Abstract
We wanted to assess the possibility that opioid activity in the central amygdala (CeA) could modulate the feeding inhibition of melanocortin stimulation of the paraventricular hypothalamus (PVN). The melanocortin system is important in both the acute regulation of satiety and feeding behavior and in the integration of long-term appetite signals. Melanotan II (MTII) is a synthetic MC3R and MC4R agonist which reduces food intake when given intracerebroventricularly (ICV) and into the PVN. Tyr-D-Ala-Gly-(me) Phe-Gly-ol (DAMGO), a μ-opioid receptor agonist, increases food intake, while opioid antagonists, like naltrexone (NTX), inhibit food intake after injection into many brain sites involved in appetite regulation, including the CeA. In food-deprived male Sprague-Dawley rats, co-injected intra-PVN MTII partially blocked the orexigenic effect of co-injected intra-CeA DAMGO. Intra-CeA NTX co-injected with intra-PVN MTII reduced food intake significantly more than either alone. NTX administered intra-CeA reduced c-Fos-immunoreactivity (IR) in nucleus accumbens neurons significantly compared to the intra-PVN MTII treated animals, animals co-injected intra-PVN with MTII and intra-CeA with NTX animals, and control animal Intra-PVN MTII induced c-Fos-IR in significantly more PVN neurons than observed in control animals. Intra-CeA NTX co-injected with intra-PVN MTII induced c-Fos-IR significantly in PVN neurons relative to control and intra-CeA NTX animals. Such data support the significance of opioid action within the CeA as a modulator of the feeding regulation action of melanocortins within the PVN, occurring within the context of a larger appetitive network.
Keywords: Opioids, Melanocortins, Food intake, Reward, Brain, Limbic, Amygdala, Hypothalamus
Introduction
Obesity is a heterogeneous disease largely resulting from a variety of maladaptive brain mechanisms within an organism in response to environmental, social, and genetic influences, that leads to an imbalance between energy intake and energy expenditure. Neuropeptides in the brain play a pivotal role in governing appetitive behavior through a complex distributed neuronal network. Within this network, these peptides have regionally specific effects. Much has been done to understand the effects of specific neuropeptides working in a particular part of the brain, but it is also important to understand how respective brain regions work together to exert a given feeding effect, as well as to know how selective neuropeptides may interact to support appetite and to elicit a concerted ingestive effect.
Through specific autonomic and emotional output, the paraventricular nucleus of the hypothalamus (PVN), the central nucleus of the amygdala (CeA), and the nucleus accumbens (Acb) are key forebrain structures involved in the regulation of ingestive behavior (1-9). The PVN is thought to primarily influence energy-based food intake (10, 11), such as when an organism has a relative energy deficit, while the CeA is a limbic structure (12) involved in reward-based food intake and affective ingestive neuromodulatory behavior (4). These nuclei are in constant communication with each other and also with other key appetite related nuclei through highly specific, organized neural projections involving various neuropeptides. Endogenous melanocortin peptides are known to act primarily in the PVN, while opioid systems play a key role in the CeA; these neuropeptides likely provide some of the signaling interaction between the PVN, CeA, and with other nuclei throughout this neural network. The Acb is a major node in the mesolimbic dopamine pathway and is important in mediating the rewarding effects of food, in part, through opioidergic neurons (13, 14).
The melanocortinergic system, which consists of the prohormone proopiomelanocortin (POMC), the POMC-derived peptide alpha-melanocyte stimulating hormone (a-MSH), and the melanocortin-3 and -4 receptors (MC3R and MC4R), has been demonstrated to play an integral role in food intake and obesity (15-20). Peripheral, central, and brain site-specific injections of α-MSH and synthetic melanocortin receptor agonists and antagonists have been shown to exert specific effects on feeding, depending on site of administration and state-dependence of the animal (2, 5, 6, 8, 9, 21, 22). Furthermore, mutations of POMC and the MC4R have been implicated in the onset of obesity in humans and animals (23-25). POMC is synthesized in the arcuate nucleus of the hypothalamus (ARC) and in the nucleus of the solitary tract (NTS), and the resultant peptide α-MSH and synthetic agonists/antagonists of the MC4R, have the most significant feeding effects in projection to the PVN. Both α-MSH and the MC4R agonist MTII have been shown to reduce food intake when injected into the PVN (2, 26).
The opioidergic system, which consists of the POMC-derived endogenous opioid receptor ligand peptides, various opioid receptors, and synthetic opioid receptor ligands, has been shown to be central to the regulation of energy homeostasis, specifically with regard to reward-based energy intake (27-29). Peripheral, central, and brain site-specific injections of opioid receptor agonists and antagonists have been shown to exert specific effects on feeding, depending on site of administration and state-dependence of the animal (3, 4, 7, 30, 31). Regional endogenous opioid tone is thought to govern meal maintenance (32-34) and the ingestion of rewarding or preferred foods (4, 35-37), as well as food ingestion in general (38-40); the behavioral feeding output is brain site-specific (13, 41). Synthetic μ-opioid receptor agonists such as Tyr-D-Ala-Gly-(me) Phe-Gly-ol (DAMGO) have been shown to increase food intake when injected into the CeA (1, 3). Conversely, naltrexone, a synthetic non-specific opioid antagonist, has been shown to reduce food intake when injected into the CeA (1, 3, 4).
