Abstract
Rotylenchulus reniformis resistant LONREN-1×FM966 breeding lines developed at Auburn University have demonstrated that the nematode resistance is accompanied by severe stunting, limited growth, and low yields. The objectives of this study were to evaluate the effects of applying nematicides to selected LONREN breeding lines on R. reniformis nematode populations, plant stunting, and yield. Four resistant breeding lines from the LONREN-1×FM966 cross, one susceptible line from the LONREN-1×FM966 cross, as well as LONREN-1, BARBREN-713, and the susceptible cultivar DP393 were evaluated with and without nematicides in the presence of R. reniformis. In the greenhouse, nematicides increased plant height across all genotypes compared with no nematicide. Rotylenchulus reniformis populations were 50% lower in the resistant lines compared with the susceptible lines at 45 days after planting (DAP). In microplot and field trials, the phenotypic stunting of all genotypes was reduced by aldicarb with increases in plant heights at 30 and 75 DAP. Increases in yields were evident across all genotypes treated with aldicarb. In all three trial environments, BARBREN-713 outperformed the LONREN-derived lines as well as ‘DP393’ in seed cotton yields, while having significantly lower R. reniformis egg densities than the susceptible genotypes.
Keywords: cotton, nematicides, reniform nematode, resistance, tolerance
The reniform nematode (Rotylenchulus reniformis Linford & Oliveira) is considered among the most damaging pests to upland cotton (Gossypium hirsutum L.) grown in Alabama (Usery et al., 2005). It causes significant yield losses in many other southeastern states of the United States (Starr et al., 2007). Several large-scale screening efforts resulted only in identifying weak to moderate resistance to R. reniformis in the G. hirsutum species (Starr et al., 2007; Weaver et al., 2007). As a result, resistant cultivars to this pathogen have not been available to growers and researchers are looking into wild cotton relatives to establish a source (Usery et al., 2005). In recent years, breeding efforts have turned to LONREN and BARBREN germplasm lines to use in crosses with upland cotton cultivars. The LONREN genetic material was developed from an exotic cotton species, Gossypium longicalyx Hutch and Lee, that is resistant to reniform nematodes. Yik and Birchfield (1984) tested four different G. longicalyx accessions for resistance and reported that all females that entered the root of these accessions never developed to the kidney shape stage and no eggs were produced by the females. This type of resistance has been described as the absence of egg production once the female has established a feeding site within the roots of G. longicalyx (Agudelo et al., 2005). Robinson et al. (2007) were able to introgress resistance to reniform nematode from G. longicalyx into upland cotton G. hirsutum. Resistance in BARBREN is derived from an exotic accession of the cotton species Gossypium barbadense L. Yik and Birchfield (1984) reported that out of six G. barbadense germplasm lines tested, the line ‘Texas 110’ was classified as highly resistant and supported only 8% of R. reniformis egg production found on the susceptible check cultivar Deltapine 16. Robinson and Percival (1997) reported that two G. barbadense accessions, TX-1347 and TX-1348 (earlier classified as G. hirsutum accessions), supported significantly fewer R. reniformis eggs than the susceptible check cultivar Deltapine 16. Later studies by Robinson et al. (2004) found that five G. barbadense accessions, GB-49, GB-13, GB-264, GB-171, and GB-713, supported less than 11% of the egg production found on susceptible check cultivar Deltapine 16; thus they classified these as resistant to R. reniformis. Furthermore, in this study only 3% of R. reniformis egg production was observed on the G. barbadense accession GB-713. Because of the high levels of R. reniformis resistance found in GB-713, scientists also included this accession in their research efforts on the introgression of resistance genes to Rotylenchulus reniformis into the upland cotton species G. hirsutum.
In April 2007, the Agricultural Research Service of the U.S. Department of Agriculture (USDA-ARS), in collaboration with Texas AgriLife and Cotton Incorporated, released two upland cotton germplasm lines, LONREN-1 and LONREN-2 (Starr et al., 2007; Bell et al., 2014). The source of resistance to reniform nematode in LONREN is derived through introgression from G. longicalyx (Robinson et al., 2007). LONREN-derived breeding lines have shown the potential to support lower reniform nematode densities in greenhouse and field trials (Bell et al., 2009; Weaver et al., 2011). In field trials, LONREN-derived lines also have shown good fiber quality when increased in fields free of the reniform nematode (Bell et al., 2009; Weaver et al., 2011; Weaver et al., 2013). It was later reported that where LONREN-derived lines were grown in fields with high levels of reniform nematode (10,000 to 50,000 per 100 cm3 of soil at planting), early-season stunting occurred indicating that these lines were intolerant to the initial attack from the reniform nematode (Nichols et al., 2010; Sikkens et al., 2011). The common response to initial nematode infection in the resistant host plants carrying a resistance gene(s) is an early hypersensitive reaction (HR) that results in cell death around the nematode feeding site, preventing nematode feeding and resulting in the death of the nematode (Das et al., 2008). Intolerance describes this nematode resistant reaction when the cell death is also damaging to the host plant (Barker, 1993). Visually plants are stunted, root systems are smaller and plant yields are compromised. These indicate that a hypersensitive response is present and has been observed in the LONREN germplasm lines (Sikkens et al., 2011), as well as in the reniform nematode resistant LONREN breeding lines (Weaver et al., 2013). Damage because of hypersensitivity occurs primarily between germination and the early seedling stage. It is not yet known if the damaging effects of a hypersensitive reaction can be overcome, entirely or partially, by providing some form of protection to seedlings through the application of nematicides or otherwise.
