Abstract
Background
Roux-en-Y gastric bypass (RYGB) imparts long-term weight loss, the mechanisms for which are not well understood. Changes in leptin and gastrointestinal (GI) hormones, including glucagon-like peptide 1 (GLP-1), peptide YY (PYY), and ghrelin, may contribute to the relative success of RYGB compared with conventional weight loss methods. This study evaluated changes in GI hormones and leptin post-RYGB. The study also evaluated whether GI hormones differed after a short-term dose of protein or fat.
Methods
GLP-1, PYY, ghrelin, and leptin were assessed in 16 women before RYGB and up to 1 year after RYGB. Plasma was collected before and at several times after a short-term equicaloric dose of protein or fat.
Results
GLP-1 area under the curve (AUC) increased at week 6 and 1 year in the fat beverage (FAT-BEV) group compared with baseline. PYY AUC remained elevated at 1 year in the FAT-BEV group. Ghrelin AUC decreased at week 2, week 6, and 1 year in the protein beverage (PRO-BEV) group compared with baseline. Ghrelin AUC was lower in the PRO-BEV group compared with the FAT-BEV group at week 6. Fasted leptin decreased at all visits in both groups and was lower in the FAT-BEV group compared with the PRO-BEV group at 1 year.
Conclusions
Changes from baseline were evident for all GI hormones and leptin; some differences were evident soon after surgery (ghrelin, leptin), whereas others were maintained long term (GLP-1, PYY, ghrelin, leptin). In response to a short-term stimulus, protein suppressed ghrelin and fat potently stimulated GLP-1 and PYY. Future work in this area is warranted.
Keywords: obesity, ghrelin, peptide YY, glucagon-like peptide 1, leptin, gastric bypass, gastrointestinal hormones
Introduction
Bariatric surgery is the most effective treatment for morbid obesity in terms of achieving major, long-term weight loss.01 Consequently, the prevalence of such surgery is increasing worldwide. Roux-en-Y gastric bypass (RYGB) is the most common bariatric procedure performed, comprising about 70% to 75% of all bariatric procedures.2 The RYGB procedure imparts substantial long-term weight loss; however, the mechanisms by which this occurs are not well understood. Restriction and bypass components characterize this procedure.3 The restrictive component is achieved by creating a gastric pouch (~20–30 mL), which promotes early satiety4,5 and thereby decreases dietary intake,5 which is considered to be the primary reason for weight loss after RYGB.6 In addition, the distal stomach, duodenum, and proximal jejunum are bypassed.4 Malabsorption of energy-yielding macronutrients is not common after RYGB, but in some cases malabsorption of micronutrients (eg, iron, vitamin B12) does occur.7,8 In addition to these physiological modifications, changes in leptin and several gastrointestinal (GI) hormones, including glucagon-like peptide 1 (GLP-1), peptide YY (PYY), and ghrelin, may explain the relative success of the RYGB procedure.9,10
GLP-1 and PYY are considered appetite-regulating hormones, given that secretion of each reduces hunger and imparts satiety.11 They are secreted from the L cells of the distal ileum and colon in response to enteral nutrient intake.12–18 The mechanism by which these hormones promote satiety is thought to be multifaceted because they slow gastric emptying,16,19,20 promote insulin release,16,21 and inhibit gastric acid secretion.19,20 Because of these effects, they are considered to play an important role in the “ileal brake” mechanism,22 which regulates the passage of nutrients through the GI tract.23 GLP-1 and PYY are secreted within 15 to 30 minutes after food intake.24–26 More specifically, data suggest that GLP-1 is stimulated by fat intake in rats27 and humans,28,29 and other data support that it is stimulated by protein intake more than carbohydrate intake.30,31 Similarly, fat32–34 and protein32,35,36 have been found to be important regulators of PYY secretion in obese32,35,36 and normal-weight individuals.33,34,36 Some data support that fasting and postprandial GLP-1 and PYY secretion are higher in normal-weight individuals compared with those who are obese or overweight.37–40 Higher GLP-1 and PYY levels have been reported in post-RYGB patients compared with pre-RYGB,9,11,41–44 normal-weight,11,44–47 over-weight,46,48,48–50 and obese individuals11,44,45,51 and those who undergo other weight loss surgeries.11,46,48,48,50
Ghrelin is the only GI hormone known to stimulate appetite, and therefore, it is considered to be orexi-genic.13–15,17 It is released both centrally (hypothalamus) and peripherally (stomach), and its antisatiating properties may be due to its biological effects to increase GI motility and decrease insulin secretion.13,52 Ghrelin levels increase in the absence of enteral intake and decrease right after meal initiation,53 and the macronutrient that induces the greatest suppression is not well defined. For instance, some research suggests that carbohydrates are suppres-sive,54,55 whereas others have found protein to be more potent in terms of prolonged suppression.30,31,55–58 Erdmann et al54 also found that fat was a potent suppressor, especially in the late postprandial state (ie, 60–180 minutes), with similar findings by Tannous dit El Khoury et al.55 In addition, increased ghrelin levels have been reported in individuals undergoing diet-induced weight loss, which might explain the difficulty in maintaining weight loss achieved with conventional methods.59 However, reduced ghrelin has been reported after RYGB weight loss compared with pre-RYGB,60–63 normal-weight,11,45,59,61,63–65 overweight,46,48 and obese 45,59,61,65,66 people and/or those who undergo other restrictive bariatric surgical procedures.46,48,65 However, not all research has found this to be the case7,9,41,44,66–73; therefore, the role of ghrelin in post-RYGB weight loss remains unclear.
The adipocytokine leptin is a product of the obesity gene (ob gene) and is understood to be involved in long-term energy balance.74 It is secreted mainly by adipocytes and, to a lesser degree, gastric chief cells75 and influences energy intake, primarily by acting on the hypothalamus17,74,76 to decrease food intake and increase energy expenditure.76 Plasma leptin concentrations are not known to be regulated by macronutrients but rather are regulated by adipose tissue mass. Leptin circulates in proportion to whole-body adipose tissue mass,74 and strong evidence exists that leptin decreases with a reduction in adipose tissue.77–80 As would be expected, decreased leptin has been reported in post-RYGB patients compared with pre-RYGB,9,63,73,78,81–91 normal-weight,92 overweight,46 and obese49,70,93 patients.
The objective of this study was to evaluate the changes in GLP-1, PYY, ghrelin, and leptin concentrations post-RYGB. In addition, we sought to determine whether there was a difference in GI hormone levels after a short-term dose of either protein or fat.
Methods
Women with class III obesity (ie, body mass index [BMI] ≥40 kg/m2) who planned to undergo the laparoscopic RYGB procedure were recruited from the Weight Loss Surgery Center at the University of Minnesota Medical Center–Fairview. Participants for this longitudinal analysis were also enrolled in a broader longitudinal study investigating body composition and metabolic changes after RYGB surgery. This study included 5 visits that were 24 hours in duration: 30 to 70 days before RYGB (baseline); 2 weeks post-RYGB (week 2); 6 weeks post-RYGB (week 6); 6 months post-RYGB (month 6); and 1 year post-RYGB (1 year). The timing of the study visits was established in order to evaluate the clinical outcomes in both the early (ie, weeks 2 and 6) and late (ie, month 6 and 1 year) postoperative stages. GI hormones and leptin were analyzed in 16 participants. Exclusion criteria included the following: use of corticosteroids, testosterone, or anabolic agents; internally placed biomedical device (eg, pacemaker); liver, renal, or heart failure; pulmonary hypertension; thyroid disease (included if treated and within normal limits); neoplastic disease; type 1 or uncontrolled type 2 diabetes mellitus (defined by hemoglobin A1c >7%); pregnancy; or previous weight loss surgery. The study protocol was reviewed and approved by the Institutional Review Board and the General Clinical Research Center (GCRC) at the University of Minnesota, and participants provided written informed consent before enrollment in the study.