Melanocortinergic and opioidergic neurons have been previously shown to have significant interaction in the regulation of food intake, yet most of the studies to date have not utilized site-specific stereotaxic microinjection techniques (42-45). Peripheral naloxone, a non-specific opioid antagonist, or NTX, blocked feeding induced by intra-ICV agouti-related peptide (AgRP), which is an endogenous melanocortin antagonist (44, 45). In addition, intra-ICV (β-End-induced feeding was significantly reduced by pretreatment with intra-ICV MTII (43). In one study (26), intra-PVN α-MSH, but not (β-End, was shown to decrease POMC gene expression in the arcuate nucleus (ARC). However, intra-PVN (β-End has been shown to increase food intake (46) and both α-MSH and (β-End are synthesized in POMC neurons in the ARC. Two studies showed increased α-MSH neuronal activation in the ARC, as indicated by c-Fos-IR expression after the stimulation (42, 45). None of these experiments specifically looked at the role of the opioid tone in the CeA in relation to melanocortinergic action in PVN, within the context of a distributed appetite network. Also, more site-specific microinjections are needed in order to refine our understanding of the relative contribution of specific nuclei to this integrated appetitive network.
Our hypothesis was that opioid activity in the CeA could modulate the feeding inhibition of melanocortin stimulation of the PVN. Therefore, the present study compares the effects of co-injection of intra-CeA naltrexone or intra-CeA DAMGO along with intra-PVN MTII on food intake and on neuronal activation in select brain regions of food-deprived rodents. We present evidence that opioid activity in the CeA can modulate the feeding inhibition of melanocortin stimulation of the PVN.
Materials and Methods
Male Sprague-Dawley rats (Charles Rivers Laboratories, Wilmington), weighing 250-350 g, were housed individually in conventional hanging wire-mesh cages with a 12:12 h light/dark schedule (lights on at 0700 h) in a temperature-controlled room (21-22 °C). Animals had ad libitum access to chow (Rodent Chow, Teklad, Indianapolis, IN), except on study days, when they were deprived of food for 21 h prior to injections; they all had libitum access to water. Food was weighed in the hopper just before injection and again 1, 2, and 4 h later and was subtracted from initial weight and spillage to calculate the amount eaten. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC).
Rats were anesthetized with a mixture of ketamine HCl (100 mg/ml) and xylazine (20 mg/ml) in a 1:1 ratio in a volume of 1 ml/kg body weight injected intraperitoneally (IP) and were fitted with unilateral 26-gauge stainless steel guide cannulae (Plastics One, Austin, TX) in the PVN and in the CeA. Stereotaxic coordinates taken from the rat brain atlas by Paxinos and Watson (1) were as follows: PVN, 1.9 mm posterior to bregma, 0.5 mm lateral to midline, and 7.0 mm below the surface of the skull; CeA, 2.3 mm posterior to bregma, 4.1 mm lateral to midline, and 7.0 mm below the surface of the skull. Dental cement was used to secure the cannulae to two screws inserted into the skull. The injector extended 1 mm beyond the tip of the cannula. Rats were allowed seven to ten days of postoperative recovery before first injection. Injections were administered in a volume of 0.5 μl over a period of 30 s, with a wait of 30 s before removal of the injector. Mock injections with artificial cerebrospinal fluid (aCSF) were carried out first in order to condition the rats for the experimental injections and rats were sorted into body-weight balanced groups prior to studies.
DAMGO (Bachem, Torrance, CA), naltrexone (NTX) (Sigma, St. Louis, MO), and MTII (Phoenix Pharmaceuticals, Belmont, CA) were dissolved in aCSF, and aCSF also served as a vehicle control in all central injection studies. The naltrexone was made up fresh daily and stored in foil-covered microcentrifuge tubes in order to protect it from light, whereas the DAMGO and MTII were made up in advance and frozen until the morning of the studies when they were thawed at room temperature and mixed again using the vortex. All injections were performed in fasted animals during the first half of the light phase, starting with the CeA and then the PVN. Approximately 30 seconds elapsed between CeA and PVN injection.
After completion of experiments, rats were sacrificed, and brains were dissected and stored in a 10% formaldehyde solution for later in order to determine cannula position in the PVN and CeA by histological examination of immunohistochemically processed brain sections (see below). Data from animals with an incorrect cannula placement (>1 mm from targeted site) were discarded.