The first germplasm line into which reniform nematode resistance present in the GB713 accession of G. barbadense was introgressed, BARBREN-713 (USDA/Mississippi State University/Cotton Incorporated), became available in spring 2012. Its development was the result of a cooperative effort between USDA-ARS, Mississippi State University, and Cotton Incorporated. In 2012, Sikkens et al. reported that BARBREN-713 did not display intolerant responses to the reniform nematode, that it supported lower populations of reniform nematode compared with susceptible cultivars, and that it had comparable yields with the susceptible cultivars in the absence of reniform nematode. Thus, the BARBREN-713 germplasm line has the potential to constitute a good alternative source for reniform resistance in G. hirsutum. The BARBREN-713 germplasm line was included in the present study for comparative reasons, though because of the novelty of this genetic material, its response to reniform nematodes is in need of more in-depth evaluation.
The overall hypothesis of this study is that nematicides applied to selected LONREN breeding lines will suppress initial nematode population densities and reduce the intolerance response and subsequent damage to cotton seedlings, by diminishing the number of hypersensitive reactions in seedlings of reniform nematode resistant lines. The objectives of this study were (i) evaluate reniform nematode population densities on LONREN-derived breeding lines with and without nematicides; (ii) evaluate the effects that applying nematicides have on early seedling stunting of LONREN breeding lines; and (iii) evaluate overall yield performances when nematicides are applied to LONREN breeding lines. The overall goal of this project is to provide protection from R. reniformis to seedlings of newly developed LONREN-derived reniform nematode resistant breeding lines.
Materials and Methods
Experimental design:
Experimental trials reported herein were conducted in the greenhouse, microplots, and field in 2011 and 2012 to determine if the application of a nematicide would benefit the growth and yield of R. reniformis resistant cotton genotypes that are intolerant of high early-season populations of the nematode. In the first year’s trials, six genotypes were evaluated in a 6 × 2 factorial design with genotype being the main factor and the second factor being the addition of a nematicide (added or not added). The genotypes entered in this study included the R. reniformis resistant germplasm line LONREN-1, resistant breeding lines A107, A122, and B219 that were derived from the cross LONREN-1בFibermax 966’ (FM966, PVP200100209, Bayer CropScience, Lubbock, TX). These resistant lines were compared with the R. reniformis susceptible cultivar Deltapine 393 [‘DP393’, PVP200400266, Delta and Pine Land Company (Monsanto), St. Louis, MO], and susceptible breeding line B211, also derived from the LONREN-1בFM966’ cross. The resistant lines selected for this study reduced nematode populations in the 2009 field trial screenings and appeared highly resistant to R. reniformis in previous greenhouse screenings (Sikkens et al., unpubl. data). In trials in fields not infested with reniform nematodes, the selected lines produced superior yields with excellent fiber qualities, especially in fiber strength compared with the other lines in the field trial (Weaver et al., 2013). The second factor in this test was the addition of a nematicide seed treatment consisting of the two nematicides, abamectin (Syngenta, Greensboro, NC) applied at 0.15 mg a.i./seed plus thiodicarb (Bayer CropScience, Research Triangle Park, NC) applied at 0.375 mg a.i./seed, or no nematicide application at all. All seeds were treated with the standard fungicides thiram at 0.002 mg a.i./seed, metalaxyl at 0.0003 mg a.i./seed, and ipconazole at 0.0001 mg a.i./seed (Bayer CropScience, Research Triangle Park, NC) to manage seedling disease, and the insecticide imidacloprid (Bayer CropScience, Research Triangle Park, NC) at 0.34 mg a.i./seed to reduce thrips damage in the first 4 wk after planting. The seed treatments were applied with a Gustafson table-top seed treater, (Bayer CropScience, Research Triangle Park, NC), mixed for 3 min in the 454-gm stainless steel bucket and allow to air dry before packaging. The 12 total genotype treatment combinations tested in 2011 were arranged in a randomized split block design with five replications and each test was repeated for a total of 120 experimental units. Identical tests were conducted in the greenhouse, microplots, and field for a total of four tests in 2011. The greenhouse test was repeated in 2011 although the microplot and field trials were only conduced once in 2011.
In the second year’s trials, eight genotypes were evaluated in an 8 × 4 factorial design in the greenhouse and field settings and an 8 × 3 factorial design in the microplots with genotypes being the main level and the second level being nematicides or no nematicide. In the microplots, the 8 × 3 factorial design excluded the combination of the nematicide seed treatments with aldicarb because of the lack of space to allow the entire test to be repeated, thus there was a total of 240 experimental units. Genotypes were expanded to include the R. reniformis resistant LONREN-1בFM966’ derived breeding line B103 and the resistant germplasm line BARBREN-713. Two additional nematicide treatments were included as well. The four nematicide treatments for greenhouse and field 2012 trials were (i) an in-furrow application at planting of aldicarb (Temik® 15 G, Bayer CropScience, Raleigh, NC) at the rate of 840 g a.i./ ha; (ii) the nematicide seed treatments abamectin and thiodicarb previously described combined with an in furrow application at planting of aldicarb of 840 g a.i./ ha; (iii) the nematicide seed treatments abamectin and thiodicarb alone, and (iv) an untreated control. In the microplots, the 8 × 3 factorial design excluded the combination of the nematicide seed treatments with aldicarb because of the lack of space. The 32 genotype by nematicide combinations were arranged in a four-row split-plot design, with five replications for a total of 320 experimental units each in the repeated field and greenhouse trials. The whole plots were the genotype and the subplots were the nematicide treatments. All greenhouse, microplot, and field trials were planted twice in different locations on the research stations in the same year for a total of six experiments in 2012.
