Abstract
One of the primary pests of bermudagrass (Cynodon spp.) on golf courses in the southeastern United States is Belonolaimus longicaudatus (sting nematode). In 2011, a commercial formulation of Bacillus firmus I-1582, Nortica 5WG, was launched in the United States for management of plant-parasitic nematodes on turfgrasses. To test the efficacy of late winter/early spring application of this biopesticide on B. longicaudatus, two field trials in 2009 compared B. firmus with fenamiphos and untreated control treatments. In 2011, two additional field trials compared treatment with B. firmus with untreated control only. These trials measured treatment effects on the population density of B. longicaudatus, turf root length, and turf percent green cover. In all four trials, treatment with B. firmus improved root length and decreased numbers of B. longicaudatus in contrast to the untreated. These results indicate that late winter/early spring application of B. firmus is an effective biopesticide treatment for management of B. longicaudatus on golf course bermudagrass.
Keywords: Bacillus firmus, Belonolaimus longicaudatus, bermudagrass, biopesticide, Cynodon spp., management, sting nematode, turfgrass
The coastal plains of the United States include some of the top golf destinations in the world. In many of these areas the golf industry is a major economic driver in the local economy. In Florida alone, golf generated more than $9.2 billion and employed 226,000 people in 2000 (Haydu and Hodges, 2002). The southeastern coast is also the native habitat of Belonolaimus longicaudatus Rau, 1958 (sting nematode), one of the most common and destructive nematodes affecting golf course turf in the region (Crow, 2005b). B. longicaudatus severely stunts roots of bermudagrass (Cynodon spp.) (Pang et al., 2011b), which is the predominate type of grass used on golf courses in the southern United States (Florkowski and He, 2008). This root stunting causes the turf to wilt, decline, and die, leading to revenue loss from reduced golf play and contributing to increased irrigation frequency, labor costs, and pesticide use.
Fenamiphos was the primary nematicide used on golf courses for several decades, but its production ceased in 2007 and will become illegal to use in October 2017 (Keigwin, 2014). 1,3-Dichloropropene (1,3-D) can be used legally for management of plant-parasitic nematodes on golf courses in several southeastern states, including Florida, under Section 24(c) Special Local Needs labels. Previous research has found 1,3-D to be very effective for management of B. longicaudatus (Crow et al., 2003, 2005). However, the utility of 1,3-D is limited by reentry, buffer, and geological restrictions. It can only be used once per year and often results in population reductions of B. longicaudatus that last only weeks or months (Crow et al., 2005), whereas conditions in much of the southeast are favorable for B. longicaudatus almost year-round (Mc Groary et al., 2009). Therefore, additional management strategies are needed.
Golf courses are under increasing pressure to manage pests using methods that are effective and perceived as being environmentally friendly (Grant, 2014). This has created favorable market conditions for development and use of biopesticides. In Florida, numerous biological nematicides have been evaluated for efficacy on turf over the past decade (Crow, 2005a, 2013; Crow et al., 2006, 2011). In 2011, Nortica 5 WG, a biological product for nematode management on turfgrasses, was launched by Bayer Environmental Science (Research Triangle Park, NC). The active ingredient in Nortica is Bacillus firmus strain I-1582, a bacterium with proven nematicidal and nemastatic effects (Keren-Zur et al., 2000; Giannakou et al., 2004; Mendoza et al., 2008). The use of this bacterial strain against nematodes in the United States was acquired by Bayer CropScience in 2008 (Anonymous, 2011). In addition to Nortica, B. firmus I-1582 is also the active ingredient in Votivo seed treatment for crop seed (Anonymous, 2011; Castillo et al., 2013). Nortica is a dry formulation that forms a suspension in water that can be sprayed directly onto the turf surface with conventional golf course spray equipment. After application, the bacteria are moved into the turf root zone by irrigation water.
During the past decade, we have conducted dozens of unpublished turfgrass field trials with B. firmus in Florida, leading to the launch of Nortica. In these trials we have evaluated many formulations, rates, and application timings. As with most nematode management tactics, it took many failures to develop a precise, effective, and consistent strategy. This paper will focus on the results from our most effective application program for management of B. longicaudatus on turf, early spring application of Nortica 5 WG at 78 kg formulation/ha. The objective of this research was to evaluate the efficacy of late winter/early spring application of this commercial formulation for management of B. longicaudatus on golf course bermudagrass in Florida.