The day before admission to the GCRC, participants were instructed to avoid caffeine, alcohol, and vigorous exercise for 24 hours before testing. Participants were also asked to be in the fasted state for at least 2 hours before GCRC admission. Participants were randomized to 1 of 2 groups: (1) PRO-BEV group: those receiving a sugar-free, high-protein beverage (20 g protein, 0 g fat, 2 g carbohydrate, 90 kcal, 8 oz) (protein: whey protein isolate, supplied by Davisco Foods International, Eden Prairie, MN), and (2) FAT-BEV group: those receiving a sugar-free , high-fat beverage (0 g protein, 9 g fat, 3 g carbohydrate, 90 kcal, 8 oz). At all visits, a baseline 4-hour fasted plasma sample was collected for the GI hormone and leptin analyses, and 15 minutes later, participants consumed the assigned beverage over a period of not more or less than 30 minutes (60 minutes at week 2 to accommodate the reduced stomach capacity). Blood draws were taken at specific time points after initiation of beverage consumption (time 0). All plasma samples were stored at −70°C before analysis of GLP-1, PYY, ghrelin, and leptin concentrations. Tables 1 and 2 depict when the hormones were sampled at each visit.
Table 1.
GI Hormone and Leptin Analysis Time Points for Baseline, Week 6, Month 6, and 1 yeara After Roux-en-Y Gastric Bypass Surgery.
| Time | |||
|---|---|---|---|
| 0 Min | +30 Min | +60 Min | +90 Min |
| Leptin | |||
| GLP-1 | GLP-1 | GLP-1 | |
| PYY | PYY | PYY | |
| Ghrelin | Ghrelin | Ghrelin | |
GI, gastrointestinal; GLP-1, glucagon-like peptide 1; PYY, peptide YY.
Analyses were conducted at baseline (ie, 30 to 70 days before Roux-en-Y gastric bypass surgery [RYGB]) and at 6 weeks, 6 months, and 1 year following RYGB.
Table 2.
GI Hormone and Leptin Analysis Time Points for Week 2a
| Time | |||
|---|---|---|---|
| 0 Min | +60 Min | +90 Min | +120 Min |
| Leptin | |||
| GLP-1 | GLP-1 | GLP-1 | |
| PYY | PYY | PYY | |
| Ghrelin | Ghrelin | Ghrelin | |
GI, gastrointestinal; GLP-1, glucagon-like peptide 1; PYY, peptide YY.
Analyses were conducted 2 weeks following Roux-en-Y gastric bypass surgery.
The longitudinal study from which these data were analyzed was originally aimed to investigate clinical outcomes, including body composition, energy metabolism, and changes in nutrition status after RYGB. One of the components of the longitudinal study was a double-blinded, randomized, 6-week supplementation period immediately after surgery to determine whether patients consuming more protein in the early postoperative period would lose less lean tissue. The participants consumed the randomly assigned beverage at each testing visit as described above and were also instructed to consume 2 of the assigned beverages daily throughout the initial 6-week postoperative period. For the purposes of this analysis, only the short-term response data from the beverage consumed at the testing visits are discussed.
Dietary Intake Assessment
Information regarding dietary intake was obtained through the use of diet records. During the week before each testing visit, participants recorded their food intake on 2 assigned weekdays and 1 weekend day. During the 6-week supplementation period (visits at weeks 2 and 6), participants were instructed to record study beverage intake. Recording errors were minimized by providing the participants with detailed instructions on how to record their intake, including estimation of portion size. Diet records were reviewed with the participants at each testing visit and were analyzed later for nutrient content using the Food Processor SQL software (version 10.4; ESHA Research, Salem, OR). To obtain an estimate of the participants’ intake during the week before the visit, an average of the recorded macronutrients and calories was calculated.
Gastrointestinal Hormone and Leptin Assays
All samples were assayed in duplicate. PYY-like immunoreactivity was measured with a specific and sensitive radioimmunoassay that measured both the full length (PYY1–36) and the fragment (PYY3–36).38,94 Plasma active GLP-1 and total ghrelin were measured by established in-house radioimmunoassay.95–97 Plasma leptin was measured with a Linco Research (Weldon Spring, MO) assay kit.
Anthropometric Measurements
Height and weight were measured using standardized procedures. Height was measured to the nearest 0.1 cm by a wall-mounted stadiometer (model S100; Ayrton Corporation, Prior Lake, MN) at baseline only. At all testing visits, weight was measured to the nearest 0.1 kg on a digital scale (model 5002; Scale-Tronix, White Plains, NY). BMI was calculated as the patient’s weight in kilograms divided by her height in meters squared (kg/m2).
Surgical Technique
All RYGB procedures were performed laparoscopically. A small gastric pouch (approximately 15 mL) was created, and the duodenum and proximal portion of the jejunum were bypassed to create a Roux limb. In most cases, the length of the Roux limb was 150 cm, according to Weight Loss Surgery Center at the University of Minnesota Medical Center–Fairview protocol.
Statistical Analysis
Study end points were defined as change from the baseline visit in body weight, fasted GI hormone levels, and fasted leptin and change from baseline in area under the curve (AUC) for GLP-1, PYY, and ghrelin. AUC for the week 2 visit was rescaled to the same length of time as the other visits for comparability. Supplement groups were compared in mixed-effects linear models with a random effect to model the correlation of repeated measurements within each participant. SAS 9.2 software (SAS Institute, Cary, NC) was used for statistical analyses.
Results
Twenty-nine women participated in the study. GI hormone and leptin levels were analyzed in only the 16 women who completed all 5 study visits and who had a sufficient amount of plasma for analyses. All participants were Caucasian, with a mean ± standard deviation age of 49 ± 9 years. At baseline, there were no statistically significant differences between the groups in terms of age, weight, height, or BMI (Table 3). In both groups, BMI and absolute body weight decreased significantly at month 6 and 1 year compared with baseline. At month 6, weight decreased by a mean 34 kg (27% weight loss), and at 1 year, weight decreased by a mean 43 kg (34% weight loss).
Table 3.
Baseline Characteristics of Study Participantsa
| Characteristic | Protein Beverage Group (n = 8) |
Fat Beverage Group (n = 8 |
|---|---|---|
| Age, y | 51 ± 7 | 47 ± 11 |
| Weight, kg | 124 ± 13 | 128 ± 15 |
| Height, cm | 166 ± 5 | 166 ± 4 |
| Body mass index, kg/m2 | 45 ± 5 | 47 ± 6 |
Data are given as mean ± standard deviation. There were no statistically significant differences between treatments.
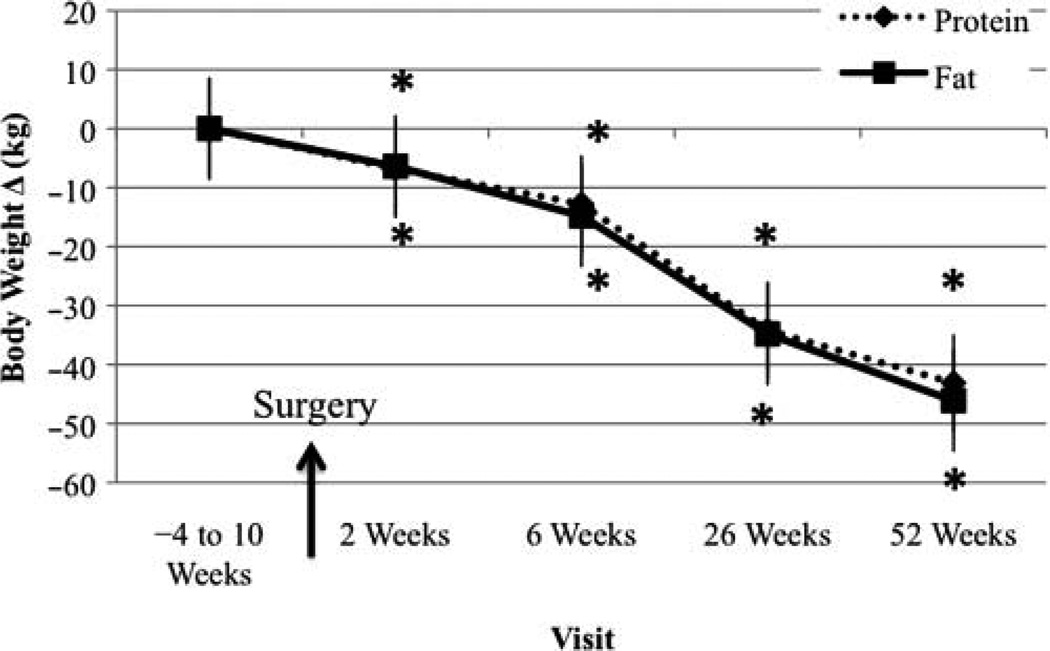
Body Weight Change From Baseline Visit
Change in body weight for all 29 participants is reported in Figure 1. Weight loss was significant at all visits in both treatment groups, with no differences between treatment groups.