Rotylenchulus reniformis inoculum preparation:
The Rotylenchulus reniformis used as inoculum in the greenhouse and microplot experiments was extracted from 60-d-old cotton stock cultures maintained in 500-cm3 polystyrene pots in the greenhouse. Juvenile and vermiform adult stages of R. reniformis were extracted from the soil using the standard gravitational sieving followed by sucrose centrifugation 1.14 sp. G (Jenkins, 1964). Eggs were collected from the roots of the cotton plants by shaking the roots on a rotary shaker for 4 min in a 0.625% NaOCl (Hussey and Barker, 1973). Eggs were washed with water over nested 75- and 25-μm-pore sieves. The R. reniformis eggs and vermiform juvenile and adult stages were enumerated at ×40 with a Nikon TSX inverted microscope (Nikon Instruments, Inc., Melville, NY). The eggs and vermiform juvenile and adult nematode stages were combined and used to augment the naturally infested soils used in greenhouse and microplot trials to standardize population averages similar to those found in the field at the Tennessee Valley Research and Experiment Center (TVREC) located near Belle Mina in northern Alabama.
Greenhouse trials:
Rotylenchulus reniformis resistant cotton genotypes, with and without nematicides, were evaluated for nematode population development and plant growth in the greenhouse at the Plant Science Research Center (PSRC) located in Auburn, AL. Experiments using the previously described 6 × 2 (2011) and 8 × 4 (2012) factorial designs were planted in 500-cm3 polystyrene pots containing a Decatur silt loam soil (23% sand, 49% silt, 28% clay) collected from the TVREC field location. This Decatur silt loam soil contained a population of R. reniformis with a population density of 3,750 vermiform juvenile and adult life stages per 500 cm3 of soil. A mixture of 1,250 R. reniformis eggs and vermiform life stages was pipetted into each polystyrene pot to increase the nematode population to 5,000 R. reniformis per 500 cm3 of soil. One seed from each of the genotypes was planted per polystyrene pot. Rotylenchulus reniformis egg populations were extracted from the roots as previously described at 45 days after planting (DAP). Plant heights and shoot and root fresh weights were recorded and numbers of eggs per gram of fresh root weight were determined.
Microplot trials:
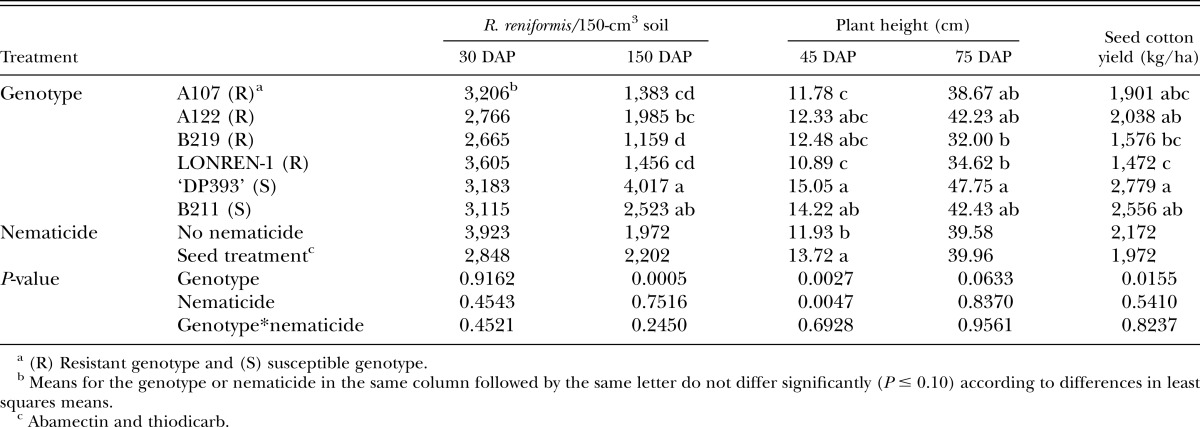
In 2011, the performance of the six cotton genotypes, with and without nematicides was evaluated for nematode density development, plant growth parameters, and yield. This microplot experiment allowed the genotypes to be evaluated in an outside environment with uniform initial nematode levels for all treatments that were somewhat similar to field conditions, thus allowing the genotypes to reach full maturity. The microplot experiment was established at the PSRC. The same 6 × 2 factorial design previously described was established in 4,000-cm3 volume microplots (30-cm wide × 35-cm deep) filled with Decatur silt loam (23% sand, 49% silt, 28% clay) natural field soil collected from the TVREC. An additional 3,500 R. reniformis egg and vermiform mixed life stages were added to each microplot to standardize the population to 5,000 R. reniformis per 500 cm3 of soil or 40,000 per microplot. Four seeds of the specific genotype were hand planted at a 2.5-cm depth in each microplot in a linear fashion to simulate a linear row foot in the field. Parameters measured in the microplot study included R. reniformis population densities, plant heights, and seed cotton yields. Soil samples were taken at 30 and 150 DAP to determine R. reniformis vermiform life-stage populations. Four soil cores, 2.5-cm wide ×15-cm deep, were taken from each microplot using a soil probe. Rotylenchulus reniformis juvenile and vermiform adult stages were extracted as previously described. Plant heights were also measured at 45 and 75 DAP. At cotton plant maturity, approximately 150 DAP, each microplot was handpicked and cotton yield was recorded as grams of seed cotton per microplot.
