Abstract
Background
RBC transfusion is a life-saving therapy, the logistical implementation of which requires RBC storage. However, stored RBCs exhibit substantial donor variability in multiple characteristics, including hemolysis in vitro and RBC recovery in vivo. The basis of donor variability is poorly understood.
Study Design and Methods
We applied a murine model of RBC storage and transfusion to test the hypothesis that genetically distinct inbred strains of mice would demonstrate strain-specific differences in RBC storage. In vivo recoveries were determined by monitoring transfused RBCs over 24 hours. Timed aliquots of stored RBCs were subjected to tandem chromatography/mass spectrometry analysis to elucidate metabolic changes in the RBCs during storage.
Results
Using independent inbred mouse strains as donors, we found substantial strain-specific differences in post-transfusion RBC recovery in vivo following standardized refrigerated storage in vitro. Poor post-transfusion RBC recovery correlated with reproducible metabolic variations in the stored RBC units, including increased lipid peroxidation, decreased levels of multiple natural antioxidants, and accumulation of cytidine. Strain-dependent differences were also observed in eicosanoid generation (i.e. prostaglandins and leukotrienes).
Conclusion
These findings provide the first evidence of strain-specific metabolomic differences following refrigerated storage of murine RBCs. They also provide the first definitive biochemical evidence for strain specific variation of eicosanoid generation during RBC storage. The molecules described that correlate with RBC storage quality, and their associated biochemical pathways, suggest multiple causal hypotheses that can be tested regarding predicting the quality of RBC units prior to transfusion and developing methods of improved RBC storage.
INTRODUCTION
Red blood cell (RBC) transfusion is a life-saving therapy, and refrigerated storage is crucial for maintaining an adequate supply of donor units. However, recent studies have focused on potential adverse clinical sequelae resulting from transfusing humans with RBC units stored for longer periods of time. Indeed, multiple observational studies in human patients provide data demonstrating inferior clinical outcomes when older, stored RBC units are transfused1. Nonetheless, this issue remains controversial because other, similarly designed human studies, show no difference in clinical outcome when comparing patients receiving transfusions of older or fresher RBC units1,2. To begin to address this controversy, several prospective human trials are currently ongoing, and one was recently completed3–5. However, it is not controversial that stored RBCs accumulate multiple factors that may be toxic when infused (e.g. microparticles, free iron, free hemoglobin, prostaglandins, and leukotrienes)6–14.
One complication in studying RBC transfusion is that there is considerable donor-to-donor variation in the effect of refrigerated storage on RBC function and quality. In addition, there is a general absence of robust analytic tests that consistently and accurately predict the quality of a given RBC unit prior to transfusion15. Due to the genetic and environmental complexity of outbred human donor populations, and the difficulty in limiting the number of independent variables in studying human RBC transfusion, we developed a robust animal model to begin to address these issues16. Using inbred mouse strains in defined environmental and dietary settings limits the experimental variability of the system, and allows for deliberate manipulation of independent variables. This was combined with metabolomic methods to determine whether variations in the levels, and/or changes in concentrations, of small molecules in vitro correlated with post-transfusion RBC recovery in vivo. In particular, we evaluated whether: 1) genetic background correlated with donor RBC storage quality, 2) metabolomic differences correlated with donor RBC storage quality, and 3) accumulation of potentially toxic molecules correlated with genetic background and/or donor RBC storage quality.
MATERIALS AND METHODS
Collection and Processing of Mouse RBC Units
H2-Kb GFP+ mice were a generous gift from Dr. Derek A. Persons (St. Jude Children’s Research Hospital; Memphis, TN) and were bred by the Emory University Division of Animal Husbandry by crossing heterozygous mice with C57BL/6J mice in excess of 10 generations17; the RBCs of these mice express Green Fluorescent Protein (GFP) and are readily enumerated by flow cytometry. These mice were used as the universal transfusion recipients in all of the described studies. C57BL/6J and FVB/J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). All mice were housed in identical conditions with identical diets and identical access to water. In an effort to model human RBC storage, mouse donor RBCs were collected and stored in a similar fashion to human RBC storage, as previously described16. All studies were carried out under protocols approved by the Institutional Animal Care and Use Committees.