Materials and Methods
The following materials and methods apply to all the trials reported herein. All trials used a randomized block design with five replications. Plots were 1.5 m2, with 0.6-m untreated borders between adjacent plots. B. firmus solution was applied using a CO2-powered backpack sprayer (Weed Systems, Hawthorn, FL) calibrated to deliver 2,421-liter solution/ha, and fenamiphos was applied using a Gandy drop-spreader (Gandy, Owatonna, MN). Immediately after treatment application, all plots including the untreated controls, were irrigated with 0.64 cm of water.
Efficacy was determined by evaluating treatment effects on B. longicaudatus and on turf health. Nematode effects were determined by comparing the population density of B. longicaudatus in treated plots with untreated plots over time. Turf health was evaluated by comparing root length and percent green cover in treated plots with untreated plots over time.
Nematode samples consisted of nine cores (1.9-cm-diam. × 10-cm-deep) from each plot that were composited into a single sample. After turf and thatch were removed, nematodes were extracted from a 100-cm3 subsample using centrifugal-flotation (Jenkins, 1964). Turf root samples consisted of two cores (3.8-cm-diam. × 15-cm-deep) from each plot that were composited (v = 350 cm3). Roots were removed manually, scanned, and root lengths measured using WinRHIZO software (Regent Instruments, Quebec City, Canada) as described by Pang et al. (2011a). Turf percent green cover was used to measure turf health. Digital images of the center 1 m2 of each plot were taken using a digital camera mounted on a custom-built photo box. The percentage of green pixels (hue 55 to 105, saturation 15 to 100) from each image was measured using a macro developed by Karcher and Richardson (2005) for use with SigmaScan Pro 5 software (SPSS, Inc., Chicago, IL). Statistical analysis was conducted using SAS 9.2 software (SAS Institute, Cary, NC).
2009 trials:
In 2009, two trials were conducted at the University of Florida Plant Science Research and Education Unit (PSREU) in Citra, FL, on a ‘Celebration’ bermudagrass (C. dactylon) golf tee and on a ‘Tifdwarf’ bermudagrass (C. dactylon × C. transvaalensis) golf green. Both sites were naturally infested with B. longicaudatus. There were three treatments in these trials: (i) untreated control, (ii) the maximum labeled rate of the industry standard nematicide fenamiphos (Nemacur 10G) at 112 kg formulation/ha, and (iii) B. firmus (Nortica 5 WG) at 78 kg formulation/ha. Treatments were applied to both trials on 8 April. Population numbers of B. longicaudatus were assayed 17 March (Pi), 22 April (Pm1), 21 May (Pm2), and 1 July (Pf). Root samples were collected 1 July. Turf percent green cover was measured at approximately 2-wk intervals starting with the day of treatment on 8 April and continuing until 1 July.
Nematode and percent green cover data were subjected to analysis of covariance (ANOVA) with the initial measurement used as the covariant. Differences indicated are shown according to the P-value generated (P ≤ 0.1, 0.05, 0.01) when the treatment was compared with the untreated control. Root length data was collected only once, so that data was subjected to analysis of variance and treatment means were separated according to Duncan’s multiple-range test (P ≤ 0.05).
2011 trials:
In 2011, two trials were conducted at PSREU on a ‘Celebration’ bermudagrass golf tee and a ‘Tifdwarf’ bermudagrass golf green that were naturally infested with B. longicaudatus. The tee location was the same, but the green location was different from those used in 2009. There were two treatments in these trials: (i) untreated control and (ii) B. firmus at 78 kg formulation/ha. Treatments were applied to the tee on 17 February and to the green on 23 April. Population densities of B. longicaudatus were assayed on the tee 1 February (Pi), 1 March (Pm1), 29 March (Pm2), and 17 May (Pf). On the green, population densities of B. longicaudatus were assayed 4 April (Pi), 6 May (Pm1), 3 June (Pm2), and 12 August (Pf). Root samples from tee were collected before treatment on 17 February and after treatment on 17 March, 12 April, and 21 June. Root samples from the green were collected before treatment on 23 April, and after treatment on 17 June and 12 August. On both trials, turf percent green cover was measured at approximately 2-wk intervals starting with the day of treatment and continuing until the trial end.