In 2012, the experimental design was expanded to a 8 × 3 factorial to include the B103 and BARBREN-713 lines as previously described. However, the combination of abamectin/thiodicarb + aldicarb treatment was omitted from the second year’s microplot trials due to the lack of available space to include all four nematicide treatments and repeat the trial. The entire microplot trial was repeated once for two separate tests in 2012. The parameters measured were identical to the first year’s trial.
Field trials:
In 2011, six genotypes, with and without nematicides, were evaluated for their effect on early-season nematode population, seedling height, and seed cotton yield. This field experiment allowed the genotypes to be evaluated in an environment similar to what is experienced in actual cotton production. The 6-ha field is located at the TVREC and has a high reniform nematode population. The same 6 × 2 factorial design previously described was set up in one-row plots that were 7.6-m long on 1.02-m centers. One hundred seed per row from each of the genotype by nematicide treatment combinations were planted with an Almaco cone planter (Nevada, IA). Soil samples were taken at planting, 30 DAP, and 150 DAP near harvest to determine R. reniformis vermiform life-stage populations in the soil. The sampling method consisted of collecting ten 2.5-cm × 20-cm cores at the base of the plants from each one-row plot. The 10 cores were combined to make up a composite sample for each plot and placed in plastic zippered bags. The samples were transported to a laboratory and a 150-cm3 subsample was extracted using the gravity sieving and sucrose centrifugation methods. Plant heights for each plot were also recorded at 45 and 75 DAP to determine what effects nematicides had on early-season growth. Four plant heights were measured in each of the one-row plots and an average plant height was calculated and recorded in centimeters. The plants were measured from the soil line to the apical meristem. All plots were machine harvested with a modified plot picker and seed cotton yields for each plot were recorded.
The 2012 field trials were expanded to include the B103 and BARBREN-713 lines in the 8 × 4 factorial design previously described. This 8 × 4 factorial test was planted in opposite corners in the R. reniformis infested field at TVREC in 2012. The in furrow application of aldicarb at the rate of 840 g a.i./ha was applied at planting with granular applicators that were attached to the planter. All the parameters measured in the 2012 trials were conducted as described in the 2011 trials with the addition of collecting roots samples to quantify egg numbers. At 100 DAP, one plant including its root system, was dug from each row with paired shovels 10 cm from the cotton stalk to determine levels of resistance for each genotype entry. The root systems were cut from the shoot and combined to make a composite sample for the whole plot (four rows). The root samples were transported to a lab where 1-g subsamples consisting of small fibrous roots were cut from each composite sample and the eggs were extracted using the 0.625% NaOCl agitation method. Egg populations were recorded as eggs per gram of fresh root for the whole plot.
Statistical analysis:
Data on R. reniformis levels were analyzed in SAS 9.2 software (SAS Institute, Carry, NC) using the GLIMMIX procedure. Student panel graphs were generated and evaluated to determine the normality of the residuals. The plant heights were analyzed using a normal distribution. The R. reniformis nematode vermiform life-stage populations extracted from the soil and the eggs collected from roots required a lognormal distribution transformation to satisfy the normality assumption. In the LSMEANS command, the PDIFF option was used to differentiate treatments at the P ≤ 0.10 significance level. The LSMEANS estimates for the lognormal distribution function were back transformed to the original value using PROC MEANS. The original mean values are presented in the tables with P values to determine statistical differences. Response data from 2011 and 2012 experiments were analyzed separately. Response data from the 2012 experiments were analyzed jointly pooling all data together when no interactions were found between repeated trials.
Results
Greenhouse trials:
In the 2011 greenhouse trials, no significant interactions occurred between the two trials thus data were pooled for analysis. There were no interactions of genotypes by nematicides evident for total egg population densities, eggs per gram of fresh root weight, or plant heights (Table 1). The abamectin/thiodicarb seed treatment reduced both total egg population densities and eggs per gram of fresh root by 51% and 50%, respectively. Both the susceptible cultivar ‘DP393’ and B211 genotype supported significantly higher total egg densities (P ≤ 0.001) and eggs per gram of root (P ≤ 0.001) than their resistant counterparts A107, A122, B219, and LONREN-1. Overall resistant genotypes supported 82% fewer eggs and 74% fewer eggs per gram of root than the susceptible genotypes (Table 1). Significant increases (P ≤ 0.001) in plant heights were observed when the abamectin/thiodicarb seed treatment was applied to all genotypes; thus no interactions were observed between the nematicide and the genotypes. The resistant genotypes A107, A122, B219, and LONREN-1 exhibited plant heights that were similar to the susceptible check B211 indicating a reduction in early-season stunting was influenced by the abamectin/thiodicarb seed treatment but the influence of the nematicide was similar across all genotypes.
Table 1.
Mean Rotylenchulus reniformis egg population densities per plant, eggs per gram of fresh root weight, and plant heights at 45 d after planting in the 2011 greenhouse trials.