Figure 1.
Body weight change from baseline visit. *Significantly different from baseline, P < .05.
Dietary Intake Assessment
At the baseline visit, the FAT-BEV group was consuming significantly more calories than the PRO-BEV group (2,305 ± 456 kcal vs 1,696 ± 457 kcal, respectively; P = .02). For the remainder of the study, no differences in caloric intake were evident between the 2 treatment groups. At week 2, participants reported consuming on average 385 kcal (range, 101–963 kcal) per day. By week 6, caloric levels increased to an average 692 kcal (range, 397–1,150 kcal) per day. At week 26, participants were consuming about 1,120 kcal (range, 645–1,565 kcal) a day, and by the 1-year follow-up visit, participants reported consuming about 1,350 kcal (range, 755–1,774 kcal) per day.
At the baseline visit, the FAT-BEV group was consuming significantly more protein than the PRO-BEV group (76 ± 26 g vs 105 ± 27 g, respectively; P < .05). However, by week 2, the PRO-BEV group was consuming significantly more protein than the FAT-BEV group (41 ± 24 g vs 11 ± 15 g; P = .01, respectively), and this difference remained significant at week 6 (PRO-BEV 57 ± 9 g vs FAT-BEV 36 ± 25 g, P = .04). This discrepancy in protein intake between the 2 groups at weeks 2 and 6 is due to the fact that we asked the participants in both groups to consume the assigned beverage for 6 weeks (ie, immediately after surgery until the week 6 visit). Participants were instructed to drink the assigned beverage twice a day in addition to consuming their regular meals and snacks. Therefore, the PRO-BEV group was potentially receiving an additional 40 g of protein per day. Protein consumption was no longer different between the groups at month 6 (59 g/d) and 1 year (65 g/d).
There were no differences in fat consumption between the groups at any visit. At baseline, participants reported consuming an average 82 g of fat per day. At week 2, fat consumption decreased and was about 12 g/d. At week 6, fat consumption increased, and participants reported an average intake of 25 g/d. Fat consumption continued to increase, and average reported intakes were 44 g at month 6 and 53 g at 1 year.
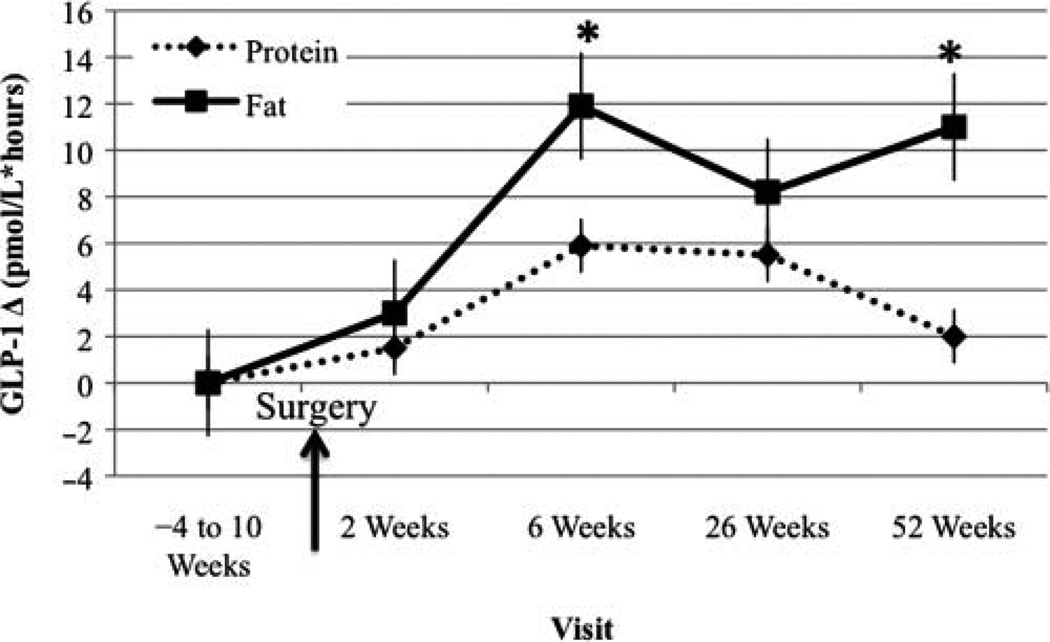
GLP-1 AUC Change From Baseline Visit
In the FAT-BEV group, GLP-1 AUC significantly increased at week 6 and 1 year post-RYGB compared with baseline (Figure 2).
Figure 2.
Glucagon-like peptide 1 (GLP-1) area under the curve change from baseline visit. *Significantly different from baseline, P < .05.
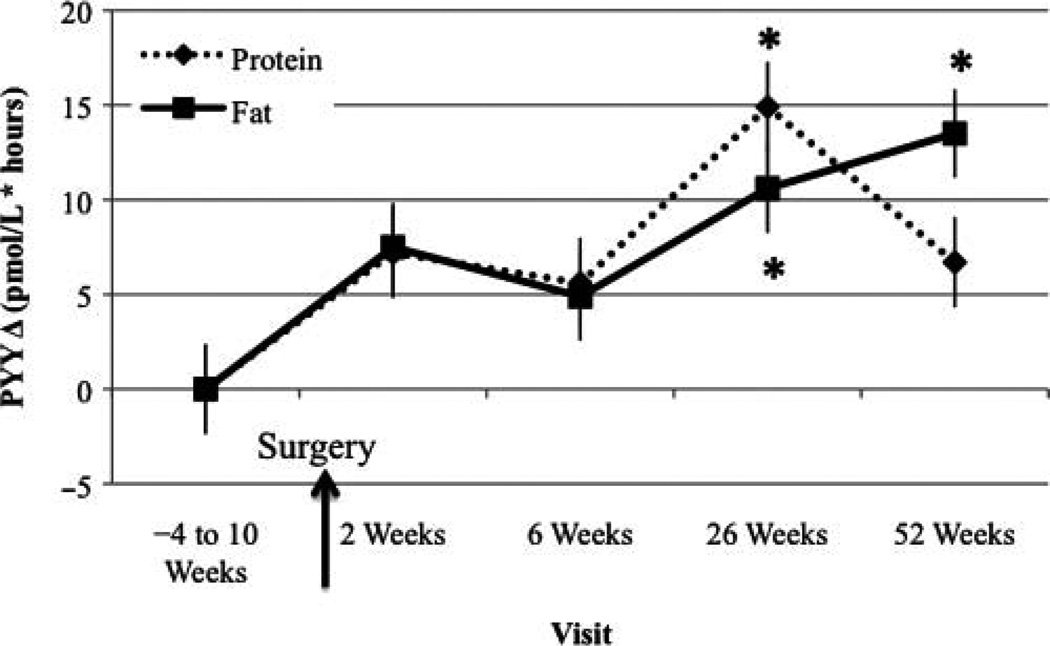
PYY AUC Change From Baseline Visit
In both the PRO-BEV and FAT-BEV groups, PYY AUC significantly increased at month 6 compared with baseline (Figure 3). In addition, PYY AUC remained higher compared with baseline at 1 year post-RYGB in the FAT-BEV group.
Figure 3.
Peptide YY (PYY) area under the curve change from baseline visit. *Significantly different from baseline, P < .05.