All data were subjected to ANOVA with the initial measurement used as the covariant. Differences indicated are shown according to the P-value generated (P ≤ 0.1, 0.05, 0.01) when the treatment was compared with the untreated control.
Results
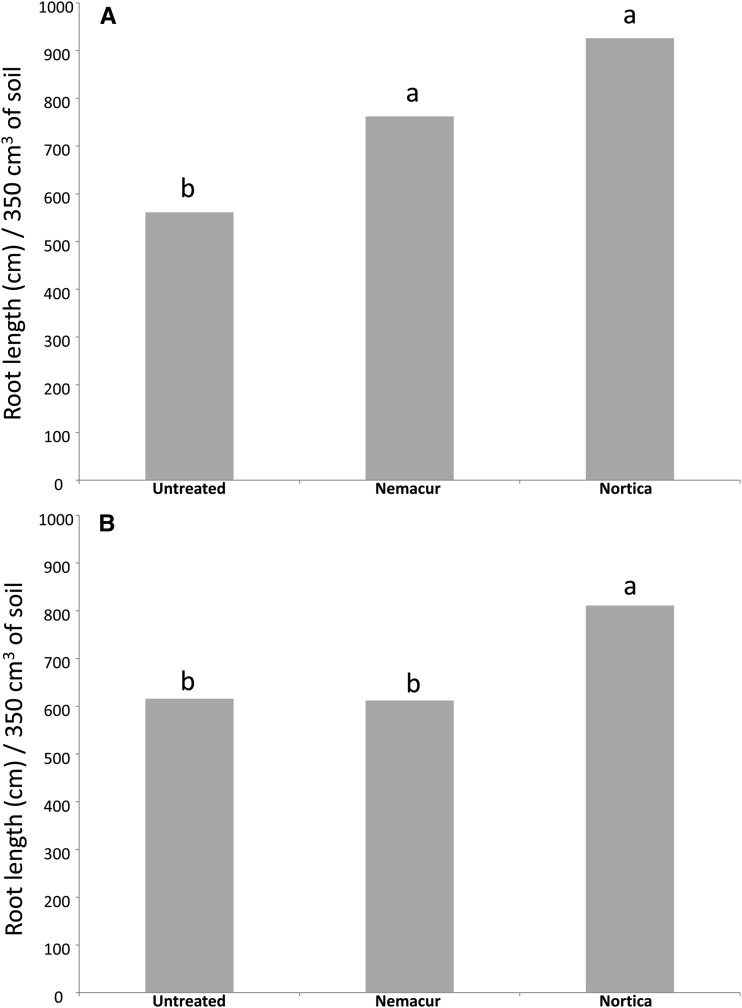
In 2009, population densities of B. longicaudatus were reduced (P ≤ 0.1) by fenamiphos 2 wk after treatment in both trials, and by B. firmus on the tee (Table 1). Eight weeks after treatment, population densities of B. longicaudatus were reduced (P ≤ 0.1) by fenamiphos and B. firmus on the green only (Table 2). Turf root length was increased (P ≤ 0.1) in both trials by B. firmus, but by fenamiphos only on the green (Fig. 1A,B). Percent green cover of the tee was improved by both fenamiphos and B. firmus (P ≤ 0.1) on most evaluation dates (Table 3). However, turf percent green cover of the green was not improved by either treatment (P > 0.1; data not shown).
Table 1.
Effects of treatment with Bacillus firmus (Nortica 5WG at 78 kg/ha) or fenamiphos (Nemacur 10G at 112 kg/ha) on population density of Belonolaimus longicaudatus/100 cm3 of soil infesting ‘Celebration’ bermudagrass (Cynodon dactylon) growing on a golf tee in 2009.