Experiment 1: Effect of intra-CeA DAMGO 2 nmol co-injection with intra-PVN MT II 50 pmol on feeding in the food-deprived rat
Drug doses were chosen based upon previous experience in our lab (1, 2, 13). Rats unilaterally cannulated in the CeA and PVN were divided into 4 injection groups (n = 8-10 per group) as follows: aCSF and aCSF, aCSF and DAMGO, aCSF and MTII, MTII and DAMGO. Rats were co-injected into the CeA and PVN in a repeated-measures, counterbalanced design in order to eliminate day effects and to allow for direct comparison of the strength of stimulation in each site within the same animal. Statistical analysis was performed using a one-way ANOVA followed by Fisher's protected LSD test. Values were considered significantly different when P < 0.05. Data were expressed as means ± SE.
Experiment 2: Effect of intra-CeA NTX 79 nmol co-injection with intra-PVN MTII 50 pmol on feeding effects in the food-deprived rat
Rats unilaterally cannulated in the CeA and PVN were divided into 4 injection groups (n = 6-10 per group) as follows: aCSF and aCSF, aCSF and NTX, aCSF and MTII, MTII and NTX. Rats were co-injected into the CeA and PVN in a repeated-measures, counterbalanced design in order to eliminate day effects and to allow for direct comparison of the strength of stimulation in each site within the same animal. Statistical analysis was performed using a one-way ANOVA followed by Fisher's protected LSD test. Values were considered significantly different when P < 0.05. Data were expressed as means ± SE.
Experiment 3: Effect of intra-CeA NTX 79 nmol co-injection with intra-PVN MTII 50 pmol on c-Fos immunoreactivity in feeding-related brain areas
Rats unilaterally cannulated in the CeA and PVN were divided into 4 groups (n = 6-9 per group) as follows: aCSF and aCSF, aCSF and NTX, aCSF and MTII, MTII and NTX. 90 minutes later, animals were deeply anesthetized (ketamine, 50 mg/kg and xylazine, 10 mg/kg IP) and perfused through the aorta with 50 ml of saline followed by 500 ml of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed and postfixed overnight in the same fixative at 4 °C. Forty-micrometer thick coronal sections were cut through the brain regions of interest and processed as free-floating sections for standard c-Fos immunostaining. Sections were pretreated for 10 min in 3% H2O2 in 10% methanol (diluted in TBS: pH 7.4-7.6) and incubated for 36 h at 4 °C in the primary goat-anti-Fos antibody (diluted 1:9000; Santa Cruz Biotechnology, CA). Sections were then incubated for 1 h at room temperature in the secondary rabbit-anti goat antibody (Vector Laboratories, Burlingame, CA). Following a 1 h incubation (room temperature) in the avidin-biotin complex, peroxidase in the tissue was visualized with 0.05% diaminobenzidine (DAB), 0.01 H2O2 and 0.3% nickel sulfate (time of reaction 12 min). The vehicle for all incubations in antibodies was a mixture of 0.25% gelatin, 0.5% Triton X-100 in TBS. Intermediate rinsing steps were done in TBS alone. Sections were mounted on gelatin-coated slides, air-dried, dehydrated in alcohols, soaked in Americlear (Baxter Diagnostics), and coverslipped in Entellan (Merck, Switzerland). A region of constant size (mm2) that completely contained the anatomical area of interest was outlined, and the number of c-Fos-immunoreactive (IR) nuclei were counted and expressed as mean number of c-Fos-IR nuclei per region of interest (in this case, PVN and Acb). The CeA sections were not available for analysis due to tissue damage during section processing. Boundaries were defined according to the atlas of Paxinos and Watson (47). Images provided by Dage-MTI DC 3CCD camera attached to a Nikon Eclipse 400 microscope were analyzed using Scion Image software. Statistical analysis was performed using a one-way ANOVA followed by Fisher's protected LSD test. Values were considered significantly different when P < 0.05. Data were expressed as means ± SE. Due to technical difficulties with brain preservation during the first c-Fos study (intra-CeA DAMGO 2 nmol co-injection with intra-PVN MT II 50 pmol), data analysis was not possible.
Results
Experiment 1: Effect of intra-CeA DAMGO 2 nmol co-injection with intra-PVN MT II 50 pmol on feeding in the food-deprived rat (Fig. 1)
Fig. 1.
The effects of co-injection of MTII and DAMGO on mean (± SE) food intake at 1, 2, and 4 hours. *denotes significantly decreased feeding at P < 0.05. **denotes significantly increased feeding at P < 0.05.
As expected, DAMGO produced a significant increase in food intake during the second hour post-injection [F(3,30) = 5.96, P = 0.0373]. MTII produced a significant decrease in food intake at four hours post-injection [F(3,30) = 10.81, P = 0.0006]. The decrease in feeding produced by MTII was significantly blocked by DAMGO at four hours post-injection [F(3,30) = 10.81, P = 0.0149), and feeding after the combination of DAMGO and MTII was also significantly reduced compared to DAMGO alone during the second hour [F(3,30) = 5.96, P = 0.0205) and during the fourth hour post-injection [F(3,30) = 10.81, P = 0.0099).
