google Scholar]
- 25.Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand grip strength: age and gender stratified normative data in a population-based study. BMC Res Notes. 2011;4:127. doi: 10.1186/1756-0500-4-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trampisch US, Franke J, Jedamzik N, Hinrichs T, Platen P. Optimal Jamar dynamometer handle position to assess maximal isometric hand grip strength in epidemiological studies. J Hand Surg. 2012;37:2368–73. doi: 10.1016/j.jhsa.2012.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


