Abstract
Biological characteristics of two strains of the entomopathogenic nematode, Heterorhabditis floridensis (332 isolated in Florida and K22 isolated in Georgia) were described. The identity of the nematode’s symbiotic bacteria was elucidated and found to be Photorhabdus luminescens subsp. luminescens. Beneficial traits pertinent to biocontrol (environmental tolerance and virulence) were characterized. The range of temperature tolerance in the H. floridensis strains was broad and showed a high level of heat tolerance. The H. floridensis strains caused higher mortality or infection in G. mellonella at 30°C and 35°C compared with S. riobrave (355), a strain widely known to be heat tolerant, and the H. floridensis strains were also capable of infecting at 17°C whereas S. riobrave (355) was not. However, at higher temperatures (37°C and 39°C), though H. floridensis readily infected G. mellonella, S. riobrave strains caused higher levels of mortality. Desiccation tolerance in H. floridensis was similar to Heterorhabditis indica (Hom1) and S. riobrave (355) and superior to S. feltiae (SN). H. bacteriophora (Oswego) and S. carpocapsae (All) exhibited higher desiccation tolerance than the H. floridensis strains. The virulence of H. floridensis to four insect pests (Aethina tumida, Conotrachelus nenuphar, Diaprepes abbreviatus, and Tenebrio molitor) was determined relative to seven other nematodes: H. bacteriophora (Oswego), H. indica (Hom1), S. carpocapsae (All), S. feltiae (SN), S. glaseri (4-8 and Vs strains), and S. riobrave (355). Virulence to A. tumida was similar among the H. floridensis strains and other nematodes except S. glaseri (Vs), S. feltiae, and S. riobrave failed to cause higher mortality than the control. Only H. bacteriophora, H. indica, S. feltiae, S. riobrave, and S. glaseri (4-8) caused higher mortality than the control in C. nenuphar. All nematodes were pathogenic to D. abbreviatus though S. glaseri (4-8) and S. riobrave (355) were the most virulent. S. carpocapsae was the most virulent to T. molitor. In summary, the H. floridensis strains possess a wide niche breadth in temperature tolerance and have virulence and desiccation levels that are similar to a number of other entomopathogenic nematodes. The strains may be useful for biocontrol purposes in environments where temperature extremes occur within short durations.
Keywords: beneficial trait, biological control, entomopathogenic nematode, Heterorhabditis floridensis
Entomopathogenic nematodes in the genera Steinernema and Heterorhabditis are biological control agents that are used to control a variety of economically important insect pests (Shapiro-Ilan et al., 2002a, 2014a; Grewal et al., 2005). The nematodes used for biocontrol purposes have a mutualistic symbiosis with a bacterium (Xenorhabdus spp. and Photorhabdus spp. for steinernematids and heterorhabditids, respectively) (Poinar, 1990; Lewis and Clarke, 2012). Infective juveniles (IJs), the only free-living stage, enter hosts through natural openings (mouth, anus, and spiracles), or in some cases, through the cuticle. After entering the host’s hemocoel, nematodes release their bacterial symbionts, which are primarily responsible for killing the host within 24 to 48 h, defending against secondary invaders, and providing the nematodes with nutrition (Lewis and Clarke, 2012). The nematodes molt and complete up to three generations within the host after which IJs exit the cadaver to find new hosts. Although entomopathogenic nematodes are (collectively) pathogenic to a wide variety of insect pests (e.g., some nematode species can infect more than 100 different hosts [Poinar, 1979]), successful commercialization has been limited to relatively few targets pests (Shapiro-Ilan et al., 2002a, 2014a; Grewal et al., 2005). Furthermore, less than one-sixth of the more than 90 entomopathogenic species that have been described to date have also been developed as commercial biocontrol products (Shapiro-Ilan et al., 2014a).
Discovery of new entomopathogenic nematode species can substantially expand or improve biological control efficacy. For example, discovery of Steinernema riobrave (Cabanillas, Poinar, and Raulston, 1994) resulted in improved control of D. abbreviatus (Duncan and McCoy, 1996; Shapiro-Ilan et al., 2002a), and discovery of Steinernema scapterisci (Nguyen and Smart, 1990) led to enhanced suppression of mole crickets, Scapteriscus spp. (Parkman et al., 1994). Additionally, discovery of endemic entomopathogenic nematodes can be beneficial in terms of finding strains or species that are well adapted to the local environment (for targeting pests in that geographic region). Once a new species is discovered, characterization of certain biological and ecological properties may facilitate an estimation of the newly described organism’s potential as a biocontrol agent (Koppenhöfer and Kaya, 1999; Koppenhöfer et al., 2000; Koppenhöfer and Fuzy, 2003; Shapiro-Ilan et al., 2005, 2009, 2012).
In a soil survey conducted in September 2011, a strain of the entomopathogenic nematode Heterorhabditis floridensis Nguyen, Gözel, Koppenhöfer, and Adams was isolated. The nematode population (designated “K22” strain) was isolated from a single composite soil sample taken from a hay field in Lizella, Georgia. Given that beneficial biological traits of H. floridensis had not yet been extensively characterized, the objective of this study was to estimate the relative biocontrol potential of this nematode species. We examined biocontrol traits in the newly discovered K22 strain as well as the original H. floridensis strain (Hf332) that was discovered in Florida (Nguyen et al., 2006). Moreover, given the importance of the bacterial partner in contributing to biocontrol success, and the fact that the bacterium associated with this nematode species had not yet been determined, we identified the nematode’s symbiont.
Materials and Methods
To estimate relative biocontrol potential, we conducted an array of laboratory bioassays. Laboratory assays that compare relative tolerance to environmental extremes and virulence to particular insect pests among nematode species may be used to help predict biocontrol abilities under field conditions (Shapiro-Ilan et al., 2002a, 2012). To measure environmental tolerance, we conducted a broad temperature range experiment to determine the scope in which H. floridensis can survive and infect; we also measured relative desiccation tolerance. Virulence to a variety of insect pests was determined including the small hive beetle, Aethina tumida Murray, plum curculio, Conotrachelus nenuphar (Herbst), Diaprepes root weevil, Diaprepes abbreviatus (L.), and the yellow mealworm, Tenebrio molitor L; these pests are commercial targets or potential targets for control using entomopathogenic nematodes (Shapiro-Ilan et al., 2002a, 2010, 2013; Ramos-Rodríguez et al., 2006; Ellis et al., 2010). For each trait, the H. floridensis strains were compared with other entomopathogenic nematodes species. Within the comparisons, at least one nematode species that was known to possess high capabilities for that trait (based on previous studies) was included.