Experiment 2: Effect of intra-CeA NTX 79 nmol co-injection with intra-PVN MTII 50 pmol on feeding effects in the food-deprived rat (Fig. 2)
Fig. 2.
The effects of co-injection of MTII and NTX on mean (± SE) food intake at 1, 2, and 4 hours. *denotes significantly decreased feeding at P < 0.05. **denotes significant difference at P < 0.05.
MTII produced a significant reduction in food intake compared to controls during the first hour [F(3,30) = 6.32, P = 0.0099), second hour [F(3,30) = 14.08, P <0.0001), and fourth hour post-injection [F(3,28) = 20.34, P <0.0001). NTX produced a significant reduction in food intake compared to controls during the first hour [F(3,30) = 6.32, P = 0.0008)], second hour [F(3,30) = 14.08, P = 0.0001)], and fourth hour post-injection [F(3,28) = 20.34, P = 0.0003)]. Co-injection of NTX and MTII produced a more significant reduction in feeding compared to NTX at the second [F(3,30) = 14.08, P= 0.0436)] and fourth hours post-injection [F(3,28) = 20.34, P = 0.0008)]; however, this effect was not seen during the first hour post-injection. Co-injection of NTX and MTII produced a more significant reduction in feeding compared to MTII at the fourth hour post-injection [F(3,28) = 20.34, P = 0.0230)]; however, this effect was not seen during the first or second hours post-injection. There was no significant difference in food intake between NTX and MTII at any of the time points; they both reduced food intake to a similar degree.
Experiment 3: Effect of intra-CeA NTX 79 nmol co-injection with intra-PVN MTII 50 pmol on c-Fos-IR in feeding-related brain areas
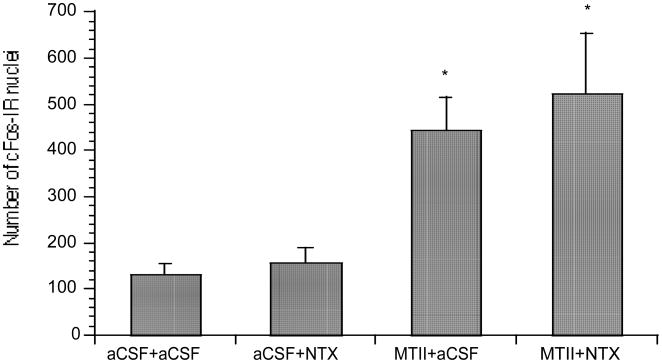
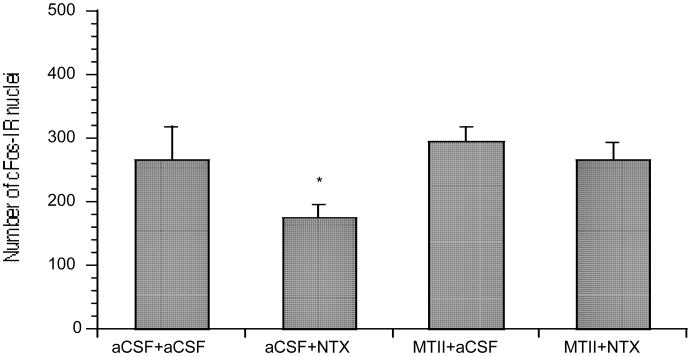
MTII treatment compared to NTX or controls caused a significant increase in the number of c-Fos-IR nuclei in the PVN [F(3,23) = 5.62, P = 0.0216 and P = 0.0131)] (Fig. 3). Co-injection of NTX and MTII compared to NTX or controls caused a significant increase in the number of c-Fos-IR nuclei in the PVN [F(3,23) = 5.62, P = 0.0056 and P = 0.0034)[. NTX treatment, compared to MTII, co-injection of NTX and MTII, and compared to controls, caused a significant decrease [F(3,25) = 3.24, P = 0.0058, P = 0.0425, and P = 0.0489)] in the number of c-Fos-IR nuclei in the Acb, but not in the PVN (Fig. 4).
Fig. 3.
Mean (± SE) number of c-Fos-immunoreactive (c-Fos-IR) nuclei in the PVN after co-injection of intra-PVN MTII 50 pmol and intra-CeA NTX 79 nmol (* P < 0.05).
Fig. 4.
Mean (± SE) number of c-Fos-immunoreactive (c-Fos-IR) nuclei in the Acb after co-injection of intra-PVN MTII 50 pmol and intra-CeA NTX 79 nmol (* P < 0.05).