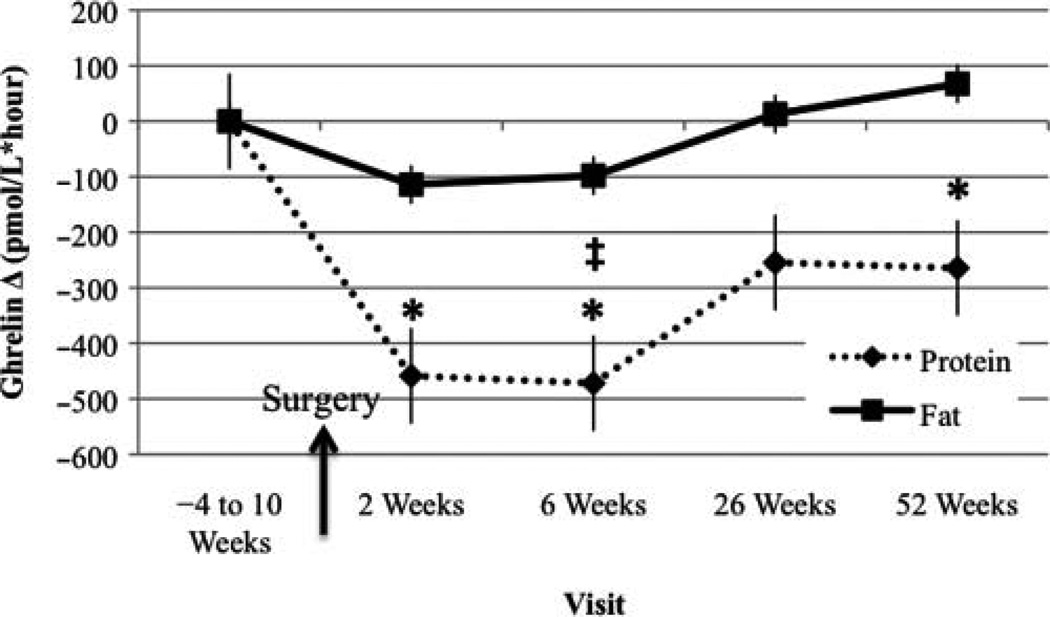
Ghrelin AUC Change From Baseline Visit
Ghrelin AUC was significantly lower at week 2 compared with baseline in the PRO-BEV group (Figure 4). Moreover, this alteration remained significant at week 6 and 1 year. Ghrelin AUC levels were significantly different between the PRO-BEV and FAT-BEV group at week 6, with lower levels in the PRO-BEV group.
Figure 4.
Ghrelin area under the curve change from baseline visit. *Significantly different from baseline, P < .05; ‡significantly different between groups, P < .05.
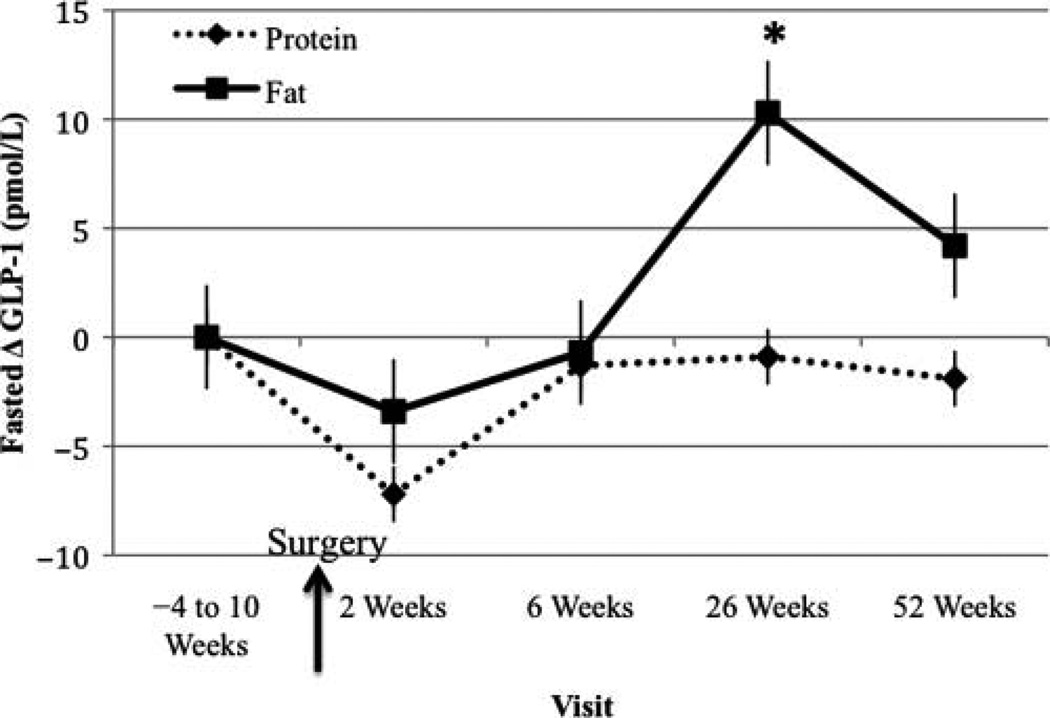
Fasted GLP-1 Change From Baseline Visit
At month 6, fasted GLP-1 increased significantly compared with baseline in the FAT-BEV group (Figure 5). No differences in fasted GLP-1 levels between treatments were evident at any visit.
Figure 5.
Fasted glucagon-like peptide 1 (GLP-1) change from baseline visit. *Significantly different from baseline, P < .05.
Fasted PYY Change From Baseline Visit
No significant differences in fasted PYY levels were evident between visits (data not presented). There was no difference in fasted PYY levels between treatment groups at any visit.
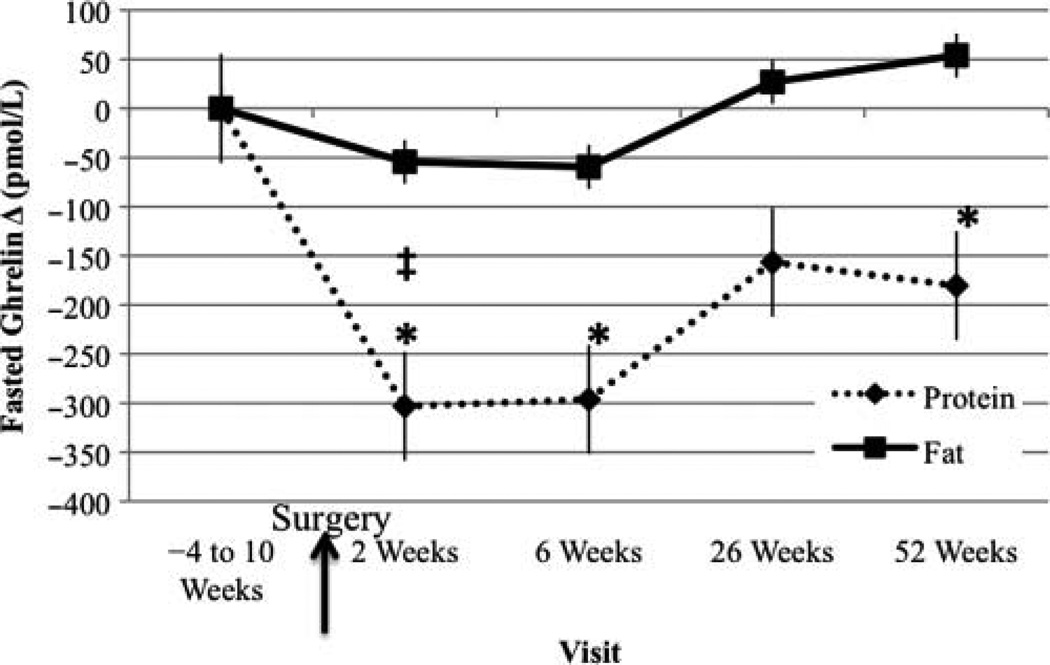
Fasted Ghrelin Change From Baseline Visit
Fasted ghrelin was significantly lower at week 2, week 6, and 1 year post-RYGB compared with the baseline visit in the PRO-BEV group (Figure 6). At week 2, fasted ghrelin was significantly different between treatments, with lower levels in the PRO-BEV group.
Figure 6.
