Abstract
Recently, application of vibrotactile noise to the wrist or back of the hand has been shown to enhance fingertip tactile sensory perception (Enders et al 2013), supporting a potential for an assistive device worn at the wrist, that generates minute vibration to help the elderly or patients with sensory deficit. However, knowledge regarding the detailed physiological mechanism behind this sensory improvement in the central nervous system, especially in the human brain, is limited, hindering progress in development and use of such assistive devices. To enable investigation of the impact of vibrotactile noise on sensorimotor brain activity in humans, a magnetic resonance imaging (MRI)-compatible vibrotactile system was developed to provide vibrotactile noise during an MRI of the brain. The vibrotactile system utilizes a remote (outside the MR room) signal amplifier which provides a voltage from −40 V to +40 V to drive a 12 mm diameter piezoelectric vibrator (inside the MR room). It is portable and is found MRI-compatible to enable its use for neurologic investigation with MRI. The system was also found to induce improvement in fingertip tactile sensation, consistent with the previous study.
Keywords: stochastic resonance, vibrotactile, piezoelectric, MRI, vibrator
1.0 Introduction
Application of vibrotactile noise has been shown to enhance skin tactile sensation via stochastic resonance (SR), not only in healthy young adults (Collins et al 1997) but also in older adults, stroke survivors, and patients with diabetic neuropathy who have sensory deficit (Liu et al 2002). SR is a phenomenon in which a system’s response to a weak signal is enhanced by the presence of subthreshold white noise (Wells et al 2005). Figure 1 shows that SR can assist a sensing system in detecting a weak signal by adding an optimal amount of noise. A weak signal that is below the threshold and thus not detected on its own (figure 1a) can cross the sensory threshold and be detected when adequate noise is added to the signal (figure 1b), while excessive noise hampers the system’s sensitivity to the signal (figure 1c). In most cases, it is preferred to eliminate noise from a signal; however, in the case of sensing a small sensory signal, an outside noise is helpful for bringing the signal above threshold and allowing the body to sense the small signal.
Figure 1.
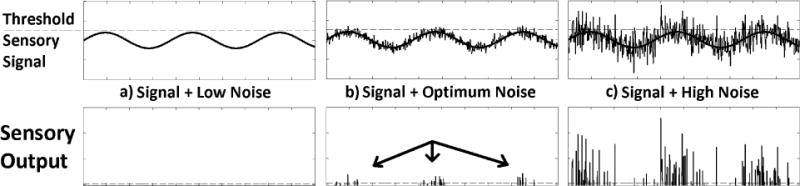
Example of stochastic resonance. (a) When the sensory signal and noise are low and do not exceed the sensory threshold, the system perceives nothing. (b) When an optimal amount of noise is added to the signal, the signal and noise exceed the sensory threshold whenever the signal reaches its peak, thus assisting the system to perceive the signal. (c) When high noise is added to the signal, the sensory threshold crossings occur most of the time irrespective of the signal shape, thus hindering the system from perceiving the signal.
The potential benefit of using vibrotactile noise as an assistive device is clear. Tactile sensation is critical for feedback motor control and dexterous movements (Augurelle et al 2003, Monzée et al 2001, Westling and Johansson 1984, Johansson and Westling 1984) that are directly relevant to motor function, functional independence, and quality of life. People with impaired tactile sensation, such as stroke survivors who often experience tactile sensory deficits in the hand (Carey 1995), suffer from resulting impairment in hand grip function, limiting their activities of daily living (Blennerhassett et al 2006). Therefore, a device generating small amounts of vibrotactile noise can be used to enhance fine motor function. For instance, a device attached to the fingertip, applying small amounts of vibrotactile noise to the cutaneous receptors in the nearby skin, not only enhanced fingertip tactile sensation but also improved dexterous grip control such as control of safety margin during grip and lift task in healthy adults (Liu et al 2013).