Discussion
Since the PVN and the CeA have distinct functions in the appetitive network, one of the main goals of this study was to test the participation of increased or decreased opioid tone within the CeA in modulating MTII's feeding effects within the PVN, in order to further differentiate these nuclei. Our results showed that both increasing and blocking opioid activity within the CeA modulates the feeding inhibition produced by melanocortin stimulation of the PVN. In previous studies, intra-PVN MTII produces anorexia in fasted and non-fasted (at start of dark cycle) rats and mice (2, 9, 48, 49). The results of this study indicate that intra-PVN MTII-induced anorexia could be partially reversed by intra-CeA DAMGO. We have previously shown that intra-CeA DAMGO produces a hyperphagic feeding response in both fasted and non-fasted rats, while intra-CeA NTX produces anorexia (1, 3, 4, 50, 51). In this study, we found that intra-CeA DAMGO-induced feeding could be partially blocked by intra-PVN MTII. Thus the two types of stimulation are integrated within their respective brain sites and act within the context of the larger appetitive network, in order to produce orexigenic or anorexigenic effects. The combination of two feeding suppressive influences, intra-CeA NTX and intra-PVN MTII, produced a statistically significant greater anorexic response than either given alone, at the 0-4 hr time point, suggesting brain integration of both energy signals. The exact location(s) of this type of energy integration cannot be elucidated without extensive c-Fos-IR study of multiple brain regions of interest. Ideally, one would want to see regionally-specific, statistically significant changes in c-Fos-IR after co-injection of sub-threshold doses.
The role of limbic structures such as the CeA in the hedonics of affective appetitive behavior has been appreciated (12, 52), as has the role of opioids (28, 29). These results accentuate the known feeding phenomena that occur within the context of increased or decreased opioid activity within the CeA because they also include the contribution of elevated melanocortinergic tone within a connected nucleus, the PVN. Historically, the PVN has been regarded as a node in the appetitive network believed to contribute more to energy driven feeding than to hedonic or emotive appetitive mechanisms (10, 11). These studies provide further evidence that anorexic feeding behavior due to increased melanocortin tone within the PVN can be modulated by opioid tone within the CeA.
Our result of decreased c-Fos-IR in the Acb after intra-CeA NTX in fasted rats is consistent with the notion that increased opioid tone is, in part, responsible for feeding behavior within these regions (50, 51, 53-55). In looking at our data (Fig. 4), one can appreciate the increased c-Fos-IR in all groups, except for the NTX group, which suggests that there is increased opiate tone throughout the Acb, as a result of food-deprivation. The NTX appears to have inhibited this increased endogenous opiate tone as evidenced by significantly decreased c-Fos-IR in the Acb, relative to all of the other groups. This hypothesis is supported by available literature. For example, increased c-Fos-IR in hypothalamic and forebrain areas has been shown in food-restricted rats regardless of injection treatment and this has been attributed to the presence of increased opioid tone (56). It is interesting to think that NTX can inhibit some c-Fos-IR in the Acb caused by energy restriction; this suggests that homeostatic information is uniquely integrated within discrete brain regions, like the Acb, that were previously thought to be more involved in the hedonism of eating, rather than linked so directly, to energy balance. It appears that opioidergic neurons within reward-based nodes, like the Acb, link energy-balance information to limbic regions, such as the CeA. Our result of intra-CeA NTX causing a reduction in c-Fos-IR in the Acb supports the notion that there is increased endogenous opioidergic neuronal communication between the CeA and Acb occurring during food-deprivation that can be inhibited by intra-CeA NTX. After all, there is known bi-directional μ-opioidergic communication between the CeA and Acb (50). It is also known that μ-opioid tone within the Acb is important for hedonically-driven feeding (55). Opioidergic neuronal contributions, within these nodes, appear to be important in free-feeding animals. Peripheral NTX prevented increases in c-Fos-IR in the Acb, CeA, and other regions induced by a meal, even when it did not block consumption of the meal (54). Peripheral NTX was found to induce c-Fos-IR within the CeA and Acb in free-feeding animals (53). DAMGO administered intra-CeA increased c-Fos-IR in the Acb in free-feeding animals, but not in any of the other brain regions studied (51).
In our study, intra-PVN MTII treatment caused a significant increase in c-Fos-IR in the PVN and co-injection of intra-CeA NTX and intra-PVN MTII produced a similar increase in c-Fos-IR in the PVN. Expression of c-Fos-IR in the PVN is expected because of the high concentration of melanocortin receptive neurons there (57) and this finding would be consistent with stimulation by MTII, as intra-ICV MTII has increased c-Fos-IR in the PVN and other brain regions (58, 59). This neuronal activation pattern is different from the suppression of feeding which we observed in this study, in that the anorexic effect by the intra-PVN MTII was seen, and this effect became significantly more anorexic with co-injection of intra-PVN MTII and intra-CeA NTX. These results indicate that the c-Fos-IR expression level in the PVN may not fully accord with the behavioral phenotype of eating, consistent with the idea that appetitive behavior is determined by the action of more than one node in the network. Our results indicate that the c-Fos-IR expression level in PVN is only part of the story. In fact, the interaction between opioidergic and melanocortinergic neurons probably occurs within multiple nodes within the larger feeding network. For instance, this interaction may be occurring within the CeA or within other higher integrating subcortical or cortical regions.