Nematodes, insects, and experimental conditions:
Nematodes used in all experiments were cultured in parallel in last instar Galleria mellonella (L.) (obtained from Vanderhorst Whole Sale Inc., Saint Marys, Ohio) according to Kaya and Stock (1997). Harvested IJs were stored at 13°C for less than 3 wk before use in experiments. Nematodes included among the various experiments were Heterorhabditis bacteriophora Poinar (Oswego strain), Heterorhabditis floridensis (332 and K22 strains), Heterorhabditis indica Poinar, Karunakar & David (Hom1 strain), Steinernema carpocapsae (Weiser) (All strain), Steinernema feltiae (Filipjev) (SN strain), Steinernema glaseri (Steiner) (4-8 and Vs strains), and S. riobrave (355, 7-12, and 9-5 strains).
Aethina tumida (wandering larvae) were provided by USDA-ARS, Gainesville, FL. Conotrachelus nenuphar (4th instars) were provided by the USDA-ARS laboratory in Kearneysville, WV, Diaprepes abbreviatus (7th to 10th instar) were supplied by the Florida Department of Agriculture, Division of Plant Industry (Gainesville, FL), and T. molitor (ca., 0.8 ± 0.05 mg per insect) were obtained from Southeastern Insectaries (Perry, GA). All bioassays (environmental tolerance and virulence) were conducted in the laboratory at approximately 25°C, arranged as completely randomized designs, and each experiment was repeated once in time (in entirety) unless otherwise stated.
Environmental tolerance—desiccation:
Desiccation tolerance was determined among nematode strains based on procedures described by Solomon et al. (1999). Approximately 2,000 IJs were pipetted onto filter paper (Whatman No. 1). Excess moisture was removed from the filter paper through vacuum filtration. The filter paper was then placed into the open lid of a 60-mm petri dish, and then into a plastic desiccator (23-cm-maximum diam. × 24-cm height, Nalgene®, Rochester, NY) that was set to 97% relative humidity (RH) based on a saturated solution of K2SO4. After 24 and 48 h of incubation at 25°C, the filter paper was removed and placed in approximately 5 ml of tap water for an additional 24 h at which time percentage nematode survival was determined based on lack of movement response when probed with a dissecting needle. Each treatment (strain) contained four replicates in a randomized block design (blocked by desiccator). In addition to the H. floridensis strains, other nematodes included in the experiment were H. bacteriophora (Oswego), H. indica (Hom1), S. carpocapsae (All), S. feltiae (SN), and S. riobrave (355). In choosing nematode treatments we considered that S. carpocapsae has been reported to be more desiccation tolerant than several other entomopathogenic nematode species (Glazer, 2002), and thus one to include for comparison.
Environmental tolerance—temperature:
Initially heat tolerance was measured by determining the ability of nematodes to survive relatively high temperatures in aqueous suspension. Procedures were based on those described by Shapiro et al. (2005, 2009) Approximately 2,000 IJs in 0.2 ml were pipetted into 5 ml of tap water in a 20-ml glass scintillation vial. The vial had already been equilibrated to 37°C before the addition of nematodes. After incubation for 4 h in a water bath shaker (rpm 70) at 37°C, 0.2 ml of the suspension was transferred to a 60-mm petri dish containing 9 ml of tap water. The dishes were incubated at 25°C for 24 h at which time the percentage nematode survival was determined as described above. Nematode treatments were the same as those described for the desiccation tolerance experiment except that two strains S. glaseri (4-8 and Vs strains) were also included. In making treatment decisions, we considered that previous studies indicated relatively high levels of heat tolerance in S. riobrave (Grewal et al., 1994) and H. indica (Shapiro and McCoy, 2000).
The experiment described above, survival in aqueous suspension, indicated promising levels of heat tolerance in the H. floridensis strains. Therefore, additional experiments on the temperature tolerance were subsequently conducted. Specifically, an experiment was conducted to estimate the range of temperatures at which H. floridensis can infect. Procedures were based on those described by Shapiro-Ilan et al. (2009). The host insect, last instar G. mellonella, was exposed to nematodes in 100-mm petri dishes lined with filter paper (Whatman No. 1). Each dish contained 10 G. mellonella and received 2,000 IJs in 1-ml tap water. The dishes were immediately placed in incubators at 10°C, 13°C, 17°C, 25°C, 30°C, or 35°C. Insect mortality was checked daily and the total percentage of dead G. mellonella was recorded daily for 7 d; the average percentage of patent infections was also recorded (Shapiro-Ilan et al., 2002b). A patent infection is defined as an overt infection exhibiting distinctive signs and symptoms of the particular disease (Lacey and Brooks, 1997; Onstad et al., 2006). There were four replicate dishes in each temperature and an equal set of no-nematode control dishes (water only) was also included at each temperature. Given that S. riobrave is known to be on the extreme end of heat tolerance, but also with a relatively wide temperature niche breadth (Grewal et al., 1994), S. riobrave (355) was chosen for comparison with the two H. floridensis strains at 10°C to 35°C. Subsequently, because all nematodes infected at temperatures up to 35°C, an additional experiment was added to test infection at 37°C and 39°C; this latter experiment was conducted in an identical manner except mortality was only recorded at 24 and 48 h, and included the two H. floridensis strains as well as three S. riobrave strains (355, 7-12, and 9-5).
Virulence assays:
All virulence assays contained four replicates of 10 insects per treatment and an equal number of nontreated (water-only) control insects. Additionally, all virulence experiments included the following nematode treatments: the two H. floridensis strains (332 and K22), H. bacteriophora (Oswego), H. indica (Hom1), S. carpocapsae (All), S. feltiae (SN), two S. glaseri strains (4-8 and Vs), and S. riobrave (355). For the sake of comparison, we considered that H. indica (HOM1) and S. riobrave (355) have shown high levels of virulence to A. tumida, D. abbreviatus, and T. molitor in previous studies (Shapiro and McCoy 2000; Ellis et al., 2010; Shapiro-Ilan et al., 2009, 2010), and S. riobrave (355) and S. feltiae (SN) were highly virulent to C. nenuphar (Shapiro-Ilan et al., 2011).
Virulence to A. tumida was assessed based on procedures described by Shapiro-Ilan et al. (2010). Arenas consisted of plastic containers (11-cm top diam., 8-cm bottom diam., 8-cm depth, with approximate 0.5-mm holes on bottom) containing 10 A. tumida each. The containers were filled with soil from the USDA-ARS pecan orchard (Byron, GA), and contained one larva each. The soil was a loamy sand (84% sand, 10% silt, 6% clay; 2.8% organic matter; pH 6.1). Nematodes were pipetted onto the soil surface of each cup in 0.5 ml of water so that the final moisture was standardized at field capacity (14%). Nematodes were applied at a rate of 2,375 IJs per container (25 IJs per cm2). Percentage insect mortality was determined 14 d after incubation.