In the 2012 greenhouse evaluations, there were no significant interactions between the two trials thus data were pooled for analysis. There were no significant interactions between genotype and nematicide for total egg population densities, eggs per gram of fresh root weight, or plant heights (Table 2). Significant differences were evident for total egg populations (P ≤ 0.001) and eggs per gram of root (P ≤ 0.001) among all genotypes. The susceptible genotypes ‘DP393’ and B211 produced total egg populations and eggs per gram of fresh root weight that were 74% and 71% as well as 26% and 54% greater than the resistant genotypes, respectively. Aldicarb alone and the combination of aldicarb + abamectin/thiodicarb provided significant reductions in total egg densities (P ≤ 0.001) and eggs per gram of root (P ≤ 0.001) compared with the untreated controls, respectively. All three nematicide treatments significantly increased plant heights compared with the no nematicide control (P ≤ 0.006). The LONREN-derived reniform nematode resistant genotypes A107, A122, and B219, as well as LONREN-1 had similar plant heights to the susceptible checks ‘DP393’ and B211 indicating that the nematicide treatments provided stunting protection to all these cotton lines. The B103 genotype that supported the lowest density of R. reniformis was also the shortest plant. The BARBREN-713 line exhibited the tallest plant heights among all genotypes while supporting of R. reniformis population densities similar to LONREN-1. The phenotypic response of the LONREN-derived genotypes was a visual trend of increasing plant heights and overall biomass where the nematicide options were applied (Fig. 1).
Table 2.
Mean Rotylenchulus reniformis egg population densities per plant, eggs per gram of fresh root, and plant heights at 45 days after planting in the 2012 greenhouse trials.

Fig. 1.
Phenotypic response of LONREN-derived resistant lines A107 (top) and B103 (bottom) at 45 d after planting in greenhouse conditions. Nematicide treatments provided significant increases in plant heights over the untreated controls.
Microplot trials:
In the 2011 microplot trial, R. reniformis initial population densities were extremely low, ranging from 39 to 93 vermiform life stages per 150 cm3 at the 30 DAP sampling period. There was no genotype by nematicide interaction for R. reniformis population densities, plant heights, or seed cotton yields (Table 3). Rotylenchulus reniformis population densities were similar among all genotypes at 30 DAP. However, by 150 DAP population densities had increased to levels that were 74% higher in the susceptible checks ‘DP393’ and B211 than those found on the resistant genotypes. The abamectin/thiodicarb seed treatments had no effect on R. reniformis population densities in the early-season sampling period at 30 or 150 DAP. Plant heights recorded at 45 and 79 DAP were not affected by abamectin/thiodicarb seed treatments and all genotypes had similar plant heights at these time periods. Seed cotton yields were not affected by nematicides and all genotypes had similar yields.
Table 3.
Mean Rotylenchulus reniformis vermiform life stages per 150-cm3 soil at 30 and 150 days after planting (DAP), plant heights at 45 and 75 DAP, and seed cotton yields in the 2011 microplot trial.

In the 2012 microplot evaluations, no significant interaction between the two trials was found so the data were pooled for analysis. As in 2011, no interaction between genotype and nematicide was detected for R. reniformis population densities, plant heights, or seed cotton yields occurred in the microplot trials (Table 4). At 30 DAP, R. reniformis population densities were low and only BARBREN-713 supported a lower population density of R. reniformis than the susceptible checks. By 150 DAP, R. reniformis population densities had increased to levels that were significantly higher (P ≤ 0.001) in the susceptible checks compared with populations found in all the resistant genotypes. Aldicarb applied as an in-furrow treatment provided a significant reduction (P < 0.001) in R. reniformis population densities in the early season at 30 DAP but no reduction was observed at the final sample at 150 DAP. The seed treatment nematicide combination did not reduce R. reniformis population densities at either sample date as compared with the no nematicide treatment. Nematicide effects were observed on plant heights at 45 and 75 DAP (Table 4). A significant increase in plant height was evident in the aldicarb treatment (P ≤ 0.079) at 45 DAP as compared with the nontreated control. The abamectin/thiodicarb seed treatment and aldicarb treatment both provided significant increases (P ≤ 0.001) in plant heights at 75 DAP. The resistant genotypes A107, A122, B219, LONREN-1, and BARBREN-713 all had plant heights that were similar to the susceptible cultivar DP393 at 45 DAP indicating that the early-season stunting of the LONREN genotypes was reduced. At 75 DAP, plant heights of these same resistant genotypes were still similar to the susceptible cultivar DP393. Seed cotton yields were affected by nematicide treatments (Table 4). The aldicarb treatment significantly enhanced seed cotton yields (P ≤ 0.062), which were 23% higher than the untreated control. All resistant genotypes exhibited seed cotton yields that were similar to the susceptible cultivar DP393.
Table 4.
Mean Rotylenchulus reniformis vermiform life stages per 150-cm3 soil at 30 and 150 days after planting (DAP), plant heights at 45 and 75 DAP, and seed cotton yields in the 2012 microplot trials.

Field trials:
In the 2011 field trial, no genotype by nematicide was observed for R. reniformis population densities, plant heights, or seed cotton yields (Table 5). Rotylenchulus reniformis populations among all genotypes were similar in the early-season sampling period at 30 DAP. A reduction of 48% of R. reniformis population densities was evident for the resistant genotypes by 150 DAP whereas the susceptible checks ‘DP393’ and B211 exhibited a slight increase in populations. Furthermore, R. reniformis population densities were significantly lower (P ≤ 0.005) in all the resistant genotypes compared with the susceptible cultivar DP393. No nematicide effects were observed on R. reniformis population densities at 30 or 150 DAP (Table 5). Early-season plant heights at 45 DAP for the resistant genotypes A122 and B219 were similar to the susceptible checks ‘DP393’ and B211. The abamectin/thiodicarb seed treatment provided a significant increase in plant height (P ≤ 0.005) at 45 DAP compared with the no nematicide control. Plant heights at 75 DAP were variable among genotypes and no plant height increases were observed with the addition of the seed treatments (Table 5). Seed cotton yields followed a similar pattern of variability with A107 and A122 exhibiting comparable yields to the susceptible checks.