Assessment of Post-Transfusion RBC Recovery and Endogenous RBC lifespan
For all RBC storage experiments, donor mouse RBCs were stored at 4°C for 14 days; this time frame was previously identified, using C57/BL6J mouse donor RBCs, as appropriately approximating the refrigerated shelf life identified by the Food and Drug Administration for human RBCs; that is, on average, 75% of donor mouse RBCs were still circulating 24 hr post-transfusion at the Day 14 “outdate.16” Therefore, at Day 14 of storage, 100 μl of stored, donor, packed RBCs (i.e. one mouse “unit”) were transfused into H2-Kb GFP+ recipient mice. At 10 min, 30 min, 1 hr, 4 hr, and 24 hr post-transfusion, peripheral blood was obtained from recipients, and transfused RBCs were enumerated by flow cytometry by gating on GFP-negative RBC events. Peripheral blood obtained from non-transfused mice was used to enumerate the low number of events in the GFP-negative gate, which were then subtracted from the analysis of transfused RBCs.
For determination of endogenous RBC lifespan, mice received 3 daily injections of NHS-biotin i.p. (Pierce, Thermo Scientific) until ~100% of circulating RBCs were reactive with avidin-allophycocyanin, as assessed by flow cytometry. Peripheral RBCs were then obtained weekly and stained with avidin-allophycocyanin, followed by enumeration of positive and negative RBCs by flow cytometry. These data were then plotted to determine RBC lifespan.
Mass Spectrometry Analysis of RBC Samples
Donor RBC samples, freshly obtained and at various times after refrigerated storage, were rapidly frozen using dry ice/ethanol and stored at 80°C. The supernatant was not stored separately nor were the RBCs washed and stored separately; thus, the results obtained evaluated the metabolites in the entire “unit.” Samples were shipped on dry ice to Metabolon Inc., where they were split into equal parts for analysis by gas-chromatography/mass spectrometry (GC/MS) and liquid chromatography-tandem mass spectrometry (LC/MS/MS). The LC/MS/MS platform was based on a Waters ACQUITY UPLC and a Thermo-Finnigan LTQ mass spectrometer, which consisted of an electrospray ionization (ESI) source and linear ion-trap (LIT) mass analyzer. The sample extract was split into two aliquots, dried, and then reconstituted in acidic or basic LC-compatible solvents, each of which contained 11 or more injection standards at fixed concentrations. One aliquot was analyzed using acidic positive-ion optimized conditions and the other using basic negative-ion optimized conditions in two independent injections using separate dedicated columns. Extracts reconstituted in acidic conditions were gradient eluted using water and methanol, both containing 0.1% Formic acid, whereas the basic extracts, which also used water/methanol, contained 6.5 mM Ammonium Bicarbonate. The MS analysis alternated between MS and data-dependent MS2 scans using dynamic exclusion. The samples destined for GC/MS analysis were re-dried under vacuum desiccation for a minimum of 24 hr prior to being derivatized under dried nitrogen using bistrimethyl-silyl-triflouroacetamide. The GC column was 5% phenyl and the temperature ramp was from 40° to 300° C in a 16 minute period. Samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Identification of known chemical entities was based on comparison to metabolomic library entries of purified standards. As of the time of analysis, more than 1000 commercially-available purified standard compounds had been acquired and registered into LIMS for distribution to both the LC and GC platforms for determination of their analytical characteristics. The combination of chromatographic properties and mass spectra gave an indication of a match to the specific compound or an isobaric entity.
The peak areas for each identified biochemical entity were log transformed, scaled to the median value for each compound observed in the experiment, and normalized to Bradford protein content; results below the limit of detection were imputed with the minimum observed value for the compound. A Two-Way ANOVA with Contrasts was used to determine the significance of variable main effects (e.g. Condition or Time/Day) and their interaction, and to identify biochemical entities that differed significantly between experimental groups (p≤0.05). An estimate of the false discovery rate (q-value) is calculated to take into account the multiple comparisons that normally occur in metabolomic-based studies.