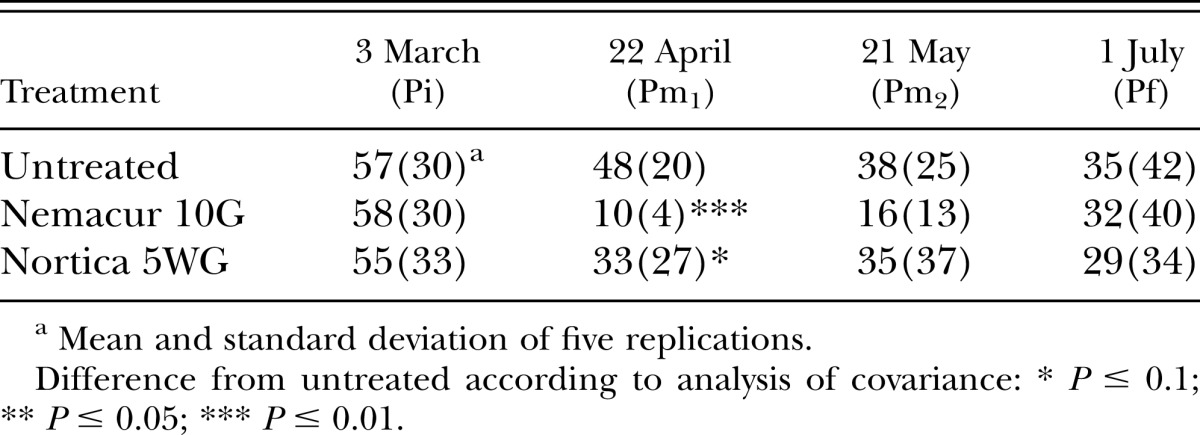
Table 2.
Effects of treatment with Bacillus firmus (Nortica 5WG) or fenamiphos (Nemacur 10G) on population density of Belonolaimus longicaudatus/100 cm3 of soil infesting ‘Tifdwarf’ bermudagrass (Cynodon dactylon × C. transvaalensis) growing on a Belonolaimus longicaudatus–infested golf green in 2009.
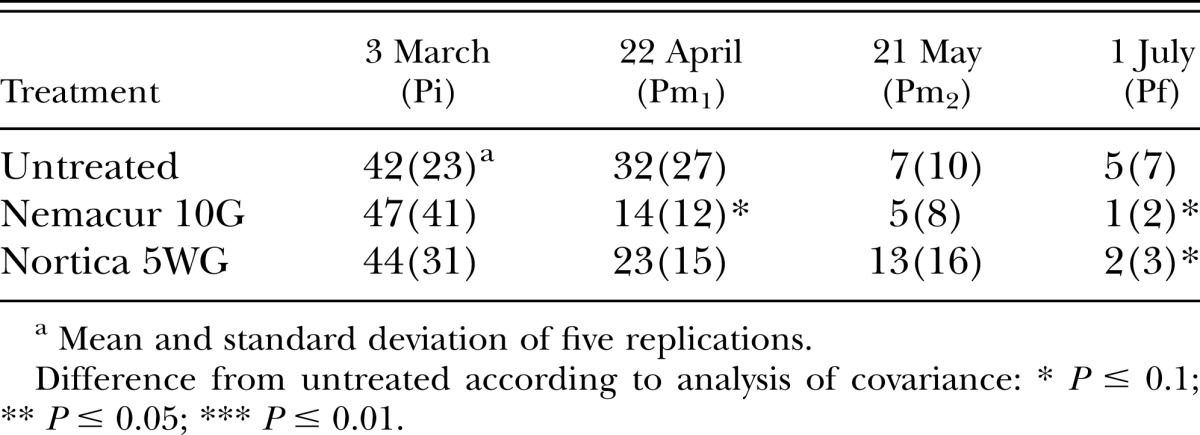
Fig. 1.
Effects of treatment with Bacillus firmus (Nortica 5WG at 78 kg/ha) or fenamiphos (Nemacur 10G at 112 kg/ha) on root length of Belonolaimus longicaudatus–infested (A) ‘Celebration’ bermudagrass (Cynodon dactylon) in a golf tee and (B) ‘Tifdwarf’ bermudagrass (C. dactylon × C. transvaalensis) in a golf green in 2009.
Table 3.
Effects of treatment with Bacillus firmus (Nortica 5WG at 78 kg/ha) or fenamiphos (Nemacur 10G at 112 kg/ha) on percent green cover of ‘Celebration’ bermudagrass (Cynodon dactylon) growing on a Belonolaimus longicaudatus–infested golf tee in 2009.