Table 5.
Mean Rotylenchulus reniformis vermiform life stages per 150-cm3 soil at 30 and 150 days after planting (DAP), plant heights at 45 and 75 DAP, and seed cotton yields in the 2011 field trial.
In the 2012 field trials, there were no significant interactions between the two trials thus data were pooled for analysis. No genotype by nematicide interaction was evident for R. reniformis soil population densities, plant heights, or yields (Table 6). Rotylenchulus reniformis population densities were similar among the LONREN-derived genotypes at 30 DAP, however BARBREN-713 had a significantly lower population density than the susceptible cultivar ‘DP393’ and the resistant lines LONREN-1 and A107. Aldicarb alone, aldicarb + abamectin/thiodicarb seed treatment, and the abamectin/thiodicarb seed treatment alone supported similar R. reniformis population densities at 30 DAP, but only the aldicarb nematicide treatment alone was significantly lower than the no nematicide treatment. By 150 DAP, resistant genotypes supported 52% lower populations of R. reniformis than the susceptible genotypes. All resistant genotypes supported significantly fewer (P ≤ 0.001) R. reniformis than the susceptible cultivar ‘DP393’ at 150 DAP. At 100 DAP, all resistant genotype entries supported significantly fewer eggs at the (P ≤ 0.10) than the susceptible cultivar ‘DP393’ (Fig. 2). The lowest egg populations were observed on the LONREN-derived genotype B103 and BARBREN-713, which supported 98% and 94% less egg production than the susceptible cultivar ‘DP393’, respectively. Early-season plant heights at 45 DAP were similar in the resistant lines A107, A122, and B219 compared with the susceptible checks ‘DP393’ and B211 (Table 6). BARBREN-713 exhibited significantly taller plants (P ≤ 0.001) at 45 DAP than all other genotypes, resistant or susceptible. Similar trends among genotypes were evident at 79 DAP with the A122 line and BARBREN-713 both exhibiting significantly taller plants than the susceptible checks. All three nematicide options provided significant increases (P ≤ 0.001) in plant height at 45 DAP for all resistant and susceptible cotton lines (Fig. 3, Table 6) with aldicarb and aldicarb + abamectin/thiodicarb resulting in taller plants than the seed treatment alone. The aldicarb alone and aldicarb + abamectin/thiodicarb treatment provided significant increases in plant heights over the seed treatment alone and the no nematicide control at 79 DAP (Table 6). Yields of the susceptible checks ‘DP393’ and B211 and the resistant lines A107, A122, B219, and LONREN-1 were similar. The BARBREN-713 resistant line exhibited significantly higher yields (P ≤ 0.001) than all other genotypes, resistant or susceptible. Aldicarb alone or in combination with abamectin/thiodicarb seed treatment enhanced yields 20% and 23%, respectively (P ≤ 0.001), compared with the untreated control. No significant yield increase was associated with the seed treatment alone.
Table 6.
Mean Rotylenchulus reniformis vermiform life stages per 150-cm3 soil at 30 and 150 days after planting (DAP), plant heights at 45 and 79 DAP, and seed cotton yields in the 2012 field trials.

Fig. 2.
Rotylenchulus reniformis egg population means at 100 d after planting in the 2012 field trial. Standard errors are shown to separate statistical difference of egg populations supported by each genotype. All resistant genotypes supported significantly fewer egg populations per gram of root compared with the cultivar ‘DP393’ at the P ≤ 0.10 significance level.
Fig. 3.
Phenotypic response of reduced stunting of LONREN-derived genotype B103 at 45 d after planting in response to nematicides. Aldicarb or abamectin/thiodicarb + aldicarb provided significant increases in early-season plant growth parameters.
Discussion
The primary goal of this study was to determine if we could protect the LONREN-derived resistant genotypes from the intolerant response of phenotypic stunting and subsequent yield losses that occur when these genotypes are planted in soils with high population densities of the reniform nematode. Results of our greenhouse, microplot, and field trials indicate applying aldicarb or aldicarb + abamectin/thiodicarb nematicides at planting to the LONREN-derived resistant lines suppressed initial nematode intolerance expressed by the cotton seedling and reduced but did not eliminate the phenotypic early-season plant stunting. These nematicide treatments provided protection to all the cotton lines, both susceptible and resistant similarly throughout the growing season. Applying aldicarb at planting for nematode management in cotton production systems has been the industry standard for nematode management for many years (Koenning et al., 2004). Previous research has found early-season plant growth stimulation when aldicarb was applied to cotton even in the absence of nematode and insect pests (Reddy et al., 1997). Similar studies have reported plant growth promotion from applying aldicarb at planting to other crops including tobacco and soybean (Barker and Powell, 1988; Barker et al., 1988). Thus the increase in plant growth to both the susceptible and resistant lines could be because of this “aldicarb kick.” The seed treatments abamectin and thiodicarb were introduced to the market in 2006 and 2007, respectively. These nematicides have provided early-season nematode management for the susceptible cotton cultivars across the cotton belt. These nematicides also provided some protection to the LONREN-derived resistant lines as they did to the susceptible lines, reducing the phenotypic stunting. However, the seed treatments abamectin and thiodicarb were most effective at reducing nematode densities and plant stunting for all genotypes tested when combined with aldicarb.