In summary, opioid activity in the CeA can modulate the feeding inhibition of melanocortin stimulation of the PVN, and there is significant opioidergic neuronal communication occurring between the CeA and Acb in fasted animals. These brain regions participate in a complex, highly integrated appetite network in order to exert their feeding effects. These findings are relevant to human obesity, in that appetitive behavior is, in part, driven by hedonics, emotion, and energy-deficits as sensed by the Acb, CeA, PVN, and other discrete brain regions. Future additional feeding studies might focus on the use of sub-threshold doses of peptides in order to further strengthen the understanding of this potential brain system interaction and energy signal integration. Then, in order to further elucidate the melanocortinergic-opioidergic neuronal architecture between these respective nuclei, more research could focus on brain-mapping by examining c-Fos-IR in the Acb, CeA, PVN, and in multiple other brain regions, after co-injection of sub-threshold doses of intra-PVN MTII and intra-CeA NTX or intra-CeA DAMGO. A comprehensive understanding of this process is useful for uncovering potential mechanisms and solutions for maladaptive eating patterns.
Acknowledgments
This research was supported by the Department of Veterans Affairs, National Institute of Drug Abuse Grant DA-03999, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-50456 and T32-DK007203. We thank Martha Grace for her unrelenting technical assistance.
References
- 1.Giraudo SQ, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat. Brain Research. 1998;782:18–23. doi: 10.1016/s0006-8993(97)01140-2. [DOI] [PubMed] [Google Scholar]
- 2.Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Research. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- 3.Giraudo SQ, Kotz CM, Billington CJ, Levine AS. Association between the amygdala and nucleus of the solitary tract in μ-opioid induced feeding in the rat. Brain Research. 1998;802:184–188. doi: 10.1016/s0006-8993(98)00602-7. [DOI] [PubMed] [Google Scholar]
- 4.Glass MJ, Billington CJ, Levine AS. Naltrexone administered to central nucleus of amygala or PVN: neural dissociation of diet and energy. Am J Physiol Regul Integ Comp Physiol. 2000;279:R86–R92. doi: 10.1152/ajpregu.2000.279.1.R86. [DOI] [PubMed] [Google Scholar]
- 5.Hansen MJ, Morris MJ. Evidence for an interaction between neuropeptide Y and the melanocortin-4 receptor on feeding in the rat. Neuropharmacology. 2002;42:792–797. doi: 10.1016/s0028-3908(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 6.Hansen MJ, Schioth HB, Morris MJ. Feeding response to a melanocortin agonist and antagonist in obesity induced by a palatable high-fat diet. Brain Research. 2005;1039:137–145. doi: 10.1016/j.brainres.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 7.Pomonis JD, Levine AS, Billington CJ. Interaction of the hypothalamic paraventricular nucleus and central nucleus of the amygdala in naloxone blockade of neuropeptide Y-induced feeding revealed by c-fos Expression. The Journal of Neuroscience. 1997;17(13):5175–5182. doi: 10.1523/JNEUROSCI.17-13-05175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raposinho PD, White RB, Aubert ML. The melanocortin agonist Melanotan-II reduces the orexigenic and adipogenic effects of neuropeptide Y but does not affect NPY-driven suppressive effects on the gonadotropic and somatotropic axes in the male rat. Journal of Neuroendocrinology. 2003;15:173–181. doi: 10.1046/j.1365-2826.2003.00962.x. [DOI] [PubMed] [Google Scholar]
- 9.Wirth MM, Olszewski PK, Yu C, Levine AS, Giraudo SQ. Paraventricular hypothalamic α-melanocyte-stimulating hormone and MTII reduce feeding without causing aversive effects. Peptides. 2001;22:129–134. doi: 10.1016/s0196-9781(00)00367-3. [DOI] [PubMed] [Google Scholar]
- 10.Billington CJ, Briggs JE, Harker S, Grace MK, Levine AS. Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am J Physiol Regul Integ Comp Physiol. 1994;266:R1765–R1770. doi: 10.1152/ajpregu.1994.266.6.R1765. [DOI] [PubMed] [Google Scholar]
- 11.Levine AS, Billington CJ. Regulation of Body Weight: Biological and Behavioral Mechanisms. New York: Wiley; 1996. Peptides in regulation of energy metabolism and body weight. [Google Scholar]
- 12.Aggleton JP, Mishkin M. Emotion: Theory, Research, Experience. London: Academic; 1986. The amygdala: sensory gateway to the emotions. [Google Scholar]
- 13.MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integ Comp Physiol. 2003;285:999–1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Research. 2004;1018:78–85. doi: 10.1016/j.brainres.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Benoit S, Schwartz M, Baskin D, Woods S, Seeley R. CNS melanocortin system involvement in the regulation of food intake. Hormones and Behavior. 2000;37:299–305. doi: 10.1006/hbeh.2000.1588. [DOI] [PubMed] [Google Scholar]