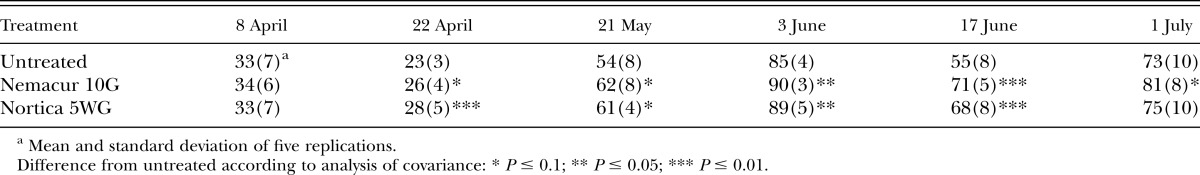
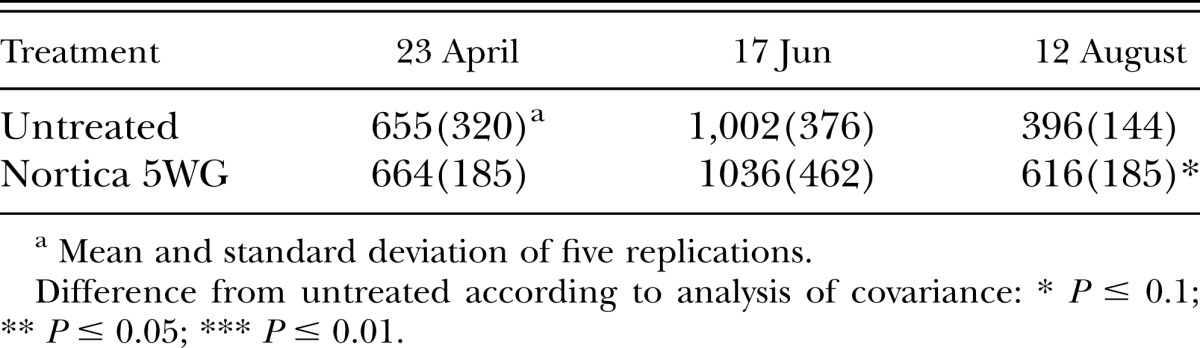
In 2011, population densities of B. longicaudatus were reduced (P ≤ 0.01) by the end of the trial by B. firmus on the tee (Table 4) and at 6 wk after treatment (P ≤ 0.05) on the green (Table 5). Root lengths on the tee were improved (P ≤ 0.05) by B. firmus on the last two sampling dates (Table 6) and on the green (P ≤ 0.1) on the last sampling date (Table 7). Treatment with B. firmus did not improve turf percent green cover on either trial in 2011 (P > 0.1; data not shown).
Table 4.
Effects of treatment with Bacillus firmus (Nortica 5WG at 78 kg/ha) on population density of Belonolaimus longicaudatus/100 cm3 of soil infesting ‘Celebration’ bermudagrass (Cynodon dactylon) growing on a golf tee in 2011.
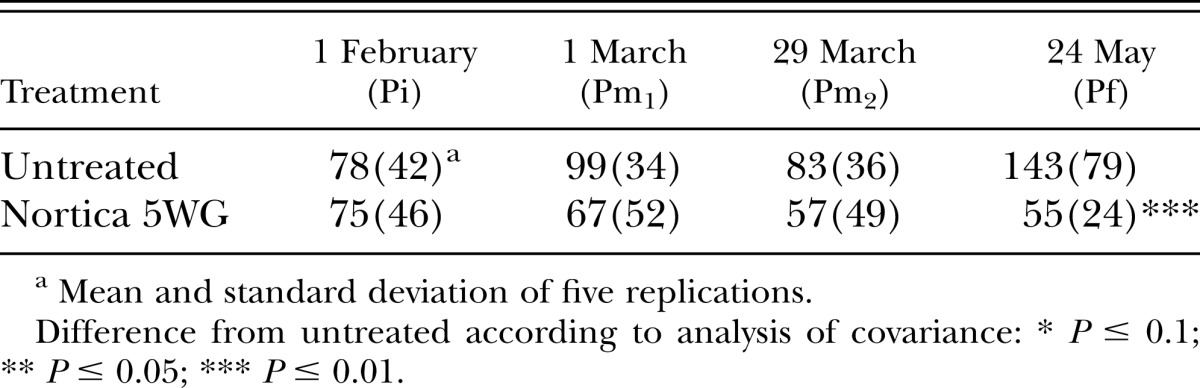
Table 5.