Nematode virulence to C. nenuphar larvae was assessed based on procedures described by Shapiro-Ilan et al. (2011). Experiments were conducted in plastic cups (Bioserv, Inc., Frenchtown, NJ). The cups (3- to 4-cm i.d., 3.5-cm deep) were filled with 20 g of soil (described above). Approximately 500 IJs were applied to each cup in 0.5-ml tap water; before addition of nematodes, tap water was added to the soil so that final moisture level in each cup was at field capacity (14%). After application of nematodes and water, one weevil larva was added to each cup. Percentage C. nenuphar mortality was determined 14-d posttreatment.
Virulence assays with D. abbreviatus (based on Shapiro-Ilan et al., 2005, 2009) were conducted in an identical same manner as described for C. nenuphar, except that the experimental units were filled with sand, which was standardized at approximately 8% moisture (field capacity).
Virulence to T. molitor was determined in 60-mm petri dishes lined with filter paper (Whatman No. 1) (Kaya and Stock, 1997; Shapiro-Ilan et al., 2009). Approximately 500 IJs were applied in 350-ul tap water. Percentage larval mortality was evaluated 2 d after application.
Identification and phylogenetic characterization of the H. floridensis and its bacterial symbiont:
H. floridensis isolates were identified based on ribosomal DNA from the internally transcribed spacer regions (ITSrDNA) of isolates K21, K22, K25, and K26 was extracted, PCR amplified and sequenced as described previously (Shapiro-Ilan et al., 2009).
To obtain the bacterial symbiont, H. floridensis (332 and K22) IJs were surface sterilized by incubating 30 min in a solution of 0.125% (w/v) methylbenzathonium chloride with gentle rocking, and then a 15-min incubation in 3% (v/v) hydrogen peroxide. The IJs were rinsed three times with sterile tap water and macerated to release their symbiotic bacteria. Photorhabdus colonies were selected based on morphological growth characteristics exhibited when cultured on Tergitol-7 Agar (BBL, Becton Dickinson and Company, Franklin Lakes, NJ) supplemented with 0.004% triphenyltetrazolium chloride. Colonies from H. floridensis 332 and K22 were noted USFL332 and K22, respectively.
Genomic DNA of bacterial strains USFL332 and K22 were extracted using a modified method of Ausubel et al. (1994). Cells of the bacterial symbiont were incubated in 600-μl cell lysis buffer (10 mM Tris-HCl, pH 8.0; 1 mM EDTA; 0.5% (w/v) sodium dodecyl sulfate; 30-μg proteinase K) for 1 h at 37°C. The DNA was separated from the cell lysate by incubation for 10 min with 150 μl of 5 M sodium chloride and 80 μl CTAB/NaCl solution (10% (w/v) cetyl trimethyl ammonium bromide in 0.7 M sodium chloride) at 65°C followed by an extraction with each phenol/chloroform/isoamyl alcohol (25:24:1) and chloroform/isoamyl alcohol (24:1). The DNA was precipitated with 0.6 volume isopropanol, washed twice with 70% (v/v) ethanol and dissolved in Tris-EDTA buffer (TE) (10 mM Tris-HCl, pH 8.0; 1 mM EDTA).
The USFL332 and K22 bacterial gyrB amplicons were ligated into a T-overhang vector using the pGEM®-T Vector System (Promega, Madison, WI), which was then transformed into MAX Efficiency® DH5α™ Competent Cells (Invitrogen, Carlsbad, CA). The cells were transferred to Luria Bertani (LB) agar plates containing ampicillin, isopropyl-beta-D-1-thiogalactopyranoside (IPTG) and X-gal and incubated overnight at 37°C. Colonies were selected and grown overnight in LB broth containing ampicillin, and the plasmids purified using the QIAprep Spin Miniprep Kit (Qiagen, Inc., Valencia, CA). The gyrB inserts were sequenced using T7 and Sp6 promoter primers at the University of Florida ICBR DNA Sequencing Facility, Gainesville, FL, with an ABI 3130 automated DNA sequencer (Life Technologies, Grand Island, New York).
The gyrB sequences of the Photorhabdus sp. type strains, Xenorhabdus bovienii T228T, Xenorhabdus nematophila ATCC19061T and Proteus mirabilis HI4320 were collected from GenBank. Xenorhabdus and Proteus were used as closely related outgroups. Sequences were aligned using MUSCLE (Edgar, 2004). Maximum-likelihood analysis (PhyML 3.0) was carried out with the general time-reversible (GTR) model of substitution with gamma-distributed rate heterogeneity and a proportion of invariant sites determined by jModelTest to give the best fit according the Akaike information criterion (Posada and Crandall, 1998). MUSCLE, PhyML, and bootstrap values (Felsenstein, 1988) were obtained from the phylogeny.fr platform (Dereeper et al., 2008).
Statistical analyses:
Treatment effects in all bioassay experiments were analyzed with two-way analysis of variance (ANOVA; Proc GLM), with the exception of heat tolerance assays measuring G. mellonella mortality and infection at different temperatures (10°C to 39°C), which were analyzed using repeated measures (Proc Mixed) with SAS 9.1 software (SAS Institute, Cary, NC). If the ANOVA detected a significant difference (P ≤ 0.05) then treatment differences were elucidated through the Student-Newman-Keuls (SNK) test (SAS software). For each experiment, data from both trials (repeated in time) were combined, and variation among trials was accounted for as a block effect. Percentage data were arcsine transformed before analysis (Southwood, 1978; Steel and Torrie, 1980, SAS, 2001); nontransformed means are presented in figures.