Greenhouse, microplot, and field trials indicate that the resistant lines do support significantly lower reniform population densities not only in the greenhouse and microplot trials but also in the natural field environment. The field trials indicate that the LONREN-1 derived R. reniformis resistant lines do lower nematode population densities at the end of the season and could reduce this pest population density to below damage levels in the field. Our results confirm suppression of R. reniformis populations observed by Bell et al. (2009) and Weaver et al. (2011). In initial nematode resistance and agronomic performance trials, LONREN lines greatly suppressed R. reniformis populations while producing superior yielding cotton compared with susceptible lines (Bell et al., 2009; Weaver et al., 2013). The same authors also reported that gene segments from G. longicalyx were responsible for an increase in fiber strength. Weaver et al. (2011) reported that the LONREN-derived resistant genotypes produced similar amounts of seed cotton to the susceptible industry cultivars ‘DP393’ and ‘FM966’ in a field without reniform nematodes. The results of our 2011 microplot study supported the findings of Weaver et al. (2011) in which intolerant responses of these LONREN-derived genotypes did not occur where high levels of R. reniformis were not present. These LONREN-derived genotypes also had excellent fiber quality in which fiber strength was greater than nonresistant sister lines. Thus, these LONREN-derived resistant lines could reduce R. reniformis populations while producing optimum yields with high-quality fiber.
Individual performances of the LONREN-derived breeding lines in this study suggest that the level of resistance was variable across resistant genotypes. The LONREN-derived resistant line A122 consistently, across all trials, supported R. reniformis population densities that were the highest among the LONREN genotypes. However, these population densities were considerably lower than those found on the susceptible cotton cultivars. Considering the egg data that were collected from the field trial at 100 DAP, this genotype would still be classified as moderately resistant when compared with the susceptible cultivar ‘DP393’. This A122 line was consistently the highest yielding LONREN genotype throughout the field studies. In contrast, the LONREN-derived genotype B103 typically supported the fewest R. reniformis and exhibited the lowest yields. The phenotypic response of B103 was always the shortest, less-vigorous plant throughout greenhouse, microplot, and field trial evaluations.
The future practicality of the LONREN source of resistance is dependent on initial reniform population density into which the genetic material is planted. In our research, aldicarb suppressed the initial nematode pressure, thus reducing the amount of damage that occurs when high populations of R. reniformis are present in the field. However, other management practices that reduce initial populations could be recommended before planting a LONREN-derived genotype such as nonhost crop rotations. Nonhost crop rotations with corn and peanut have been reported to significantly reduce R. reniformis populations to a level that would be low enough the following season that the LONREN-derived genotypes would not be damaged (Gazaway et al., 2000; Moore et al., 2010). In Sikkens et al. (2011), it was reported that as R. reniformis population levels increased from 0 to 50,000 per 150 cm3 of soil, plant growth parameters of shoot and root dry mass declined and severe stunting of the plant occurred. Sikkens et al. (2011) also reported that plant height was comparable with susceptible checks when reniform levels were below 5,000 vermiform nematodes per pint of soil. The findings from the Sikkens et al. (2011) study and our study indicate that if R. reniformis initial populations are low, generally below 1,000 vermiform life stages per 500 cm3 of soil, selected LONREN-derived resistant genotypes could be incorporated as a preventative measure in a management practice.
The BARBREN-713 germplasm line that was included in the experimental design allowed a new source of R. reniformis resistance to be compared with the LONREN-derived genotypes. In our microplot and field trials, there was no evidence of any intolerant response to the R. reniformis nematode with this genetic material. This line performed well with or without nematicide treatments and produced the highest seed cotton yields out of all the genotypes entered, including the resistant and susceptible lines. Rotylenchulus reniformis population densities at harvest were much lower for the BARBREN-713 line than for the susceptible entries. Similar results were also reported by Sikkens et al. (2012) in which BARBREN-713 supported much lower nematodes than susceptible checks while exhibiting the highest seed cotton yields. The results of this study and our study indicate that the BARBREN-713 source of resistance will replace the LONREN source of resistant in future R. reniformis resistance research and breeding.
Literature Cited
- Agudelo P, Robbins RT, Stewart JM, Bell A, Robinson AF. Histological observations of Rotylenchulus reniformis on Gossypium longicalyx and interspecific cotton hybrids. Journal of Nematology. 2005;37:444–447. [PMC free article] [PubMed] [Google Scholar]
- Barker KR. Resistance/tolerance and related concepts/terminology in plant nematology. Plant Disease. 1993;77:111–113. [Google Scholar]
- Barker KR, Koenning SR, Bostain AL, Ayers AR. Growth and yield responses of soybean to aldicarb. Journal of Nematology. 1988;20:421–431. [PMC free article] [PubMed] [Google Scholar]
- Barker KR, Powell NT. Influence of aldicarb on the growth and yield of tobacco. Journal of Nematology. 1988;20:432–438. [PMC free article] [PubMed] [Google Scholar]
- Bell AA, Robinson AF, Quintana J, Dighe ND, Menz MA, Stelly DM, Zheng X, Jones JE, Overstreet C, Burris E, Cantrell RG, Nichols RL. Registration of LONREN-1 and LONREN-2 germplasm lines of upland cotton resistant to reniform nematode. Journal of Plant Registrations. 2014;8:187–190. [Google Scholar]
- Bell AA, Starr JL, Jones JE, Lemon R, Nichols RL, Overstreet C, Stelly DM. 2009. Nematode resistant and agronomic performance of LONREN- and NEMSTACK lines. Proceedings of the Beltwide Cotton Conferences 1:178. Memphis, TN: National Cotton Council of America.