Effects of treatment with Bacillus firmus (Nortica 5WG at 78 kg/ha) on population density of Belonolaimus longicaudatus/100 cm3 of soil infesting ‘Tifdwarf’ bermudagrass (Cynodon dactylon × C. transvaalensis) growing on a golf green in 2011.
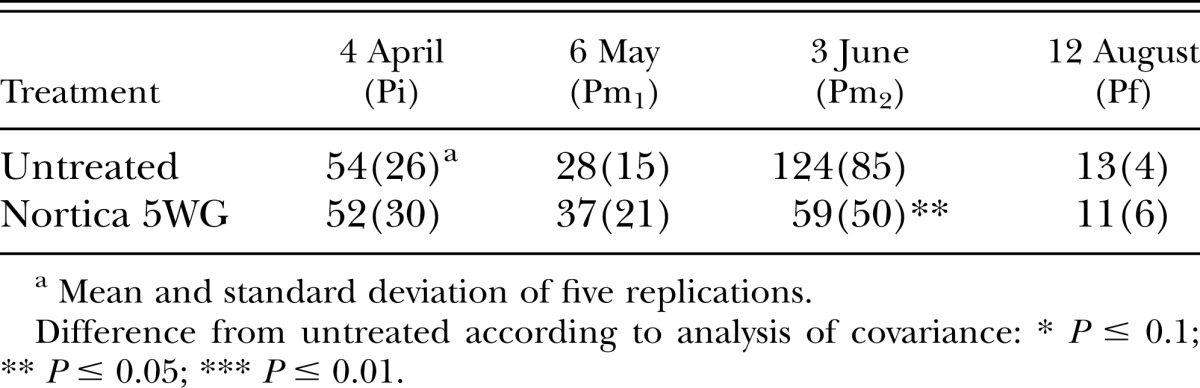
Table 6.
Effects of treatment with Bacillus firmus (Nortica 5WG at 78 kg/ha) on root length/350 cm3 of soil of ‘Celebration’ bermudagrass (Cynodon dactylon) in a Belonolaimus longicaudatus–infested golf tee in 2011.
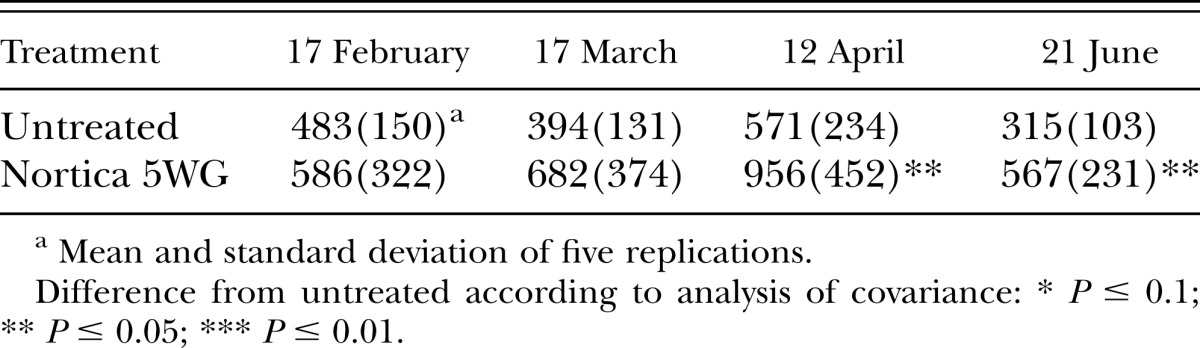
Table 7.
Effects of treatment with Bacillus firmus (Nortica 5 WG at 78 kg/ha) on root length/350 cm3 of soil of ‘Tifdwarf’ bermudagrass (Cynodon dactylon × C. transvaalensis) growing on a Belonolaimus longicaudatus–infested golf green in 2011.