Results
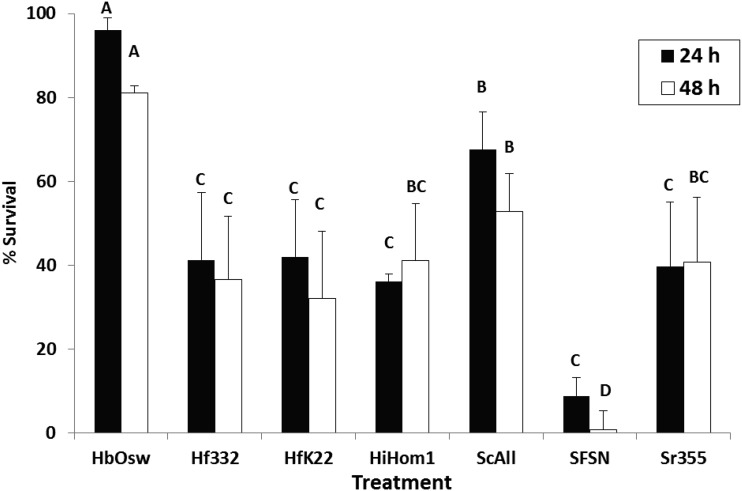
Differences in desiccation tolerance, as indicated by nematode survival, were observed among nematode treatments after 24 h (F = 14.41; df = 6, 44; P < 0.0001) and 48 h (F = 201.89; df = 6, 44; P < 0.0001) exposure to 97% RH (Fig. 1). Heterorhabditis bacteriophora (Oswego) exhibited the highest desiccation tolerance followed by S. carpocapsae (All) and the lowest tolerance was observed in S. feltiae (SN) (Fig 1). The other treatments including the H. floridensis strains exhibited intermediate levels of desiccation tolerance (Fig. 1).
Fig. 1.
Mean percentage mortality (± SE) of infective juvenile nematodes following exposure to 97% RH for 24 or 48 h. Treatments included Heterorhabditis bacteriophora (Oswego strain), H. floridensis (332 and K22 strains), H. indica (Hom1 strain), Steinernema carpocapsae (All strain), S. feltiae (SN strain), and S. riobrave (355 strain). Different letters above bars indicate statistical differences (SNK test, α = 0.05).
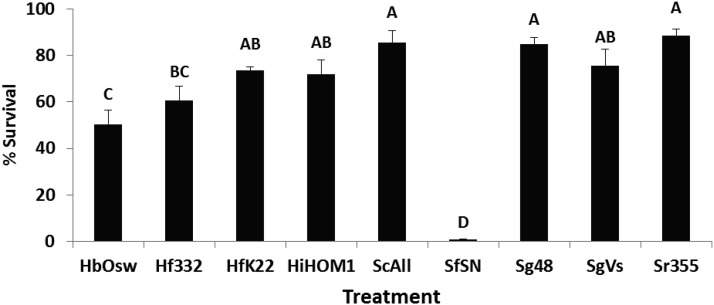
Incubation in aqueous suspension at 37°C indicated survival differences among nematode treatments (F = 38.32; df = 8, 44; P < 0.0001) (Fig. 2). Steinernema carpocapsae (All), S. glaseri, and S. riobrave (355), exhibited higher survival than S. feltiae (SN), H. floridensis (332), and H. bacteriophora (Oswego) but were not different from H. floridensis (K22), H. indica (Hom1), and S. glaseri (Vs). S. feltiae (SN) had the lowest survival followed by H. bacteriophora (Oswego) (Fig. 2).
Fig. 2.
Mean percentage mortality (± SE) of infective juvenile nematodes following exposure to 37°C for 4 h. Treatments included Heterorhabditis bacteriophora (Oswego strain), H. floridensis (332 and K22 strains), H. indica (Hom1 strain), Steinernema carpocapsae (All strain), S. feltiae (SN strain), S. glaseri (4-8 and Vs strains), and S. riobrave (355 strain). Different letters above bars indicate statistical differences (SNK test, α = 0.05).
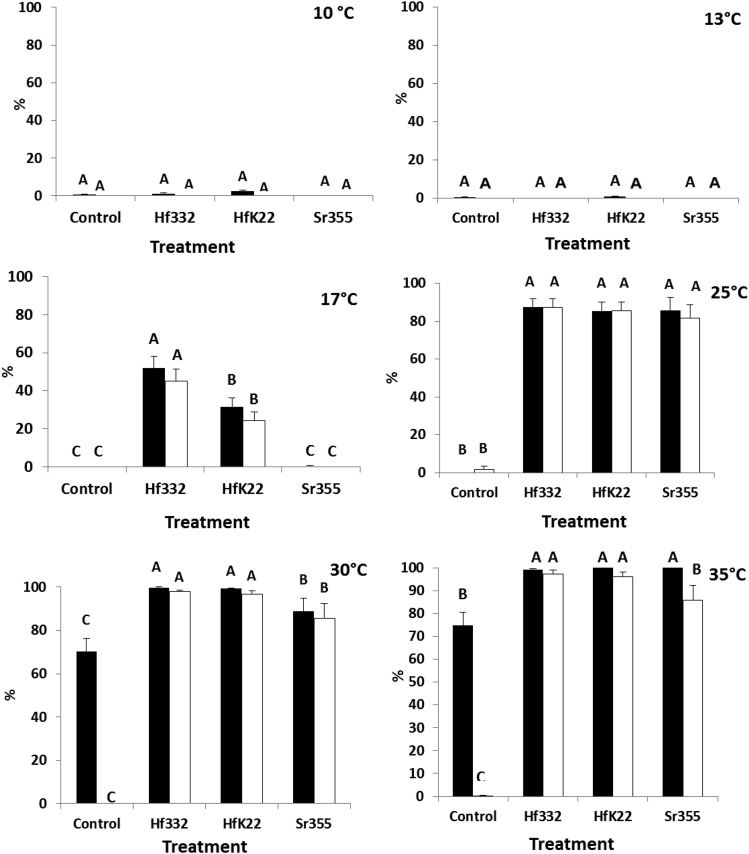
At various temperatures between 10°C and 35°C, the ability to infect G. mellonella differed among H. floridensis strains and S. riobrave (355) (Fig. 3). At 10°C and 13°C, no differences in percentage mortality were observed relative to the control (F = 1.86; df = 3, 9; P = 0.2075 for 10°C and F = 0.78; df = 3, 9; P = 0.5354 for 13°C), and patent infections were not observed at these temperatures (Fig. 3). At 17°C, both H. floridensis strains (332 and K22) caused higher mortality and infections than S. riobrave (355) and the control (which were not different from each other); mortality caused by the 332 strain was higher than that of the K22 strain (F = 43.92; df = 3, 9; P < 0.0001 for mortality and F = 44.93; df = 3, 9; P < 0.0001 for infection) (Fig. 3). At 25°C, no differences among nematode treatments were observed, and all treatments caused higher mortality than the control (F = 507.35; df = 3, 9; P < 0.0001 for mortality and F = 360.81; df = 3, 9; P < 0.0001 for infection) (Fig. 3). At 30°C, all treatments caused higher mortality and infection than the control, yet mortality and percentage of patent infection was higher in the H. floridensis treatments than S. riobrave (355) (F = 80.95; df = 3, 9; P < 0.0001 for mortality, and F = 3318.09; df = 3, 9; P < 0.0001 for infection) (Fig. 3). At 35°C, the H. floridensis strains caused a higher percentage patent infection than S. riobrave (355), yet the nematode treatments did not differ in percentage mortality, and all treatments caused higher mortality than the control (F = 200.85; df = 3, 9; P < 0.0001 for mortality and F = 1374.33; df = 3, 9; P < 0.0001 for infection) (Fig. 3).