- Das S, De Mason DA, Ehlers JD, Close TJ, Roberts PA. Histological characterization of root-knot nematode resistance in cowpea and its relation to reactive oxygen species modulation. Journal of Experimental Botany. 2008;59:1305–1313. doi: 10.1093/jxb/ern036. [DOI] [PubMed] [Google Scholar]
- Gazaway WS, Akridge JR, McLean K. 2000. Impact of various crop rotations and various winter cover crops on reniform nematode in cotton. Proceedings of the Beltwide Cotton Conferences 1:162–163. Memphis, TN: National Cotton Council of America.
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Koenning SR, Kirkpatrick TL, Starr JL, Walker NA, Wrather JA, Mueller JD. Plant-parasitic nematodes attacking cotton in the United States: Old and emerging production challenges. Plant Disease. 2004;88:100–113. doi: 10.1094/PDIS.2004.88.2.100. [DOI] [PubMed] [Google Scholar]
- Moore SR, Gazaway WS, Lawrence KS, Goodman B, Akridge JR. 2010. Value of rotational crops for profit increase and reniform nematode suppression with and without nematicide in Alabama. Proceedings of the Beltwide Cotton Conferences 1:160–168. Memphis, TN: National Cotton Council of America.
- Nichols RL, Bell A, Stelly D, Dighe N, Robinson F, Menz M, Starr J, Agudelo P, Jones J, Overstreet C, Burris E, Cook C, Lemon R, Fang D. 2010. Phenotypic and genetic evaluation of LONREN- germplasm. Proceedings of the Beltwide Cotton Conferences 1:798–799. Memphis, TN: National Cotton Council of America. [Google Scholar]
- Reddy VR, Wang Z, Reddy KR. Growth responses of cotton to aldicarb and temperature. Environmental and Experimental Botany. 1997;38:39–48. [Google Scholar]
- Robinson AF, Bell AA, Dighe NC, Menz MA, Nichols RL, Stelly DM. Introgression of resistance to nematode Rotylenchulus reniformis into upland cotton (Gossypium hirsutum) from Gossypium longicalyx. Crop Science. 2007;47:1865–1877. [Google Scholar]
- Robinson AF, Percival AE. Resistance to Meloidogyne incognita race 3 and Rotylenchulus reniformis in wild accessions of Gossypium hirsutum and G. barbadense from Mexico. Journal of Nematology. 1997;29:746–755. [PMC free article] [PubMed] [Google Scholar]
- Robinson AF, Bridges AC, Percival AE. New sources of resistance to the reniform (Rotylenchulus reniformis) and root-knot (Meloidogyne incognita) nematode in upland (Gossypium hirsutum L.) and sea island (G. barbadense L.) cotton. Journal of Cotton Science. 2004;8:191–197. [Google Scholar]
- Sikkens RB, Bell AA, Wheeler TA, Overstreet C, Weaver DB, Lawrence KS, Nichols RL. 2012. Performance evaluation of LONREN- and BARBREN reniform nematode resistant germplasm lines. Proceedings of the Beltwide Cotton Conferences 1:761–767. Memphis, TN: National Cotton Council of America. [Google Scholar]
- Sikkens RB, Weaver DB, Lawrence KS, Moore SR, van Santen E. LONREN upland cotton germplasm response to Rotylenchulus reniformis inoculum level. Nematropica. 2011;41:68–74. [Google Scholar]
- Starr JL, Koenning SR, Kirkpatrick TL, Robinson AF, Roberts PA, Nichols RL. The future of nematode management in cotton. Journal of Nematology. 2007;39:283–294. [PMC free article] [PubMed] [Google Scholar]
- Usery SR, Lawrence KS, Lawrence GW, Burmester CH. Evaluation of cotton cultivars for resistance and tolerance to Rotylenchulus reniformis. Nematropica. 2005;35:121–133. [Google Scholar]
- Weaver DB, Lawrence K, van Santen E. Reniform nematode resistance in upland cotton germplasm. Crop Science. 2007;47:19–24. [Google Scholar]
- Weaver DB, Sikkens RB, Sharpe RR, Moore SR, Lawrence KS. 2011. LONREN- x FM966 progeny evaluation in a field infested with reniform nematodes. Proceedings of the Beltwide Cotton Conferences 1:737–741. Memphis, TN: National Cotton Council of America. [Google Scholar]
- Weaver DB, Sikkens RB, Lawrence KS, Sürmelioğlu Ç, van Santen E, Nichols RL. RENlon and its effects on agronomic and fiber quality traits in upland cotton. Crop Science. 2013;53:913–920. [Google Scholar]
- Yik CP, Birchfield W. Resistant germplasm in Gossypium species and related plants to Rotylenchulus reniformis. Journal of Nematology. 1984;16:146–153. [PMC free article] [PubMed] [Google Scholar]