Discussion
These studies reveal that late winter/early spring applications of 78 kg/ha of the B. firmus product Nortica 5WG consistently improve roots of bermudagrass growing in soil infested with B. longicaudatus in northern Florida. In addition, our results also indicate that this treatment can reduce population densities of B. longicaudatus and, less consistently, improve turf cover. However, our unpublished data indicate that B. firmus treatments applied later in the year are not as effective as late winter/early spring ones. Likewise, unpublished results indicate that higher rates do not improve results, and that frequent applications of lower rates are not as effective as a single application of 78 kg/ha. All the studies presented herein focus on management of B. longicaudatus, although a number of other plant-parasitic nematode species also cause damage to golf course bermudagrass in the region. Efficacy of B. firmus against certain of these nematodes, particularly Meloidogyne graminis, with a different formulation and application schedule, will be the focus of a future paper.
Bacillus firmus I-1582 has been shown to reduce egg hatch, induce paralysis, and cause mortality of various species of plant-parasitic nematodes. Results of Mendoza et al. (2008) indicated that the nematicidal and nemastatic effects of B. firmus are primarily attributable to active metabolites produced and not direct antagonism by the bacteria. According to the internal research of Bayer, B. firmus colonizes the root rhizosphere and consumes root exudates, thereby preventing nematode attraction to the root (Anonymous, 2011). Bayer claims that the bacterium produces enzymes that break down nematode egg shells and phytohormones that stimulate root growth (Anonymous, 2011).
In our trials, the most consistent benefit from B. firmus was improved root growth. In 2009, the root length increases from B. firmus exceeded those from fenamiphos. This could be caused by root growth stimulation, by prolonged reduction in feeding by B. longicaudatus, or a combination of both factors. Although reduced population densities of B. longicaudatus following application of B. firmus were observed in all four trials, in three of them the impact on nematodes increased over time and was greatest at the end of the trial, which is in contrast with typical results from a conventional nematicide, which impacts are greatest at the beginning and then dissipate over time, as was observed from fenamiphos in 2009. The nematode results observed from B. firmus are consistent with a root-protection mode of action, where nematode population densities decline slowly over time as the nematodes are unable to feed. Therefore, it appears that B. firmus might colonize the root rhizosphere and protect the roots from B. longicaudatus by potentially one or more means.
In the 2009 golf green trial, population densities of B. longicaudatus declined to near zero in all treatments. This happened in the entire green, not just in the research area. In previous years, populations of B. longicaudatus in this green remained high year-round, making it an ideal nematicide trial site. Although the cause for this decline is unknown, it should be noted that at the present time, 5 yr later, B. longicaudatus have never recovered in this green. However, this area is not suppressive to several other plant-parasitic nematode species that remain at high numbers on this green, particularly Meloidogyne graminis, M. marylandi, and Mesocriconema ornatum. The cause of this B. longicaudatus suppressive soil is unknown at this time but is believed to be fungal in origin.
Based on these results, B. firmus can be an effective management tool for B. longicaudatus on golf course bermudagrass when used early in the year as a preventative treatment. In Florida, it has not been effective as a late-season curative treatment for turf already exhibiting damage from B. longicaudatus (W. T. Crow, unpubl. data). Bacillus firmus is a good foundation treatment in IPM programs for B. longicaudatus. In areas with a history of damage from B. longicaudatus, B. firmus can be applied early and then nematode populations can be monitored to determine need for chemical nematicide treatment later in the year. In many cases, application of B. firmus should reduce the frequency of chemical nematicide application. Research is currently underway evaluating the efficacy of monitoring and calendar-based IPM strategies that include B. firmus for management of B. longicaudatus on tolerant and susceptible bermudagrasses (Crow and Kenworthy, 2013).
Literature Cited
- Anonymous 2011. Bio-protection against nutrient robbers. Bayer Research 23:58–61.