Fig. 3.
Mean (± SE) percentage mortality (black bars) and percentage of patent infections (white bars) in Galleria mellonella larvae following exposure to entomopathogenic nematodes at various temperatures. Mortality and infection was recorded daily up to 7 d posttreatment. Treatments included Heterorhabditis floridensis (332 and K22 strains), S. riobrave (355 strain), and a nontreated control. Different letters above bars indicate statistical differences (SNK test, α = 0.05).
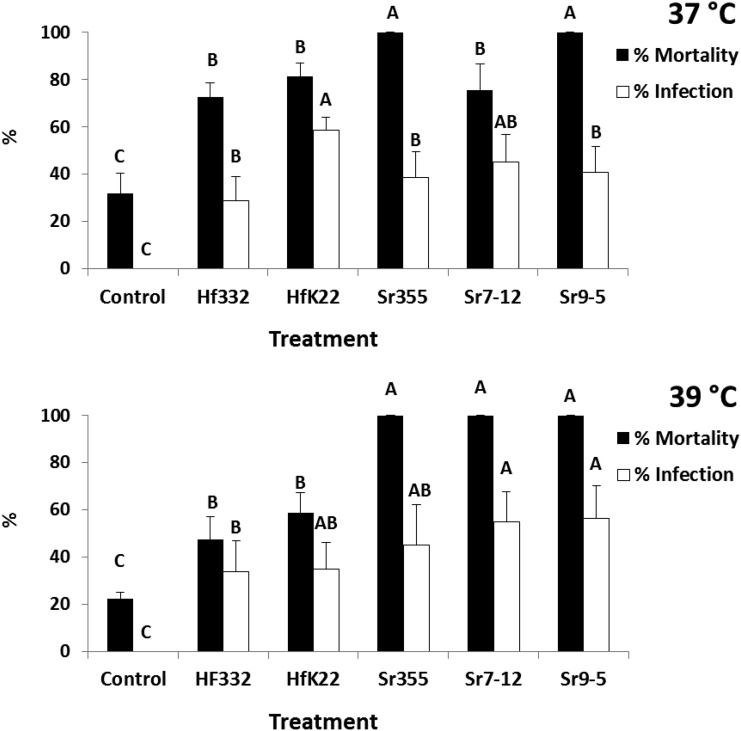
At 37°C and 39°C, both H. floridensis strains (332 and K22) as well as three strains of S. riobrave (355, 7-12, and 9-5) caused significant mortality and infection in G. mellonella (for mortality F = 19.24; df = 5, 15; P < 0.0001 at 37°C, and F = 41.86; df = 5, 15; P < 0.0001 at 39°C; for infection F = 16.65; df = 5, 15; P < 0.0001 at 37°C, and F = 17.84; df = 5, 15; P < 0.0001 at 39°C) (Fig. 4). Mortality caused by the S. riobrave strains was higher than H. floridensis strains at both temperatures with exception that S. riobrave (9-5) was not different at 37°C (Fig 4). Percentage of patent infections caused by H. floridensis (K22) at 37°C was higher than all other nematodes except S. riobrave (7-12) (Fig. 4). At 39°C, percentage infection in the H. floridensis (332) treatment was reduced relative to the 7-12 and 9-5 strains of S. riobrave (Fig. 4).
Fig. 4.
Mean (± SE) percentage mortality (black bars) and percentage of patent infections (white bars) in Galleria mellonella larvae after 48 h following exposure to entomopathogenic nematodes at 37°C and 39°C. Treatments included Heterorhabditis floridensis (332 and K22 strains), S. riobrave (355, 7-12, and 9-5 strains), and a nontreated control. Different letters above bars indicate statistical differences (SNK test, α = 0.05).
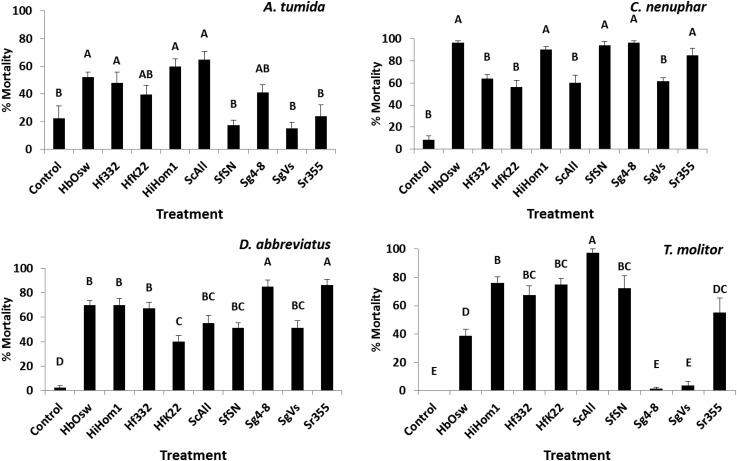
Virulence among nematode treatments varied among insect hosts (Fig. 5). Only four nematode treatments were pathogenic to A. tumida relative to the control: H. bacteriophora (Oswego), H. floridensis (332), H. indica (Hom1), and S. carpocapsae (All) (F = 6.48; df = 9, 69; P < 0.0001) (Fig. 5). Five nematode treatments cause higher mortality than the control in Conotrachelus nenuphar. H. bacteriophora (Oswego), H. indica (Hom1), S. riobrave (355), S. feltiae (SN), and S. glaseri (4-8) (F = 28.04; df = 9, 69; P < 0.0001) (Fig. 5). All nematodes caused greater mortality than the control in D. abbreviatus with S. riobrave (355) and S. glaseri exhibiting the highest virulence; H. floridensis (K22) was in the lowest virulence grouping (F = 24.85; df = 9, 69; P < 0.0001) (Fig. 5). All nematodes were pathogenic to T. molitor except S. glaseri (4-8 and Vs); S. carpocapsae was the most virulent and H. floridensis strains exhibited intermediate virulence (F = 33.94; df = 9, 69; P < 0.0001) (Fig. 5).
Fig. 5.