RESULTS
Variation Among Inbred Mouse Strains in Post-Transfusion RBC Recovery After Refrigerated Storage
For the purposes of this study, “RBC storage quality” was defined as the extent of post-transfusion recovery of the stored RBCs; higher recovery was defined as higher quality. Thus, to test the hypothesis that donor-specific variation in RBC quality after refrigerated storage exists in non-human systems, mice were chosen as the experimental model. After screening 6 common inbred laboratory strains of mice from Jackson labs (C57BL6/J, FVB/J, C3H/J, BALBc/J, SJL/J, DBA2/J) and observing variation in RBC storage quality between strains (data not shown), we focused our studies on a direct comparison of the one that exhibited the best storage quality (C57BL/6J; i.e. B6), and the one that exhibited the worst RBC storage quality (FVB/NJ; i.e. FVB). To this end, peripheral blood RBCs were obtained from B6 and FVB mice, filter leukoreduced, stored for 14 days, transfused, and evaluated for post-transfusion recovery using a method that models human RBC storage16; these prior studies demonstrated that 14 days of storage provided results with B6 RBCs that mimicked those found with healthy human blood donors after 42 days of storage (i.e. ~75% post-transfusion recovery). To allow post-transfusion tracking without labeling or otherwise modifying the donor RBCs, a single strain of recipient mice was utilized that expressed transgenic GFP in their RBCs; in this way, no artifactual damage to the donor RBCs was introduced by any further pre-transfusion manipulation or any labeling process. The circulating, transfused donor RBCs were then quantified by flow cytometry by gating on the GFP-negative population.
RBCs from each donor strain were stored at the same hematocrit, and the same volume of RBCs was transfused from each strain into naïve recipients. All donor RBCs were transfused intravenously through the tail vein. As is typically the case when transfusing stored human or murine RBCs into either human or murine recipients, respectively, there was a rapid clearance phase followed by a significantly slower clearance phase (Figure 1A). The early phase clearance in humans has been interpreted as the rapid extravascular clearance of significantly storage-damaged RBC18, which has been demonstrated to be the case in mice, since depletion of phagocytes prevents the early clearance from occurring11. At 10 min post-transfusion, approximately 40% fewer transfused FVB RBCs were still circulating in peripheral blood, as compared with transfused B6 RBCs (Figure 1A). This statistically significant difference persisted over time, with FVB RBCs having only ~50% of the 24-hour post-transfusion recovery of B6 RBCs (Figure 1A). The post-transfusion recovery of 14-day old B6 RBCs was comparable to that seen previously16. Taken together, these data demonstrate a strain-specific difference in donor RBC storage quality and suggest that a genetic difference(s) may account for the observed effects.
Figure 1.
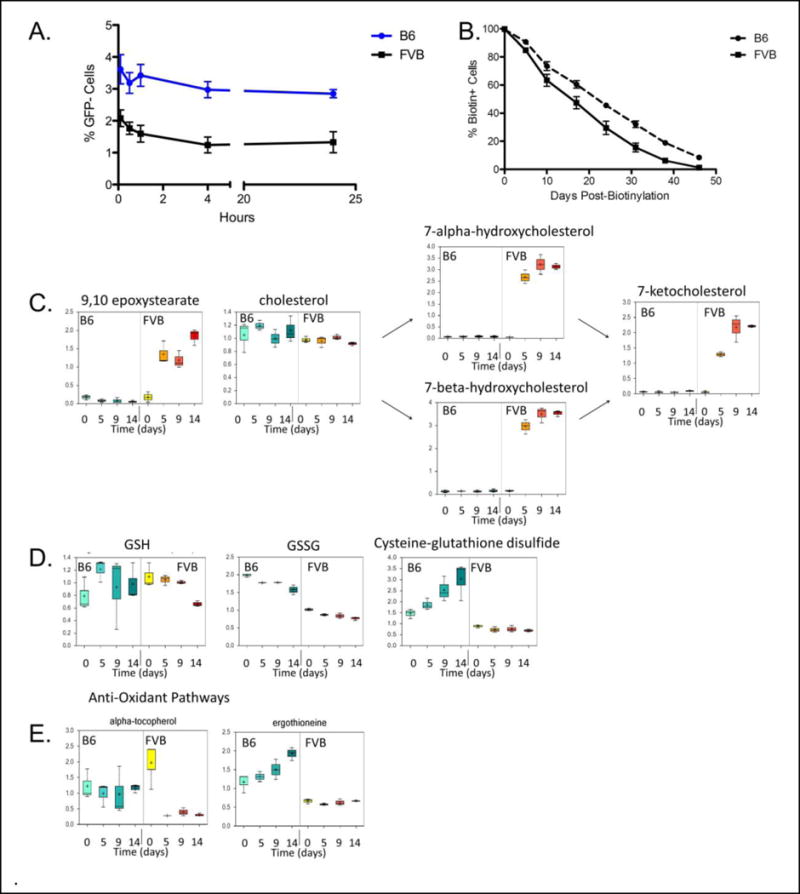
Post-Transfusion Recovery of Stored Murine RBC units, Endogenous RBC Lifespan, and Metabolomic Analysis of Markers of Oxidative Stress During RBC storage. Murine RBCs were collected and stored (as described in Methods) for 14 days, and then transfused into H2-Kb GFP mice that express transgenic GFP on all hematopoietic cells, including RBCs. (Panel A) Peripheral blood of recipient mice was sampled at indicated times post-transfusion and recovery of transfused, stored RBCs was determined by flow cytometric enumeration of GFP-negative RBCs. (Panel B) B6 or FVB mice underwent biotinylation in vivo until 100% of their RBCs were avidin reactive. Peripheral blood samples were then stained with avidin-APC at the indicated time points and the percentage of avidin-reactive RBCs was enumerated, which allowed a determination of endogenous RBC lifespan. (Panels C–E) Aliquots of stored RBC units obtained from FVB and B6 mouse donors were frozen at the indicated time points and were then analyzed by GC/MS and LC/MS/MS, as appropriate (as described in Methods). Results are reported for products of lipid peroxidation (C), for glutathione pathway components (D), and for two natural anti-oxidants (E). These metabolomic data represent the combined results from 3 separate experiments; in each experiment, post-transfusion recovery was determined at Day 14 of storage, with similar results to those in Panel A in each case.