Fasted ghrelin change from baseline visit. *Significantly different from baseline, P < .05; ‡significantly different between groups, P < .05.
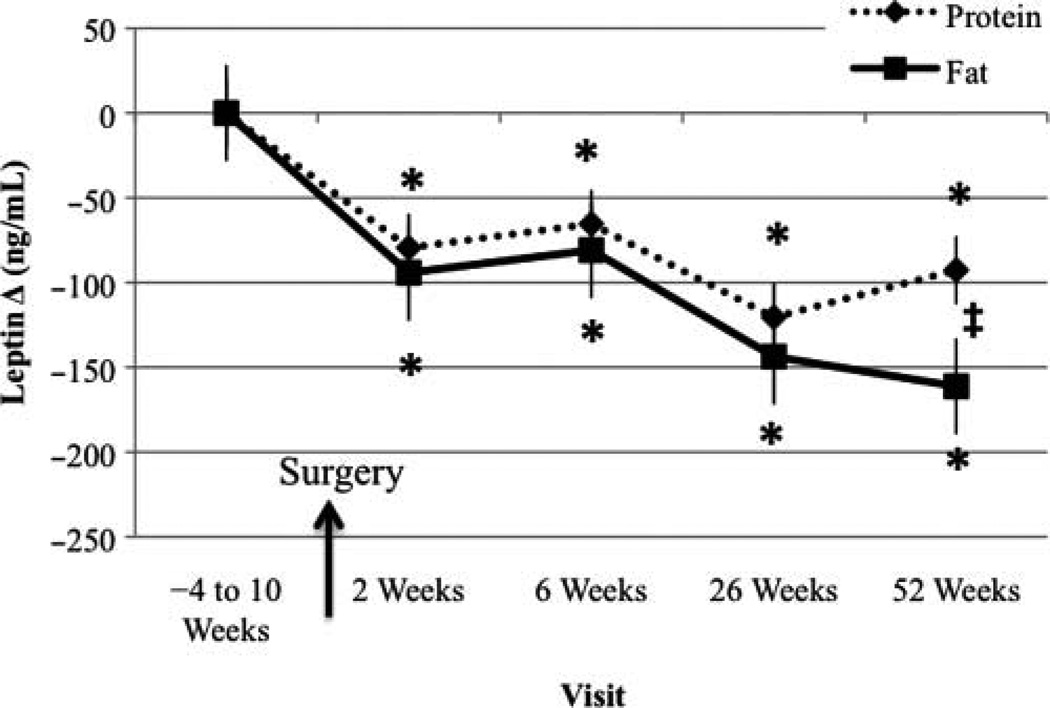
Fasted Leptin Change From Baseline Visit
In both the PRO-BEV and FAT-BEV groups, leptin significantly decreased compared with baseline at all time points after RYGB (Figure 7). Leptin levels were significantly different between the PRO-BEV and FAT-BEV groups at 1 year, with lower leptin in the FAT-BEV group.
Figure 7.
Fasted leptin change from baseline visit. *Significantly different from baseline, P < .05; ‡significantly different between groups, P < .05.
Discussion
This study investigated the effect of the RYGB procedure on body weight and levels of GLP-1, PYY, ghrelin, and leptin. We also evaluated the effect of short-term administration of protein and fat on GI hormone levels. In patients with morbid obesity, RYGB resulted in significant weight loss, which was maintained for at least 1 year after surgery. In addition to the favorable change in weight, alterations in GI hormones and leptin also occurred, and in some cases these changes were macronutrient specific.
General Findings for Both Beverage Groups
Similarities between treatment groups were evident for fasted leptin at all visits and PYY AUC at month 6. In regard to fasted leptin, the adipocytokine decreased from baseline at all visits. This was an expected result and similar to what has been previously reported. Decreased leptin has consistently been found after a reduction in adipose tissue mass after weight loss, either by conventional methods78–80 or via RYGB.9,46,49,63,70,73,78,81–93 Of interest, however, we observed that fasted leptin was significantly different as soon as 2 weeks post-RYGB, before significant changes in absolute body weight were observed. This immediate change was also found by Rubino et al85 at 3 weeks post-RYGB, before significant weight changes and might be a result of the RYGB procedure itself. Leptin is secreted in proportion to adipose tissue mass,74 and it would be expected that this change would not occur until a later follow-up visit (eg, month 6). In addition to adipocytes, leptin is secreted from the stomach,75 which is substantially altered after RYGB. This manipulation might explain the decreased fasted leptin levels we found soon after surgery.
PYY AUC was higher in both groups at 6 months post-RYGB compared with baseline. Our results are similar to those who have evaluated PYY level at 6 months post-RYGB.7,9 However, we are not aware of any study that has looked at the effect of different macronutrients on PYY level in the post-RYGB state. Given what has been reported in overweight and normal-weight individuals, we expected PYY to increase after a short-term dose of either protein32,35,36 or fat,32–34 which is in line with our results.
Specific Findings—Protein Beverage Group
In the PRO-BEV group, a short-term dose significantly lowered ghrelin AUC at 2 weeks, 6 weeks, and 1 year post-RYGB compared with baseline levels. In terms of the ghrelin AUC results at 2 weeks, this early change is congruent with what has been found in previous research 30 minutes to 10 days after surgery60,62 and, similar to leptin, occurred before a significant change in absolute body weight occurred, suggesting that a component of the RYGB procedure is involved in this result. Through repeated measurements before, during, and after the RYGB procedure, Lin et al62 found that the greatest decline in ghrelin occurs after complete division of the stomach, offering an explanation as to why this favorable change was observed so soon after surgery. In terms of the macronutrient effect on ghrelin AUC, the fact that we found an effect in the PRO-BEV group after RYGB is a novel result. To our knowledge, no study has compared the effect of macronutrients on ghrelin in the post-RYGB state. We expected that protein would suppress ghrelin AUC in our patient population at 2 weeks post-RYGB, because research done in overweight and obese individuals indicates that ghrelin is lower after protein intake compared with the respective baseline levels,55,57 carbohydrate intake,30,31,55,56 and/or fat intake.55 However, more research is needed in this area to clarify whether the ghrelin response post-RYGB is macronutrient specific. Such research has the potential to better define the diet recommendations for patients post-RYGB.
Ghrelin AUC was also significantly reduced at 6 weeks post-RYGB compared with both baseline and the FAT-BEV group. By this time point, participants had a significant change in body weight yet were still considered to be obese. Limited research has evaluated ghrelin at 6 weeks post-RYGB, and contrary findings are evident. At 4 weeks post-RYGB, Sundbom et al69 reported an increase in ghrelin to levels comparable with pre-RYGB, whereas 2 studies63,78 did not find a significant ghrelin suppression compared with pre-RYGB levels. In addition, le Roux et al41 found an insignificant increase in ghrelin at 6 weeks compared with baseline. Discrepancies might be related to differences in study protocol. For example, Morínigo et al63 measured ghrelin after a mixed 398-kcal liquid meal with 14.5% protein, and le Roux et al41 measured ghrelin after a mixed 400-kcal meal (protein amount not specified), whereas Sundbom et al69 measured only fasted ghrelin. Olivan et al78 measured ghrelin after a 50-g oral glucose tolerance test. We measured ghrelin after a 90-kcal protein beverage (88.9% protein), and our significant finding might be due to the higher proportion of protein. Studies that evaluated patients of similar weight status (obese and/or overweight) have observed protein to potently suppress ghre-lin.30,31,56,57 Although fat has been found to suppress ghrelin,54,55 we did not find this at week 6 and instead observed significantly lower ghrelin in the PRO-BEV group compared with the FAT-BEV group. Perhaps the best explanation is that protein may be more suppressive of the GI hormone than fat,55,57 especially in post-RYGB patients. Satiety research suggests that protein is the most satiating, and fat the least satiating,98 which may account for our findings.