However, a vibrotactile noise device attached to the fingertip physically interferes with dexterous hand and finger movement and cannot be a viable solution for impaired sensation and hand dexterity. In lieu of the direct noise, vibrotactile noise applied remotely from the fingertip, such as to the wrist, was recently shown to have a promise to enhance fingertip tactile sensation (Enders et al 2013) and hand dexterity (Kosmopoulos et al 2014). It is postulated that the noise from the wrist may be integrated with the signal from the fingertip within the central nervous system, through existing nerve connections (Merzenich et al 1983; Bjorkman et al 2004; Hidaka et al 2000). This postulation needs to be physiologically tested in order to support further development of a remote vibrotactile noise device to enhance finger tactile sensation. One of the testing beds is Magnetic Resonance Imaging (MRI) of the brain, to identify brain areas whose activity changes with remote vibrotactile noise. To physiologically elucidate how the remote noise-induced improvement in tactile sensation and hand function are represented in human brains as seen by MRI, an MRI-compatible vibrotactile system was designed as follows. While MRI provides great insight into the usage of the brain, the strong magnetic fields greatly limit the use of standard vibrators, thus necessitating a custom-made MRI-compatible vibrotactile system. This system is expected to assist understanding of the neural working of remote vibrotactile noise, thereby furthering the development of assistive devices using remote vibrotactile noise to enhance hand function.
2.0 Methods
2.1 Overall design
In order to avoid extra complications due to the MRI-compatibility requirement, the system was set up so that the noise source (audio file on a laptop, a 10 Hz to 1 kHz random noise, which was created by LabVIEW and was found effective in enhancing fingertip tactile sensation in Enders et al 2013) and the driving circuit (noise amplifier) were located outside of the MRI room, with shielded wires running into the MRI room. The BNC connector was chosen to connect to the interface panel that separates the MR room from the computer control room. Coaxial wire was chosen because shielded wires minimize interference from the magnetic field of the MRI.
2.2 Vibrator
Because the vibrotactile system had to be MRI compatible, we could not use the magnetic material in common solenoid style vibrators. We selected a 12 mm diameter piezoelectric vibrator from a Piezoelectronic Buzzer (PS1240P02BT, TDK Corporation). It required up to 80 Vp-p to yield adequate vibration.
To prevent any potential electrical shock from the amplified signal sent to the piezoelectric vibrator in the event that the vibrator was placed upside down and the two wires contact the human skin, the two electrical leads were coated with a nonconductive hot-melt adhesive (All-Purpose Clear Mini Glue Sticks, Arrow Fastener, Saddle Brook, NJ).
2.3 Vibrotactile Amplifier: Fly back converter
A battery system was chosen to allow the device to be portable for use in various test environments. We used a DC-DC converter to boost the voltage to 80 Vp-p required for adequate vibration. The flyback converter is based on the buck-boost converter, with the switching realized using a Q1 MOSFET and diode. If the inductor is added to a transformer, a buck-boost converter is changed to a flyback converter (Erickson and Maksimovic 2004).
Figure 2 shows this design in detail. The control chip is a Texas Instruments TL384. The control chip requires a frequency greater than or equal to 300 kHz to yield a stable output and an input voltage between 10 V and 30 V. In the current device, a frequency of 300 kHz is used as well as a power source that uses two series 9 V batteries which yield an 18 V supply. A Wurth 750311889 transformer with a 1:5 step up ratio steps up the voltage.
Figure 2.