Mean (± SE) percentage mortality of Aethina tumida, Conotrachelus nenuphar, Diaprepes abbreviatus, and Tenebrio molitor larvae following exposure to entomopathogenic nematodes. Treatments included Heterorhabditis bacteriophora (Oswego strain), H. indica (Hom1 strain), Heterorhabditis floridensis (332 and K22 strains), Steinernema carpocapsae (All strain), S. feltiae (SN strain), S. glaseri (4-8 and Vs strains), S. riobrave (355 strain), and a nontreated control. Different letters above bars indicate statistical differences (SNK test, α = 0.05).
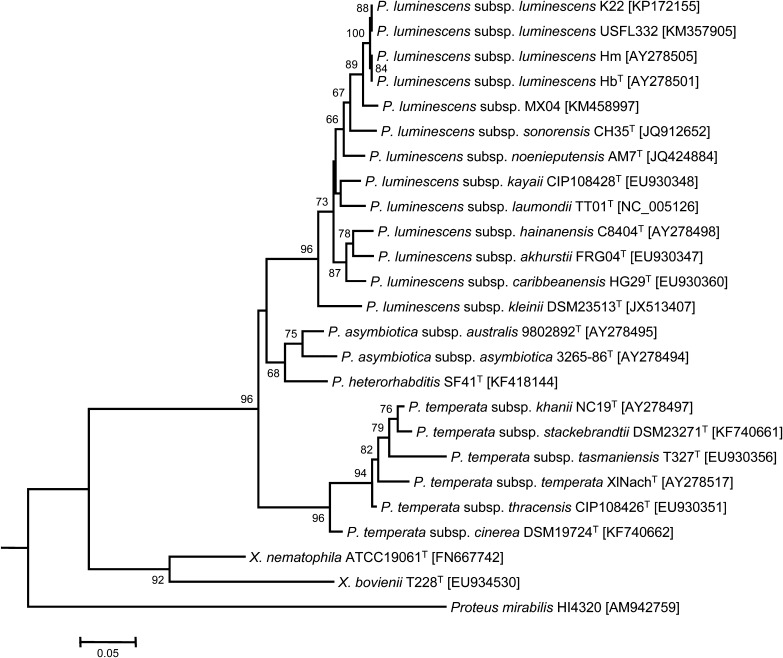
The phylogenetic analysis (Fig. 6) shows that the gyrB sequences of bacterial strains USFL332 and K22 are grouped with those of P. luminescens subsp. luminescens strains HbT (Poinar et al., 1977) and Hm (Bleakley and Nealson, 1988). The four gyrB sequences are highly similar (>99.6% identity on 703 aligned nucleotide positions). The sequences only differed in one nucleotide. So far, only these two latter P. luminescens subsp. luminescens strains were described and their host species were not determined with certainty (Akhurst et al., 2004). The phylogenetic position of H. floridensis using ITS sequences shows that this species shares a common ancestor with H. sonorensis and H. mexicana for which the symbiotic bacteria were isolated and characterized. In the same way as for their host, the respective symbiotic bacterial strains share also a common ancestor suggesting coevolutionary events (Maneesakorn et al., 2011). Amongst the genus Heterorhabditis, nematode populations belonging to the same species can carry different Photorhabdus species and subspecies. In the case of H. floridensis, both populations in this study are associated with only P. luminescens subsp. luminescens. The ITS rDNA sequences for the H. floridensis strains (K22) was identical to H. floridensis FL-332 (GenBank accession DQ372922.1).
Fig. 6.
Maximum-likelihood phylogenetic tree showing the position of bacterial strains USFL332 and K22 within the genus Photorhabdus. The analysis is based on 703 aligned nucleotide positions of the gyrB sequences. Xenorhabdus and Proteus are used as closely related outgroup taxa. Sequences of species type strains are indicated by T. GenBank accession numbers are in brackets. Bootstrap values of more than 50% (from 100 replicates) are indicated at the nodes. Bar: 5% divergence.
Discussion
Our results indicate temperature tolerance in H. floridensis (332 and K22 strains) covers a wide range. Temperature tolerance in the H. floridensis strains was compared with a number of nematodes including S. riobrave, which is known to be a heat-tolerant species (Grewal et al., 1994; Shapiro and McCoy 2000; Shapiro-Ilan et al., 2009). Grewal et al. (1994) measured temperature tolerance in 12 entomopathogenic nematode strains and reported that only S. riobrave was able to infect G. mellonella at 39°C. In our study, similar to S. riobrave (355), the H. floridensis strains were capable of infecting a host up to 39°C, and the mortality level and or proportion of patent infections produced by the H. floridensis strains was higher than S. riobrave at 30°C and 35°C. Relative to other entomopathogenic nematode species, particularly other heterorhabditids, the observed level of H. floridensis infectivity at higher temperatures is unusual (Grewal et al., 1994; Ganguly and Singh, 2000; Shapiro-Ilan et al., 2009; Mukuka et al., 2010; Zadji et al., 2014). These data are, however, in agreement with those obtained on the capability of the symbiotic bacteria associated to these nematode species to cultivate in nutrient broth at 38°C to 39°C for P. luminescens subsp luminescens (Fischer-Le Saux et al., 1999) and 39°C to 40°C for X. cabanillasii (Tailliez et al., 2006). In addition to infecting at higher temperatures, the H. floridensis strains also infected at 17°C, whereas S. riobrave did not. Thus, H. floridensis appears to be quite heat tolerant and also possesses a modest level of cold tolerance.
Beyond temperature tolerance, H. floridensis (332 and K22) exhibited mostly intermediate levels of beneficial traits relative to other nematode treatments. In desiccation tolerance, as well as virulence to C. nenuphar, D. abbreviatus, and T. molitor, other nematode strains displayed superior levels of each trait compared with H. floridensis (332 and K22). One exception was virulence to A. tumida, in which H. floridensis strains were in the highest statistical grouping.
Regarding comparisons among previously characterized species, most of our results, though not all, are consistent with earlier studies. For example, similar to previous studies, we observed high levels of heat tolerance in S. riobrave and a lack of heat tolerance in S. feltiae (Grewal et al., 1994; Shapiro and McCoy, 2000; Shapiro-Ilan et al., 2009), and superior desiccation tolerance in S. carpocapsae (Shapiro-Ilan et al., 2009). Also consistent with previous studies, we observed S. riobrave (355), H. indica (Hom1), and S. feltiae (SN) to be highly virulent to C. nenuphar, and S. riobrave to be highly virulent to D. abbreviatus (Shapiro and McCoy, 2000; Shapiro-Ilan et al., 2011). Moreover, results of the present study are consistent with that of Shapiro-Ilan et al. (2009), in which a limited characterization of the 332 strain of H. floridensis was made regarding virulence to D. abbreviatus, and T. molitor (A. tumida and C. nenuphar were not tested in the previous study nor was environmental tolerance assessed).