- 16.Pritchard LE, Turnbull AV, White A. Pro-opiomelancortin processing in the hypothalamus: impact on melanocortin signalling and obesity. Journal of Endocrinology. 2005;172:411–421. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- 17.Rafflin-Sanson ML, Keyzer Yd, Bertagna X. Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. European Journal of Endocrinology. 2003;149:79–90. doi: 10.1530/eje.0.1490079. [DOI] [PubMed] [Google Scholar]
- 18.Ramos EJB, Meguid MM, Campos ACL, Coelho JCU. Neuropeptide Y, α-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition. 2005;21:269–279. doi: 10.1016/j.nut.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Wardlaw SL. Obesity as a neuroendocrine disease: lessons to be learned from proopiomelancortin and melanocortin receptor mutations in mice and men. The Journal of Clinical Endocrinology & Metabolism. 2001;86(4):1442–1446. doi: 10.1210/jcem.86.4.7388. [DOI] [PubMed] [Google Scholar]
- 20.Yang YK, Harmon CM. Recent developments in our understanding of melanocortin system in the regulation of food intake. Obesity Reviews. 2003;4:239–248. doi: 10.1046/j.1467-789x.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 21.Benoit SC, Clegg DJ, Barrera JG, Seeley RJ, Woods SC. Learned meal initiation attenuates the anorexic effects of the melanocortin agonist MTII. Diabetes. 2003;52:2684–2688. doi: 10.2337/diabetes.52.11.2684. [DOI] [PubMed] [Google Scholar]
- 22.McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular α–MSH on food intake, adiposity, and c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integ Comp Physiol. 2000;279:R695–R703. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- 23.Biebermann H, Krude H, Elsner A, Chubanov V, Gudermann T, Gruters A. Autosomal dominant mode of inheritance of a melancortin-4 receptor mutation in a patient with severe early onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes. 2003;52:2984–2988. doi: 10.2337/diabetes.52.12.2984. [DOI] [PubMed] [Google Scholar]
- 24.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melancortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 25.Krude H, Beibermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nature Genetics. 1998:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 26.Kim EM, Grace MK, O'Hare E, Billington CJ, Levine AS. Injection of α-MSH, but not B-endorphin, into the PVN decreases POMC gene expression in the ARC. NeuroReport. 2002;13:497–500. doi: 10.1097/00001756-200203250-00028. [DOI] [PubMed] [Google Scholar]
- 27.Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: The molecular face of hedonism? Brain Research Reviews. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Levine AS. The animal model in food intake regulation: Examples from the opioid literature. Physiology & Behavior. 2006;89:92–96. doi: 10.1016/j.physbeh.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Levine AS, Billington CJ. Opioids as agents of reward-related feeding: a consideration of the evidence. Physiology & Behavior. 2004;82:57–61. doi: 10.1016/j.physbeh.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Yeomans MR, Gray RW. Selective Effects of Naltrexone on Food Pleasantness and Intake. Physiology & Behavior. 1995;60(2):439–446. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 31.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behavior. Neuroscience and Biochemical Reviews. 2002;26:713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 32.Cooper SJ, Turkish S. Effects of naltrexone on food preference and concurrent behavioral responses in food-deprived rats. Pharmacology Biochemistry & Behavior. 1989;33:17–20. doi: 10.1016/0091-3057(89)90422-x. [DOI] [PubMed] [Google Scholar]
- 33.Glass MJ, Grace MK, Cleary JP, Billington CJ, Levine AS. Naloxone's effect on meal microstructure of sucrose and cornstarch diets. Am J Physiol Regul Integ Comp Physiol. 2001;281:R1605–R1612. doi: 10.1152/ajpregu.2001.281.5.R1605. [DOI] [PubMed] [Google Scholar]
- 34.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: When reward outweighs homeostasis. Physiology & Behavior. 2007;91(5):506–512. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12(16):3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 36.Glass MJ, Grace MK, Cleary JP, Billington CJ, Levine AS. Potency of naloxone's anorectic effect in rats is dependent on diet preference. Am J Physiol Regul Integ Comp Physiol. 1996;271(1 pt 2):R217–R221. doi: 10.1152/ajpregu.1996.271.1.R217. [DOI] [PubMed] [Google Scholar]
- 37.Levine AS, Weldon DT, Grace MK, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol Regul Integ Comp Physiol. 1994:R248–R252. doi: 10.1152/ajpregu.1995.268.1.R248. [DOI] [PubMed] [Google Scholar]