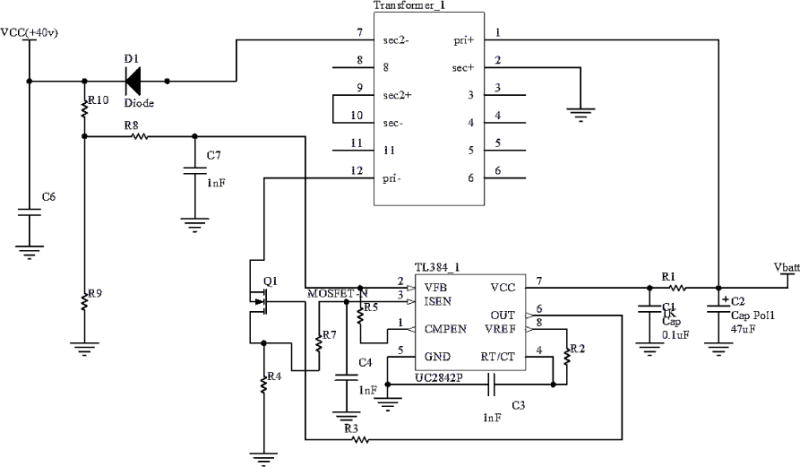
The flyback converter voltage v boosts battery voltage Vbatt = 18 V, yielding VCC = +40 V (Erickson and Maksimovic 2004)
R1 = 5 Ω, R2 = 5.7 kΩ, C3 = 1 nF, R3 = 5 Ω, R4 = 0.05 Ω, R5 = 100 kΩ, R7 = 30 Ω, R8 = 10 kΩ, C7 = 1 nF, VCC = 2.5 V*(R9 + R10)/R9, R10 = 1.5 MΩ, R9 = 100 kΩ for VCC = 40 V, C1 = 47 μF, C2 = 0.1 μF. C6 = 47 μF, D1 = BZW50–100B, Q1 = MOS-FET K2256. Vbatt= 18 V, VCC is 40 V.
2.4 Amplifier
We amplified 0.1 V input signal to 80 Vp-p output using a two stage amplifier, The OPA452 Power amp included High Pass Filters (HPF) with a corner frequency of 5 Hz, between each stage.
2.5 Final Design
An external laptop fed the amplifier, which fed the MR room internal cable and vibrator.
2.6 MR Safety Testing and Phantom Testing
First, initial inspection outside the MR room was performed on the cable and vibrator to detect any pull against the strong magnet. Second a safety test inside the MR room was performed by bringing the cable and vibrator into the MR room to test for any magnetic pull.
A multimeter was used to confirm zero conductance between the two electrical leads on the piezoelectric vibrator insulated with hot-melt. A temperature probe placed against the vibrator while the MRI scanner was operating confirmed negligible heating.
The anatomical scans (SPGR) were run without the device present in the MR room, with the device present in the MR room but turned off, and with the device turned on thus generating vibration in the MR room, to confirm intactness of the image.
2.7 Device Performance Testing
We examined amplifier gain and confirmed that equal amplification is applied across the frequency range. We confirmed that the voltage fed to the vibrator was not affected by the MRI scan while the MRI scan was off and on.
We confirmed that the actual vibration level applied to the skin does not change with the MRI scan by measuring the sensory threshold outside and inside the MR room. We measured with vibration intensity just below the sensory threshold, to see if the MRI would increase the vibration intensity for the subject to perceive the vibration. We also measured just above the sensory threshold, to see if the MRI would decrease the vibration intensity for the subject to not be able to perceive the vibration.
To demonstrate that the system could enhance fingertip tactile sensation remotely as in the previous study (Enders et al 2013), we compared fingertip tactile sensation without noise to that with vibrotactile noise applied to the wrist. We measured fingertip tactile sensation as the minimum intensity of tactile stimulus on the fingertip that subjects could perceive. We applied tactile stimulus to the fingertip using the C-3 Tactor (Engineering Acoustics, Inc, Casselberry, FL) using five healthy young adults.
We placed the vibrator flat to the hand skin and attached using sticky tapes. We applied no additional pressure on the vibrator. Direct measurement of vibration amplitudes in the MR room was not possible as such measurement devices are not MRI-safe.