In contrast to our results, Grewal et al., (1994) observed S. riobrave infection at 10°C and 15°C. Perhaps this discrepancy was because of the use of different strains (the study by Grewal et al. used the RGV strain). Also, unlike our observations, S. riobrave was reported to be virulent to A. tumida in a previous study by Ellis et al. (2010); conceivably, the discrepancy is because of differing assay conditions.
Incidentally, given that novel comparisons among nematode species and strains were made, our results revealed several unique findings in addition to those concerning the H. floridensis strains. For instance, we discovered that H. bacteriophora (Oswego) is highly desiccation tolerant at 97% RH; however, the same strain did not display exceptional tolerance to 85% RH in a previous study (Shapiro-Ilan et al., 2014b). Apparently, relative desiccation tolerance varies depending on the stress level. Also we observed (perhaps not surprising) that the 9-5 strain of S. riobrave is highly heat tolerant, which is consistent with previous observations for the other strains tested (Shapiro-Ilan et al., 2014b). Finally, we discovered that the 4-8 strain of S. glaseri possesses high levels of several beneficial traits; the strain was in the highest grouping for heat tolerance (in aqueous suspension) and in all virulence tests (i.e., to A. tumida, C. nenuphar, and D. abbreviatus) except in the assay with T. molitor.
In summary, we found H. floridensis strains 332 and K22 to be extremely heat tolerant and also possess modest cold tolerance. In most other beneficial traits that were measured, the two H. floridensis strains tended to exhibit only moderate levels relative to other nematodes. Nonetheless, the broad temperature tolerance of H. floridensis could be advantageous in biocontrol applications, e.g., in systems that experience rapid temperature changes. We also discovered significant biocontrol potential in S. glaseri (4-8). Field and greenhouse trials will be required to determine the efficacy of these strains under natural conditions.
Literature Cited
- Akhurst RJ, Boemare NE, Janssen PH, Peel MM, Alfredson DA, Beard CE. Taxonomy of Australian clinical isolates of the genus Photorhabdus and proposal of subspecies Photorhabdus asymbiotica subsp. asymbiotica subsp. nov. and Photorhabdus asymbiotica subsp. australis subsp. nov. International Journal of Systematic and Evolutionary Microbiology. 2004;54:1301–1310. doi: 10.1099/ijs.0.03005-0. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. 1994. Current protocols in molecular biology, vol. 1. New York: John Wiley and Sons. [Google Scholar]
- Bleakley B, Nealson KH. Characterization of primary and secondary forms of Xenorhabdus luminescens strain Hm. FEMS Microbiology and Ecology. 1988;53:241–250. [Google Scholar]
- Cabanillas HE, Poinar GO, Jr, Raulston JR. Steïnernema riobravis n. sp. (Rhabditida: Steinernematidae) from Texas. Fundamental and Applied Nematology. 1994;17:123–131. [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research. 2008;36:W465–9. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, McCoy CW. Vertical distribution in soil, persistence, and efficacy against citrus root weevil (Coleoptera: Curculionidae) of two species of entomogenous nematodes (Rhabditida: Steinernematidae: Heterorhabditidae) Environmental Entomology. 1996;25:174–178. [Google Scholar]
- Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JD, Spiewok S, Delaplane KS, Buchholz S, Neumann P. Susceptibility of Aethina tumida (Coleoptera: Nitidulidae) larvae and pupae to entomopathogenic nematodes. Journal of Economic Entomology. 2010;103:1–9. doi: 10.1603/ec08384. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: Inference and reliability. Annual Review of Genetics. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Fischer-Le Saux M, Viallard V, Brunel B, Normand P, Boemare NE. Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov., P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperata subsp. temperata subsp. nov. and P. asymbiotica sp. nov. International Journal of Systematic Bacteriology. 1999;49:1645–1656. doi: 10.1099/00207713-49-4-1645. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Singh LK. Optimum thermal requirements for infectivity and development of an indigenous entomopathogenic nematode, Steinernema thermophilum. Indian Journal of Nematology. 2000;31:148–152. [Google Scholar]
- Glazer I. 2002. Survival biology. Pp. 169–188 in R. Gaugler, ed. Entomopathogenic nematology. New York: CABI Publishing.
- Grewal PS, Selvan S, Gaugler R. Thermal adaptation of entomopathogenic nematodes—niche breadth for infection, establishment and reproduction. Journal of Thermal Biology. 1994;19:245–253. [Google Scholar]
- Grewal PS, Ehlers RU, Shapiro-Ilan DI, editors. 2005. Nematodes as biocontrol agents. New York: CABI Publishing. [Google Scholar]
- Kaya HK, Stock SP. 1997. Techniques in insect nematology. Pp. 281–324 in L. A. Lacey, ed. Manual of techniques in insect pathology. San Diego, CA: Academic Press.
- Koppenhöfer AM, Fuzy EM. Ecological characterization of Steinernema scarabaei, a scarab-adapted entomopathogenic nematode from New Jersey. Journal of Invertebrate Pathology. 2003;83:139–148. doi: 10.1016/s0022-2011(03)00056-9. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer AM, Kaya HK. Ecological characterization of Steinernema rarum. Journal of Invertebrate Pathology. 1999;73:120–128. doi: 10.1006/jipa.1998.4822. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer AM, Ganguly S, Kaya HK. Ecological characterization of Steinernema monticolum, a cold-adapted nematode from Korea. Nematology. 2000;2:407–416. [Google Scholar]
- Lacey LA, Brooks WM. 1997. Initial handling and diagnosis of diseased insects. Pp. 1–15 in L. A. Lacey, ed. Manual of techniques in insect pathology. San Diego, CA: Academic Press.
- Lewis EE, Clarke DJ. 2012. Nematode parasites and entomopathogens. Pp. 395–424 in F. E. Vega and H. K. Kaya, eds. Insect pathology, 2nd ed. Amsterdam: Elsevier.