The suppressed ghrelin AUC levels observed at week 2 and 6 were maintained long-term in this study. Ghrelin AUC was significantly suppressed at 1 year post-RYGB compared with baseline levels. At this time point, almost half of the participants were considered to be overweight (n = 7) with the remainder still classified as obese (n = 9). Reduced ghrelin levels have been reported in RYGB patients 1 to 2 years postsurgery, compared with other surgical procedure patients65 and obese45,65 and normal-weight individuals.45,65 This finding of suppressed ghrelin levels at 1 year post-RYGB is encouraging and might offer a long-term explanation for the relative success of the procedure. Future work should compare weight loss outcomes with ghrelin levels to better establish ghrelin’s role in weight loss after RYGB.
Fasted ghrelin was significantly lower at weeks 2 and 6 and 1 year compared with baseline in only the PRO-BEV group and was significantly lower than in the FAT-BEV group at week 2. Treatment-specific alterations in the fasted ghrelin levels were unexpected and prompted us to investigate our diet data for possible explanations. As described in the Methods, this analysis was part of a larger study evaluating body composition changes and the impact of protein intake on those changes in the RYGB patient population and was not initially designed to evaluate GI hormones and leptin. Consequently, participants in this study only fasted for 4 hours before the baseline blood draw. At weeks 2 and 6, analysis of the meal eaten 4 to 6 hours before the baseline blood draw indicated that the percentage of calories from protein was significantly greater in the PRO-BEV group compared with the FAT-BEV group (week 2: PRO-BEV 42%, FAT-BEV 20%, P = .05; week 6: PRO-BEV 31%, FAT-BEV 14%, P < .05). Ghrelin, being orexigenic, increases in the absence of enteral intake and, in this study, was higher in the FAT-BEV group. Protein has been suggested to be the most satiating of the 3 macronutrients,99 and perhaps a sustained reduced ghrelin level between meals is a mechanism for this. Because the study participants fasted for between 4 and 6 hours before the study, it might be more appropriate to consider them to be in a postprandial rather than fasting state. It is certainly possible that our levels of baseline fasting ghrelin were susceptible to some previous meal effects.
Although we observed an apparent difference between the treatment groups in fasting ghrelin concentrations at some of the postoperative time points, we hesitate to make general recommendations regarding the clinical significance and implication of these findings, for several reasons. First, we had a small sample size (n = 8 in each group) and might not have had enough power to detect real differences. Second, because this study was not originally designed to evaluate GI hormones, the fasting time period might not have been long enough to ensure an adequate preprandial state. Overall, we cannot be certain whether the difference in fasted ghrelin concentrations between groups was true or spurious, and thus further work is needed to elucidate the GI hormone profile in response to different macronutrients in the post-RYGB patient population.
Specific Findings—Fat Beverage Group
GLP-1 AUC was greater at 6 weeks and 1 year post-RYGB in the FAT-BEV group compared with baseline levels. Other research has investigated GLP-1 between 4 weeks9,78,100,101 and 6 weeks41,42,51,78,102 post-RYGB, and overall, and the findings were similar to ours. Most researchers have found significantly increased levels at this time point compared with baseline,41,42,51,100,101 although 2 studies did not find such an effect.9,102 Clements et al102 may not have found increased GLP-1 at 6 weeks post-RYGB because they only measured the GI hormone in the fasted state, and GLP-1 is released in response to nutrient intake. The changes that we and others observed occurred when patients were still considered obese; therefore, the weight loss is not driving the changes in GLP-1. Instead, increased GLP-1 levels post-RYGB are thought to occur because of a surgical component that promotes more rapid delivery of nutrients to the distal gut.103,104
The increased GLP-1 AUC levels found at week 6 were maintained long term in our study (ie, 1 year post-RYGB). Few other studies have evaluated GLP-1 changes at this specific time point post-RYGB. Korner et al7 found GLP-1 increased significantly at 1 year post-RYGB compared with baseline, whereas Morínigo et al42 did not find this. Increased GLP-1 levels have been found at post-RYGB in studies of longer duration (eg, >1 year) compared with those who underwent other weight loss procedures11,48,50 and nonsurgical controls,10,11,49,50,105 and this alteration has been hypothesized to play a role in the success of the procedure. le Roux et al41 evaluated those who had “good” and “poor” weight loss outcomes, defined as 40% and 20% weight loss after RYGB, respectively (RYGB was preformed 25.3 ± 1.7 months before the study). Patients considered to have poor weight loss had significantly lower GLP-1 levels compared with those who were considered to have good weight loss.41
In contrast to the lack of significant change in GLP-1 AUC levels in the PRO-BEV group from baseline to all time points following surgery, we found that GLP-1 AUC levels were increased in response to short-term administration of fat at 6 weeks and 1 year post-RYGB. This was a novel finding, as no other study to our knowledge has looked at GLP-1 changes in response to different macronutrients before and after RYGB. We found no significant differences at any of the time points between the groups in GLP-1 AUC. Graphic representation of the data suggests that the short-term response to protein was not stimulatory of GLP-1, and there might have been a difference between groups that we were unable to detect because of an insufficient sample size. Although it has been reported that both protein and fat can potently stimulate GLP-1 release, fat has been reported to stimulate GLP-1 more potently than protein in normal-weight individuals28 and obese boys.29 Lipids are thought to be the most potent stimulus for GLP-1 secretion, and ingestion of fats leads to sustained secretion.18 From our data in the RYGB patient population, fat ingestion more potently stimulated GLP-1 compared with protein. More work is needed in this area to establish the most effective post-RYGB diet recommendations.
Fasted GLP-1 levels were significantly greater at 6 months compared with baseline in only the FAT-BEV group. This result was unexpected, and we evaluated the macronutrient content of the meal eaten 4 to 6 hours before the baseline blood draw in order to better understand the mechanism involved. Compared with either protein or carbohydrate, fat most potently slows GI transit.106 In addition, GLP-1 secretion peaks after meal termination and remains elevated for several hours afterward.11,48–51,78,100,101,105 Although both protein30,31 and fat28,29 stimulate GLP-1 secretion, it is possible that the FAT-BEV group had an elevated GLP-1 level secondary to the meal eaten before the visit, which was higher in the percentage of calories from fat compared with the PRO-BEV group and might not have been completely digested and absorbed because of the slower transit time of fat (FAT-BEV 46% vs PRO-BEV 33%).
At 1 year post-RYGB, PYY AUC increased significantly compared with baseline levels. Studies that have specifically looked at this time point have reported similar findings.7,47 Likewise, cross-sectional studies that compared PYY levels in pre-RYGB patients,107 other weight loss surgical patients,11,48 and nonsurgical controls10,11,44,45, 48 with those of patients who were at least 1 year post-RYGB found higher PYY in the post-RYGB group. It is thought that increased PYY levels, similar to GLP-1, mediate the long-term weight loss/maintenance found in RYGB patients. In the le Roux et al41 study that classified post-RYGB patients according to their weight loss outcome, those who were considered to have good weight loss had significantly higher PYY compared with the poor weight loss group.
This is the first study we know of that evaluated the PYY response after different macronutrients in the pre-RYGB and post-RYGB patient population. We found that at 1 year post-RYGB, PYY AUC only increased in response to the short-term administration dose of fat. Prior work evaluating the PYY response to different macronutrients in normal-weight individuals found that PYY is secreted in response to fat33,34 and protein,36 and we are not sure why we did not see such an effect at 1 year for protein in our study. PYY AUC in the PRO-BEV group reached its peak at 6 months and by 1 year had decreased to a value similar to what was seen at week 2. Based on these results, we suggest that PYY is more sensitive to fat in the post-RYGB patient population, but more research in this area is needed before a definitive conclusion can be made.
Limitations
Several limitations need to be considered when evaluating our results. First, we had a small sample size (n = 8 in each treatment group) and might not have had enough power to detect real differences. Second, our study sample included only Caucasian women, and therefore, the findings are not generalizable to the population. Third, because of cost and time constraints, participants were not able to serve as their own controls for the 2 treatments, and instead they received only 1 beverage type for all 5 visits. Fourth, this analysis was part of a larger study evaluating body composition changes in the RYGB patient population and was not initially designed to evaluate leptin and the GI hormones. Consequently, participants in this study were only fasted for 4 hours before the baseline blood draw for GI hormones and leptin. The possibility of altered results secondary to the meal eaten 4 hours before the analyses cannot be ruled out.