3.0 Results and Discussion
3.1 MR Safety Testing and Phantom Testing
The BNC coaxial cable and piezoelectric vibrator passed the initial inspection outside the MR room using a strong magnet. The BNC connector was affected by the magnet. Since the BNC connector is to stay at the interface panel between the MR room and the computer room which is located outside the 10 gauss (1 mT) line, it was recommended that care should be taken with installing the BNC connector to the interface panel, by not bringing the connector close to the 10 gauss (1 mT) line. The cable and vibrator also passed the second safety test inside the MR room, as there were no detectable forces or magnetic effects in the MR room. These tests assured that the device does not pose a risk to the MRI scanner.
Patient safety testing confirmed no hazard of electric shock or risk of skin burning. The anatomical scans showed no signs of the device interfering with the MRI scans. Upon passing these safety and phantom tests, the vibrotactile device was approved for use within the short bore 3.0 T MR room at the Medical College of Wisconsin.
3.2 Device Performance with MRI
The device works well in the MRI room. Similar power spectral density when MRI is ON and OFF showed the MRI does not interfere with the device.
When the voltage input to the vibrator was just below the sensory threshold level that was determined outside the MR room, the subject did not feel any vibration during the MRI scans, which indicates that the vibration intensity did not increase during the MRI scans. When the voltage input to the vibrator was just above the sensory threshold level that was determined outside the MR room, the subject constantly felt the vibration during the MRI scans, indicating that the vibration intensity did not decrease during the MRI scans. Thus, the subject could not perceive any changes in vibration amplitudes, suggesting that the vibration amplitude was not affected by the MRI scans.
Improvement in fingertip tactile sensation with remote vibrotactile noise was demonstrated using the developed vibrotactile system. Consistently with Enders et al (2013), application of this vibrator generating subthreshold vibrotactile noise at the wrist resulted in improved index finger sensation (Figure 3). The improvement in the index finger sensation is shown as a decrease in sensory threshold on the index finger pad.
Figure 3.
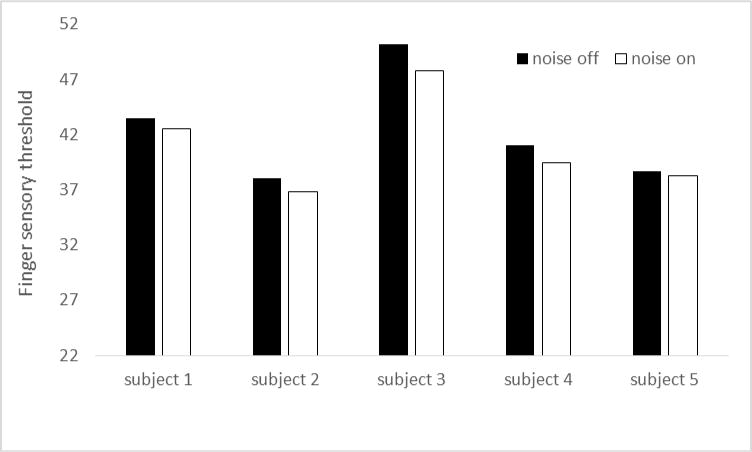
Demonstration of fingertip tactile sensory enhancement using the remote vibrotactile noise applied using the developed device. Consistent with Enders et al (2013), all five subjects’ fingertip sensation improved with noise applied to the wrist using the developed device. Improved sensation was indicated by the decreased sensory threshold (minimum level of tactile stimulus needed for the subject to perceive the stimulus, expressed in % stimulus intensity).
Conclusion
The developed vibrotactile system drives a 12 mm diameter piezoelectric vibrator that can be safely used inside the MR room. The amplifier can amplify the MP3 noise signal to a satisfactory level for the subject in the MRI room, and doesn’t interact with the MRI machine nor distort the MRI scans. The vibrator function does not appear to be affected by the MRI, either. Using the developed vibrotactile system, we were also able to replicate the previous finding of improved fingertip tactile sensation by applying vibrotactile noise to the wrist in five healthy adults. We are currently using the vibrotactile system in MRI investigations with healthy adults as well as stroke patients, without safety or technical issues. This vibrotactile system can be used for many other applications to apply vibrotactile stimulus to human skin or other systems during MRI scans.