- Castillo JD, Lawrence KS, Kloepper JW. Biocontrol of the reniform nematode by Bacillus firmus GB-126 and Paecilomyces lilacinus 251 on cotton. Plant Disease. 2013;97:967–976. doi: 10.1094/PDIS-10-12-0978-RE. [DOI] [PubMed] [Google Scholar]
- Crow WT. Alternatives to fenamiphos for management of plant-parasitic nematodes on bermudagrass. Journal of Nematology. 2005a;37:477–482. [PMC free article] [PubMed] [Google Scholar]
- Crow WT. How bad are nematode problems on Florida’s golf courses? Florida Turf Digest. 2005b;22:10–12. [Google Scholar]
- Crow WT. Effects of a commercial formulation of Paecilomyces lilacinus strain 251 on overseeded bermudagrass infested with Belonolaimus longicaudatus. Journal of Nematology. 2013;45:223–227. [PMC free article] [PubMed] [Google Scholar]
- Crow WT, Kenworthy KE. Integrated pest management of plant-parasitic sting nematodes on bermudagrass. Turfgrass and Environmental Research Online. 2013;12:33. [Google Scholar]
- Crow WT, Giblin-Davis RM, Lickfeldt DW. Slit injection of 1,3-dichloropropene for management of Belonolaimus longicaudatus on established bermudagrass. Journal of Nematology. 2003;35:302–305. [PMC free article] [PubMed] [Google Scholar]
- Crow WT, Lickfeldt DW, Unruh JB. Management of sting nematode (Belonolaimus longicaudatus) on bermudagrass putting greens with 1,3-dichloropropene. International Turfgrass Society Research Journal. 2005;10:734–741. [Google Scholar]
- Crow WT, Luc JE, Giblin-Davis RM. Evaluation of Econem, a formulated Pasteuria sp. bionematicide, for management of Belonolaimus longicaudatus on golf course turf. Journal of Nematology. 2011;43:101–109. [PMC free article] [PubMed] [Google Scholar]
- Crow WT, Porazinska DL, Giblin-Davis RM, Grewal PS. Entomopathogenic nematodes are not an alternative to fenamiphos for management of plant-parasitic nematodes on golf courses in Florida. Journal of Nematology. 2006;38:52–58. [PMC free article] [PubMed] [Google Scholar]
- Florkowski WJ, He S. 2008. Preference of golf-course operators for various turf varieties and their perceived importance of selected problems in turf maintenance. Pp. 3–26 in M. Pessarakli, ed. Handbook of turfgrass management and physiology. Boca Raton, FL: CRC Press.
- Giannakou IO, Karpouzas DG, Prophetou-Athanasiadou D. A novel non-chemical nematicide for the control of root-knot nematodes. Applied Soil Ecology. 2004;26:69–79. [Google Scholar]
- Grant J. Environmentally friendly golf: Reducing chemical use and adapting best management practices can make golf courses playable and environmentally friendly. Golf Course Management. 2014;82:100–104. [Google Scholar]
- Haydu JJ, Hodges AW. 2002. Economic impact of the Florida golf course industry. Economic Information Report EIR02–4. Gainesville, FL: University of Florida.
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Karcher DE, Richardson MD. Batch analysis of digital images to evaluate turfgrass characteristics. Crop Science. 2005;45:1536–1539. [Google Scholar]
- Keigwin RP., Jr Fenamiphos: Amendment to existing stocks provision of use deletion and product cancellation order. Federal Register. 2014;79:59261–59262. [Google Scholar]
- Keren-Zur M, Antonov J, Bercovitz A, Feldman K, Husid A, Kenan G, Markov N, Rebhun M. Bacillus firmus formulations for the safe control of root-knot nematodes. Proceedings of the Brighton Crop Protection Conference on Pests and Diseases. 2000;2A:47–52. [Google Scholar]
- Mc Groary P, Crow WT, McSorley R, Giblin-Davis RM, Cisar JL. Seasonal fluctuations of Belonolaimus longicaudatus in bermudagrass. Nematropica. 2009;39:99–110. [Google Scholar]
- Mendoza AR, Kiewnick S, Sikora RA. In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Science and Technology. 2008;18:377–389. [Google Scholar]
- Pang W, Crow WT, Luc JE, McSorley R, Giblin-Davis RM, Kenworthy KE, Kruse JK. Comparison of water displacement and WinRHIZO software for root parameter assessment. Plant Disease. 2011a;95:1308–1310. doi: 10.1094/PDIS-01-11-0026. [DOI] [PubMed] [Google Scholar]
- Pang W, Luc JE, Crow WT, Kenworthy KE, Giblin-Davis RM, McSorley R, Kruse JK. Bermudagrass cultivar responses to sting nematode. Crop Science. 2011b;51:2199–2203. [Google Scholar]