Conclusions
We have shown that, in addition to substantial weight loss, multiple changes in GI hormones and leptin occur after RYGB. Some differences were evident quite soon after surgery (2 weeks: ghrelin, leptin) whereas others were maintained long-term (1 year: GLP-1, PYY, ghrelin, and leptin). Overall, the success of the procedure was likely attributable to the synergistic effect of these changes. To our knowledge, this is the first study to evaluate the effect of different macronutrients on GI hormones in the post-RYGB patient population. Significant differences between macronutrient groups in terms of GI hormones and leptin were evident for ghrelin and leptin. In addition, differences were observed within treatment groups. Notably, we found that protein suppressed ghrelin, whereas fat was a potent stimulator of GLP-1 and PYY. This line of research has particular relevance to nutrition support practitioners because it may facilitate improvements in the nutrition care of post-RYGB patients. Future work in this area has the potential to better define post-RYGB macronutrient recommendations to impart greater satiety and thereby promote more effective weight loss and/or weight maintenance in this growing patient population.
Clinical Relevancy Statement.
Alterations in gastrointestinal hormones and leptin occur after Roux-en-Y gastric bypass (RYGB) surgery, and in some instances, they can be macronutrient-specific. From a clinical perspective, this is useful information that might allow clinicians to make improved macronutrient recommendations for patients who have undergone RYGB. Future work is needed in this area to better define the optimal diet for the RYGB patient population.
Acknowledgments
We thank the study participants for their commitment to our study and the team at the Weight Management Center at the University of Minnesota Medical Center– Fairview and the GCRC at the University of Minnesota for their support of this study.
Financial disclosure: Funding for this study was provided by the Rhoads Research Foundation of the American Society for Parenteral and Enteral Nutrition (C. Earthman); grant MO1-RR00400 from the National Center for Research Resources; the National Institutes of Health, which supported the General Clinical Research Center at the University of Minnesota; the Minnesota Agricultural Experiment Station (C. Earthman); and the Midwest Dairy Association (C. Earthman).
References
- 1.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–2796. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 2.Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Endocrinol Metab Clin North Am. 2008;37:943–964. doi: 10.1016/j.ecl.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Saliba J, Wattacheril J, Abumrad NN. Endocrine and metabolic response to gastric bypass. Curr Opin Clin Nutr Metab Care. 2009;12:515–521. doi: 10.1097/MCO.0b013e32832e1b14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchwald H, Consensus Conference Panel Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005;1:371–381. doi: 10.1016/j.soard.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132:2253–2271. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 6.Pilkington TR, Gazet JC, Ang L, Kalucy RS, Crisp AH, Day S. Explanations for weight loss after ileojejunal bypass in gross obesity. Br Med J. 1976;1:1504–1505. doi: 10.1136/bmj.1.6024.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25:1150–1156. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 10.Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Edén Engström B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 2008;32:1640–1646. doi: 10.1038/ijo.2008.157. [DOI] [PubMed] [Google Scholar]
- 11.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 15.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf) 2008;69:173–179. doi: 10.1111/j.1365-2265.2007.03164.x. [DOI] [PubMed] [Google Scholar]
- 17.Dhillo WS. Appetite regulation: an overview. Thyroid. 2007;17:433–445. doi: 10.1089/thy.2007.0018. [DOI] [PubMed] [Google Scholar]
- 18.Dube PE, Brubaker PL. Nutrient, neural and endocrine control of glucagon-like peptide secretion. Horm Metab Res. 2004;36:755–760. doi: 10.1055/s-2004-826159. [DOI] [PubMed] [Google Scholar]
- 19.Frezza EE, Wachtel MS, Chiriva-Internati M. The multiple faces of glucagon-like peptide-1-obesity, appetite, and stress: what is next? A review. Dig Dis Sci. 2007;52:643–649. doi: 10.1007/s10620-006-9096-2. [DOI] [PubMed] [Google Scholar]
- 20.le Roux CW, Bloom SR. Peptide YY, appetite and food intake. Proc Nutr Soc. 2005;64:213–216. doi: 10.1079/pns2005427. [DOI] [PubMed] [Google Scholar]
- 21.Vincent RP, Ashrafian H, le Roux CW. Mechanisms of disease: the role of gastrointestinal hormones in appetite and obesity. Nat Clin Pract Gastroenterol Hepatol. 2008;5:268–277. doi: 10.1038/ncpgasthep1118. [DOI] [PubMed] [Google Scholar]
- 22.Jayasena CN, Bloom SR. Role of gut hormones in obesity. Endocrinol Metab Clin North Am. 2008;37:769–787. xi. doi: 10.1016/j.ecl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Van Citters GW, Lin HC. The ileal brake: a fifteen-year progress report. Curr Gastroenterol Rep. 1999;1:404–409. doi: 10.1007/s11894-999-0022-6. [DOI] [PubMed] [Google Scholar]
- 24.Coppini LZ, Waitzberg DL, Campos AC. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr Opin Clin Nutr Metab Care. 2005;8:329–332. doi: 10.1097/01.mco.0000165013.54696.64. [DOI] [PubMed] [Google Scholar]
- 25.Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept. 2005;128:117–124. doi: 10.1016/j.regpep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 27.Yoder SM, Yang Q, Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol. 2009;G297:G299–G305. doi: 10.1152/ajpgi.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 29.Maffeis C, Surano MG, Cordioli S, Gasperotti S, Corradi M, Pinelli L. A high-fat vs a moderate-fat meal in obese boys: nutrient balance, appetite, and gastrointestinal hormone changes. Obesity (Silver Spring) 2010;18:449–455. doi: 10.1038/oby.2009.271. [DOI] [PubMed] [Google Scholar]
- 30.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91:2913–2919. doi: 10.1210/jc.2006-0609. [DOI] [PubMed] [Google Scholar]
- 31.Bowen J, Noakes M, Clifton PM. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int J Obes (Lond) 2007;31:1696–1703. doi: 10.1038/sj.ijo.0803665. [DOI] [PubMed] [Google Scholar]
- 32.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab. 2007;92:4052–4055. doi: 10.1210/jc.2006-2273. [DOI] [PubMed] [Google Scholar]
- 33.Maljaars J, Romeyn EA, Haddeman E, Peters HP, Masclee AA. Effect of fat saturation on satiety, hormone release, and food intake. Am J Clin Nutr. 2009;89:1019–1024. doi: 10.3945/ajcn.2008.27335. [DOI] [PubMed] [Google Scholar]
- 34.Seimon RV, Feltrin KL, Meyer JH, et al. Effects of varying combinations of intraduodenal lipid and carbohydrate on antropy-loroduodenal motility, hormone release, and appetite in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009;296:R912–R920. doi: 10.1152/ajpregu.90934.2008. [DOI] [PubMed] [Google Scholar]
- 35.Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3–36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab. 2008;52:188–195. doi: 10.1159/000138122. [DOI] [PubMed] [Google Scholar]
- 36.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 38.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 39.le Roux CW, Batterham RL, Aylwin SJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez Bartolome M, Borque M, Martinez-Sarmiento J, et al. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12:324–327. doi: 10.1381/096089202321088084. [DOI] [PubMed] [Google Scholar]
- 41.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 42.Morinigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 43.Whitson BA, Leslie DB, Kellogg TA, et al. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res. 2007;141:31–39. doi: 10.1016/j.jss.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 45.Chan JL, Mun EC, Stoyneva V, Mantzoros CS, Goldfine AB. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring) 2006;14:194–198. doi: 10.1038/oby.2006.25. [DOI] [PubMed] [Google Scholar]
- 46.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 47.Morinigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–275. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 48.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 49.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglyco-penia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 50.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morínigo R, Moizé V, Musri M, et al. Glucagon-like peptide-1, pep-tide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 52.Zwirska-Korczala K, Konturek SJ, Sodowski M, et al. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol. 2007;58(suppl 1):13–35. [PubMed] [Google Scholar]