Acknowledgments
This work was supported by the 2012–2013 University of Wisconsi-Madison/University of Wisconsin-Milwaukee Intercampus Research Incentive Grants Program and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
PMEA/485915/PAP/7051 resubmission to Physiological Measurement http://iopscience.iop.org/09673334/
Physics and Astronomy Classification Scheme: 87.19.lt, *43.64.Vm, *43.66.Wv
Contributor Information
Fa Wang, Email: fwang57@wisc.edu.
Kishor Lakshminarayanan, Email: lakshmi4@uwm.edu.
Gregory P. Slota, Email: gslota@vt.edu.
Na Jin Seo, Email: najinseo@uwm.edu.
John G Webster, Email: webster@engr.wisc.edu.
References
- Augurelle AS, Smith AM, Lejeune T, Thonnard JL. Importance of cutaneous feedback in maintaining a secure grip during manipulation of hand-held objects. J Neurophysiol. 2003;89:665–71. doi: 10.1152/jn.00249.2002. [DOI] [PubMed] [Google Scholar]
- Bjorkman A, Rosen B, Lundborg G. Acute improvement of hand sensibility after selective ipsilateral cutaneous forearm anaesthesia. Eur J Neurosci. 2004;20:2733–6. doi: 10.1111/j.1460-9568.2004.03742.x. [DOI] [PubMed] [Google Scholar]
- Blennerhassett JM, Carey LM, Matyas TA. Grip force regulation during pinch grip lifts under somatosensory guidance: comparison between people with stroke and healthy controls. Arch Physical Med Rehab. 2006;87:418–29. doi: 10.1016/j.apmr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Carey LM. Somatosensory loss after stroke. Crit Rev Physical Rehab Med. 1995;7:51–91. [Google Scholar]
- Collins JJ, Thomas TI, Grigg P. Noise-mediated enhancements and decrements in human tactile sensation. Physical Rev E. 1997;56:923–6. [Google Scholar]
- Enders LR, Hur P, Johnson MJ, Seo NJ. Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance. J NeuroEng Rehab. 2013;10:105. doi: 10.1186/1743-0003-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RW, Maksimovic D. Fundamentals of Power Electronics. Second. New York: Springer; 2004. p. 161. [Google Scholar]
- Hidaka I, Nozaki D, Yamamoto Y. Functional stochastic resonance in the human brain: Noise induced sensitization of baroreflex system. Phys Rev Lett. 2000;85:3740–3. doi: 10.1103/PhysRevLett.85.3740. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–64. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Kosmopoulos ML, Hur P, Enders LR, Seo NJ. 7th World Congress of Biomechanics. Boston, MA: 2014. Effect of remote subthreshold vibrotactile noise on hand function post-stroke. [Google Scholar]
- Kurita Y, Shinohara M, Ueda J. Wearable sensorimotor enhancer for fingertip based on stochastic resonance effect. IEEE Transactions on Human-Machine Systems. 2013;43:333–337. [Google Scholar]
- Liu W, Lipsitz LA, Montero-Odasso M, Bean J, Kerrigan DC, Collins JJ. Noise-enhanced vibrotactile sensitivity in older adults, patients with stroke, and patients with diabetic neuropathy. Arch Phys Med Rehabil. 2002;83:171–6. doi: 10.1053/apmr.2002.28025. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983;10:639–65. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Monzée J, Lamarre Y, Smith A. The effects of digital anesthesia on force control using a precision grip. J Neurophysiol. 2001;89:672–83. doi: 10.1152/jn.00434.2001. [DOI] [PubMed] [Google Scholar]
- Wells C, Ward LM, Chua R, Inglis JT. Touch noise increases vibrotactile sensitivity in old and young. Psychological Science. 2005;16:313–20. doi: 10.1111/j.0956-7976.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res. 1984;53:277–84. doi: 10.1007/BF00238156. [DOI] [PubMed] [Google Scholar]