- Maneesakorn P, An RS, Daneshvar H, Taylor K, Bai X, Adams BJ, Grewal PS, Chandrapatya A. Phylogenetic and cophylogenetic relationships of entomopathogenic nematodes (Heterorhabditis: Rhabditida) and their symbiotic bacteria (Photorhabdus: Enterobacteriaceae) Molecular Phylogenetics and Evolution. 2011;59:271–280. doi: 10.1016/j.ympev.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Mukuka J, Strauch O, Hoppe C, Ehlers R-U. Improvement of heat and desiccation tolerance in Heterorhabditis bacteriophora through cross-breeding of tolerant strains and successive genetic selection. BioControl. 2010;55:511–521. [Google Scholar]
- Nguyen KB, Smart GC. Steinernema scapterisci n. sp. (Rhabditida: Steinernematidae) Journal of Nematology. 1990;22:187–199. [PMC free article] [PubMed] [Google Scholar]
- Nguyen KB, Gozel U, Koppenhöfer HS, Adams BJ. Heterorhabditis floridensis n. sp. (Rhabditida: Heterorhabditidae) from Florida. Zootaxa. 2006;1177:1–19. [Google Scholar]
- Onstad DW, Fuxa JR, Humber RA, Oestergaard J, Shapiro-Ilan DI, Gouli VV, Anderson RS, Andreadis TG, Lacey LA. 2006. An abridged glossary of terms used in invertebrate pathology, 3rd ed. Society for Invertebrate Pathology. http://www.sipweb.org/glossary.
- Parkman JP, Frank JH, Nguyen KB, Smart GC., Jr Inoculative release of Steinernema scapterisci (Rhabditida: Steinernematidae) to suppress pest mole crickets (Orthoptera: Gryllotapidae) on golf courses. Environmental Entomology. 1994;23:1331–1337. [Google Scholar]
- Poinar GO., Jr 1979. Nematodes for biological control of insects. Boca Raton, FL: CRC Press.
- Poinar GO., Jr 1990. Biology and taxonomy of Steinernematidae and Heterorhabditidae. Pp. 23–62 in R. Gaugler and H. K. Kaya, eds. Entomopathogenic nematodes in biological control. Boca Raton, FL: CRC Press.
- Poinar GO, Thomas GM, Hess R. Characteristics of the specific bacterium associated with Heterorhabditis bacteriophora (Heterorhabditidae: Rhabditida) Nematologica. 1977;23:97–102. [Google Scholar]
- Posada D, Crandall KE. Modeltest: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Ramos-Rodriguez O, Campbell JF, Ramaswamy SB. Pathogenicity of three species of entomopathogenic nematodes. Journal of Stored Products Research. 2006;42:241–252. [Google Scholar]
- Shapiro DI, McCoy CW. Virulence of entomopathogenic nematodes to Diaprepes abbreviatus (Coleoptera: Curculionidae) in the laboratory. Journal of Economic Entomology. 2000;93:1090–1095. doi: 10.1603/0022-0493-93.4.1090. [DOI] [PubMed] [Google Scholar]
- Shapiro DI, Cate JR, Pena J, Hunsberger A, McCoy CW. Effects of temperature and host age on suppression of Diaprepes abbreviatus (Coleoptera: Curculionidae) by entomopathogenic nematodes. Journal of Economic Entomology. 1999;92:1086–1092. [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Koppenhöfer AM. 2002a. Factors affecting commercial success: Case studies in cotton, turf and citrus. Pp. 333–356 in R. Gaugler, ed. Entomopathogenic nematology. Wallingford, UK: CABI Publishing.
- Shapiro-Ilan DI, Gaugler R, Tedders WL, Brown I, Lewis EE. Optimization of inoculation for in vivo production of entomopathogenic nematodes. Journal of Nematology. 2002b;34:343–350. [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Stuart RJ, McCoy CW. Characterization of biological control traits in the entomopathogenic nematode Heterorhabditis mexicana (MX4 strain) Biological Control. 2005;32:97–103. [Google Scholar]
- Shapiro-Ilan DI, Mbata GN, Nguyen KB, Peat SM, Blackburn D, Adams BJ. Characterization of biocontrol traits in the entomopathogenic nematode Heterorhabditis georgiana (Kesha strain), and phylogenetic analysis of the nematode’s symbiotic bacteria. Biological Control. 2009;51:377–387. [Google Scholar]
- Shapiro-Ilan DI, Morales-Ramos JA, Rojas MG, Tedders WL. Effects of a novel entomopathogenic nematode–infected host formulation on cadaver integrity, nematode yield, and suppression of Diaprepes abbreviatus and Aethina tumida under controlled conditions. Journal of Invertebrate Pathology. 2010;103:103–108. doi: 10.1016/j.jip.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Leskey TC, Wright SE. Virulence of entomopathogenic nematodes to plum curculio, Conotrachelus nenuphar: Effects of strain, temperature, and soil type. Journal of Nematology. 2011;43:187–195. [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Bruck DJ, Lacey LA. 2012. Principles of Epizootiology and Microbial Control. Pp. 29–72 in F. E. Vega and H. K. Kaya, eds. Insect pathology, 2nd ed. Amsterdam: Elsevier.
- Shapiro-Ilan DI, Wright SE, Tuttle AF, Cooley DR, Leskey TC. Using entomopathogenic nematodes for biological control of plum curculio, Conotrachelus nenuphar: Effects of irrigation and species in apple orchards. Biological Control. 2013;67:123–129. [Google Scholar]
- Shapiro-Ilan DI, Han R, Qiu X. 2014a. Production of entomopathogenic nematodes. Pp. 321–356 in J. Morales-Ramos, G. Rojas, and D. I. Shapiro-Ilan, eds. Mass production of beneficial organisms: Invertebrates and entomopathogens. Amsterdam: Academic Press.
- Shapiro-Ilan DI, Brown I, Lewis EE. Freezing and desiccation tolerance in entomopathogenic nematodes: Diversity and correlation of traits. Journal of Nematology. 2014b;46:27–34. [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Paperna I, Glazer I. Desiccation survival of the entomopathogenic nematode Steinernema feltiae: Induction of anhydrobiosis. Nematology. 1999;1:61–68. [Google Scholar]
- Southwood TRE. 1978. Ecological methods: with particular reference to the study of insect populations. London: Chapman and Hall.
- Steel RGD, Torrie JH. 1980. Principles and procedures of statistics. New York: McGraw-Hill.
- Tailliez P, Pagès S, Ginibre N, Boemare N. New insight into diversity in the genus Xenorhabdus, including the description of ten novel species. International Journal of Systematic and Evolutionary Microbiology. 2006;56:2805–2818. doi: 10.1099/ijs.0.64287-0. [DOI] [PubMed] [Google Scholar]
- Zadji L, Baimey H, Afouda L, Moens M, Decraemer W. Characterization of biocontrol traits of heterorhabditid entomopathogenic nematode isolates from South Benin targeting the termite pest Macrotermes bellicosus. BioControl. 2014;59:333–344. [Google Scholar]