- 53.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 54.Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul Pept. 2003;116:101–107. doi: 10.1016/s0167-0115(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 55.Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006;50:260–269. doi: 10.1159/000091684. [DOI] [PubMed] [Google Scholar]
- 56.Bowen J, Noakes M, Trenerry C, Clifton PM. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab. 2006;91:1477–1483. doi: 10.1210/jc.2005-1856. [DOI] [PubMed] [Google Scholar]
- 57.Foster-Schubert KE, Overduin J, Prudom CE, et al. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blom WA, Lluch A, Stafleu A, et al. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr. 2006;83:211–220. doi: 10.1093/ajcn/83.2.211. [DOI] [PubMed] [Google Scholar]
- 59.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 60.Couce ME, Cottam D, Esplen J, Schauer P, Burguera B. Is ghrelin the culprit for weight loss after gastric bypass surgery? A negative answer. Obes Surg. 2006;16:870–878. doi: 10.1381/096089206777822151. [DOI] [PubMed] [Google Scholar]
- 61.Tritos NA, Mun E, Bertkau A, Grayson R, Maratos-Flier E, Goldfine A. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res. 2003;11:919–924. doi: 10.1038/oby.2003.126. [DOI] [PubMed] [Google Scholar]
- 62.Lin E, Gletsu N, Fugate K, et al. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch Surg. 2004;139:780–784. doi: 10.1001/archsurg.139.7.780. [DOI] [PubMed] [Google Scholar]
- 63.Morínigo R, Casamitjana R, Moizé V, et al. Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res. 2004;12:1108–1116. doi: 10.1038/oby.2004.139. [DOI] [PubMed] [Google Scholar]
- 64.Christou NV, Look D, McLean AP. Pre- and post-prandial plasma ghrelin levels do not correlate with satiety or failure to achieve a successful outcome after Roux-en-Y gastric bypass. Obes Surg. 2005;15:1017–1023. doi: 10.1381/0960892054621071. [DOI] [PubMed] [Google Scholar]
- 65.Leonetti F, Silecchia G, Iacobellis G, et al. Different plasma ghre-lin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J Clin Endocrinol Metab. 2003;88:4227–4231. doi: 10.1210/jc.2003-030133. [DOI] [PubMed] [Google Scholar]
- 66.Engstrom BE, Ohrvall M, Sundbom M, Lind L, Karlsson FA. Meal suppression of circulating ghrelin is normalized in obese individuals following gastric bypass surgery. Int J Obes (Lond) 2007;31:476–480. doi: 10.1038/sj.ijo.0803440. [DOI] [PubMed] [Google Scholar]
- 67.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 68.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–3183. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 69.Sundbom M, Holdstock C, Engstrom BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 70.Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–350. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- 71.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 72.Mancini MC, Costa AP, de Melo ME, et al. Effect of gastric bypass on spontaneous growth hormone and ghrelin release profiles. Obesity (Silver Spring) 2006;14:383–387. doi: 10.1038/oby.2006.51. [DOI] [PubMed] [Google Scholar]
- 73.Vendrell J, Broch M, Vilarrasa N, et al. Resistin, adiponectin, ghre-lin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 74.Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- 75.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2008;24:659–664. doi: 10.1097/MOG.0b013e328311a65f. [DOI] [PubMed] [Google Scholar]
- 76.Bloomgarden ZT. Gut and adipocyte peptides. Diabetes Care. 2006;29:450–456. doi: 10.2337/diacare.29.02.06.dc06-0006. [DOI] [PubMed] [Google Scholar]
- 77.Konopko-Zubrzycka M, Baniukiewicz A, Wroblewski E, et al. The effect of intragastric balloon on plasma ghrelin, leptin, and adi-ponectin levels in patients with morbid obesity. J Clin Endocrinol Metab. 2009;94:1644–1649. doi: 10.1210/jc.2008-1083. [DOI] [PubMed] [Google Scholar]
- 78.Olivan B, Teixeira J, Bose M, et al. Effect of weight loss by diet or gastric bypass surgery on peptide YY3–36 levels. Ann Surg. 2009;249:948–953. doi: 10.1097/SLA.0b013e3181a6cdb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ratliff J, Mutungi G, Puglisi MJ, Volek JS, Fernandez ML. Carbohydrate restriction (with or without additional dietary cholesterol provided by eggs) reduces insulin resistance and plasma leptin without modifying appetite hormones in adult men. Nutr Res. 2009;29:262–268. doi: 10.1016/j.nutres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Weigle DS, Breen PA, Matthys CC, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 81.Czupryniak L, Pawlowski M, Kumor A, Szymanski D, Loba J, Strzelczyk J. Predicting maximum Roux-en-Y gastric bypass-induced weight reduction—preoperative plasma leptin or body weight? Obes Surg. 2007;17:162–167. doi: 10.1007/s11695-007-9042-1. [DOI] [PubMed] [Google Scholar]
- 82.Fruhbeck G, Diez-Caballero A, Gil MJ, et al. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg. 2004;14:606–612. doi: 10.1381/096089204323093363. [DOI] [PubMed] [Google Scholar]
- 83.Molina A, Vendrell J, Gutierrez C, et al. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes Surg. 2003;13:615–621. doi: 10.1381/096089203322190844. [DOI] [PubMed] [Google Scholar]
- 84.Ramos AP, de Abreu MR, Vendramini RC, Brunetti IL, Pepato MT. Decrease in circulating glucose, insulin and leptin levels and improvement in insulin resistance at 1 and 3 months after gastric bypass. Obes Surg. 2006;16:1359–1364. doi: 10.1381/096089206778663706. [DOI] [PubMed] [Google Scholar]
- 85.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, et al. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–1911. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das SK, Roberts SB, McCrory MA, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 88.Whitson BA, Leslie DB, Kellogg TA, et al. Adipokine response in diabetics and nondiabetics following the Roux-en-Y gastric bypass: a preliminary study. J Surg Res. 2007;142:295–300. doi: 10.1016/j.jss.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 89.Bobbioni-Harsch E, Morel P, Huber O, et al. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–4700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 90.Riedl M, Vila G, Maier C, et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008;93:2307–2312. doi: 10.1210/jc.2007-2383. [DOI] [PubMed] [Google Scholar]
- 91.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab. 2002;87:1184–1188. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- 92.Faraj M, Jones P, Sniderman AD, Cianflone K. Enhanced dietary fat clearance in postobese women. J Lipid Res. 2001;42:571–580. [PubMed] [Google Scholar]
- 93.Hickey MS, Pories WJ, MacDonald KG, Jr, et al. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg. 1998;227:637–644. doi: 10.1097/00000658-199805000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 96.Adrian TE, Bloom SR, Bryant MG, Polak JM, Heitz PH, Barnes AJ. Distribution and release of human pancreatic polypeptide. Gut. 1976;17:940–944. doi: 10.1136/gut.17.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR. Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab. 2005;90:2205–2211. doi: 10.1210/jc.2004-1641. [DOI] [PubMed] [Google Scholar]
- 98.Westerterp-Plantenga MS, Luscombe-Marsh N, Lejeune MPGM, et al. Dietary protein, metabolism, and body-weight regulation: dose-response effects. Int J Obes. 2006;30:S16–S23. [Google Scholar]
- 99.Soenen S, Westerterp-Plantenga MS. Proteins and satiety: implications for weight management. Curr Opin Clin Nutr Metab Care. 2008;11:747–751. doi: 10.1097/MCO.0b013e328311a8c4. [DOI] [PubMed] [Google Scholar]
- 100.Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incre-tin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–5. [PubMed] [Google Scholar]
- 103.Näslund E, Kral J. Impact of gastric bypass surgery on gut hormones and glucose homeostasis in type 2 diabetes. Diabetes. 2006;55(suppl 2):S92–S97. [Google Scholar]
- 104.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 105.Vidal J, Nicolau J, Romero F, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94:884–891. doi: 10.1210/jc.2008-1620. [DOI] [PubMed] [Google Scholar]
- 106.Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8:367–373. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- 107.Pournaras DJ, Osborne A, Hawkins SC, et al. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg. 2009;20:56–60. doi: 10.1007/s11695-009-9989-1. [DOI] [PubMed] [Google Scholar]