- 38.Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, μ and κ opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Research. 1995;700:205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- 39.Kotz CM, GLass MJ, Levine AS, Billington CJ. Regional effect of naltrexone in the nucleus of the solitary tract in blockade of NPY-induced feeding. Am J Physiol Regul Integ Comp Physiol. 2000;278:R499–R503. doi: 10.1152/ajpregu.2000.278.2.R499. [DOI] [PubMed] [Google Scholar]
- 40.Kotz CM, Grace MK, Briggs J, Levine AS, Billington CJ. Effects of opioid antagonists naloxone and naltrexone on neuropeptide Y-induced feeding and brown fat thermogenesis in the rat. Journal of Clinical Investigation. 1995;96:163–170. doi: 10.1172/JCI118017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn JG, O'Hare E, Levine AS, Kim EM. Evidence for a μ-opioid-opioid connection between the paraventricular nucleus and ventral tegmental area in the rat. Brain Research. 2003;991:206–211. doi: 10.1016/j.brainres.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Bomberg EM, Grace MK, Levine AS, Olszewski PK. Functional interaction between nociceptin/orphanin FQ and α-melanocyte-stimulating hormone in the regulation of feeding. Peptides. 2006;27:1827–1834. doi: 10.1016/j.peptides.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Grossman HC, Hadjimarkou MM, Silva RM, Giraudo SQ, Bodnar RJ. Interrelationships between m opioid and melanocortin receptors in mediating food intake in rats. Brain Research. 2003;991:240–244. doi: 10.1016/s0006-8993(03)03442-5. [DOI] [PubMed] [Google Scholar]
- 44.Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP- (83-132) on food intake and food selection. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2001;280(3):R814–21. doi: 10.1152/ajpregu.2001.280.3.R814. [DOI] [PubMed] [Google Scholar]
- 45.Olszewski PK, Wirth MM, Grace MK, Levine AS, Giraudo SQ. Evidence of interactions between melanocortin and opioid systems in regulation of feeding. NeuroReport. 2001;12(8):1727–1730. doi: 10.1097/00001756-200106130-00042. [DOI] [PubMed] [Google Scholar]
- 46.Liebowitz SF, Hor L. Endorphinergic and α-noradrenergic systems in the paraventricular nucleus: Effects on eating behavior. Peptides. 1982;3:421–428. doi: 10.1016/0196-9781(82)90102-4. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, W C. The Rat Brain in Stereotaxic Coordinates. 3rd. San Diego: Academic Press; 1997. [Google Scholar]
- 48.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 49.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385 doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 50.Kim EM, Quinn JG, Levine AS, O'Hare E. A bi-directional μ–opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Research. 2004;1029:135–139. doi: 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Levine AS, Olszewski PK, Mullett MA, Pomonis JD, Grace MK, Kotz CM, et al. Intra-amygdalar injection of DAMGO: effects on c-Fos levels in brain sites associated with feeding behavior. Brain Research. 2004;1015:9–14. doi: 10.1016/j.brainres.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 52.Rogan MT, LeDoux JE. Emotion: systems, cells, synaptic plasticity. Cell. 1996;85:469–475. doi: 10.1016/s0092-8674(00)81247-7. [DOI] [PubMed] [Google Scholar]
- 53.Carr KD, Kutchukhidze N, Park TH. Differential effects of μ and κ opioid antagonists on Fos-like immunoreactivity in extended amygala. Brain Research. 1999;822:34–42. doi: 10.1016/s0006-8993(99)01088-4. [DOI] [PubMed] [Google Scholar]
- 54.Park TH, Carr KD. Neuranatomical patterns of Fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline-and naltrexone-treated rats. Brain Research. 1998;805:169–180. doi: 10.1016/s0006-8993(98)00719-7. [DOI] [PubMed] [Google Scholar]
- 55.Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ. Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. European Journal of Neuroscience. 2006;23:1605–1613. doi: 10.1111/j.1460-9568.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- 56.Carr KD, Park TH, Zhang Y, Stone EA. Neuroanatomical patterns of Fos-like immunoreactivity induced by naltrexone in food-restricted and ad libitum fed rats. Brain Research. 1998;779:26–32. doi: 10.1016/s0006-8993(97)01074-3. [DOI] [PubMed] [Google Scholar]
- 57.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 58.Thiele TE, Van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, et al. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol Regul Integ Comp Physiol. 1998:R248–R254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- 59.Trivedi P, Jiang M, Tamvakopoulos CC, Shen X, Yu H, Mock S, et al. Exploring the site of anorectic action of peripherally administered synthetic melanocortin peptide MT-II in rats. Brain Research. 2003;977:221–230. doi: 10.1016/s0006-8993(03)02683-0. [DOI] [PubMed] [Google Scholar]