To test if RBC quality after refrigerator storage in vitro correlated with the endogenous RBC lifespan in the donor mice in vivo, the total blood volumes of B6 and FVB mice were each labeled by biotinylation in vivo. In this method, any new RBCs that are generated do not have biotin on their surface; thus, RBC lifespan can be determined by the rate of decrease of avidin-reactive RBCs over time19. Using this approach, FVB RBCs had a shorter endogenous lifespan as compared to B6 RBCs, correlating with the trend seen in refrigerated RBC storage quality (Figure 1B). However, the natural differences in endogenous lifespan were not as pronounced as the differences in post-transfusion recovery of stored RBCs. Taken together, these data demonstrate strain-dependent differences in endogenous RBC lifespan, which correlate with amplified differences in post-transfusion recovery resulting from refrigerated RBC storage.
Analysis of Anti-oxidant Pathways and Accumulation of Oxidative Damage During RBC Storage
Mass spectrometric analysis captured multiple types of metabolites that were tracked during storage. The current report focuses on several illustrative types of metabolites and their associated biosynthetic and catabolic pathways. Levels of 9,10 epoxystearate (as an indicator of lipid oxidation) increased progressively in refrigerator-stored FVB RBC units, but remained largely at baseline levels in stored B6 RBC units (Figure 1C). Likewise, oxidation of cholesterol into both 7-alpha-hydroxycholesterol and 7-beta-hydroxycholesterol, and their subsequent conversion into 7-ketocholesterol, progressively increased in FVB RBC units, but not in B6 units (Figure 1C). However, cholesterol levels in both FVB and B6 units did not differ and did not change significantly with storage time. Taken together, these data demonstrate that increased lipid oxidation correlated with poor storage quality of FVB RBCs.
Reduced glutathione (GSH) is generally considered to be the most robust anti-oxidant in RBCs. Although no statistically significant differences in GSH levels were observed when comparing stored RBC units from B6 and FVB mice (Figure 1D), B6 RBC units had consistently higher levels of oxidized glutathione (GSSG) and cysteine-glutathione disulfides (Figure 1D); the latter also increased progressively with storage in B6 units. In addition, although levels of the natural anti-oxidant alpha-tocopherol (i.e. Vitamin E) were equivalent at the time of blood collection, alpha-tocopherol levels dropped rapidly in FVB, but not B6, RBC units (Figure 1E). Moreover, levels of another anti-oxidant, ergothioneine, were significantly higher on the day of collection in B6 RBC units, as compared to FVB RBC units (Figure 1E); these levels also increased during storage in B6, but not FVB, RBC units.
Analysis of multiple components of the GSH biosynthetic pathway revealed that B6 RBC units accumulated higher levels of homocysteine and cysteine during storage, as compared to FVB RBC units (Figure 2); these could potentially provide more substrate for GSH synthesis de novo in the RBC units by combining with glutamate and glycine. Nonetheless, no differences in glutamate or glycine levels were observed when comparing FVB and B6 RBC units, either at baseline or during storage. In addition, the sum of GSH and GSSG levels was higher in B6, as compared to FVB, RBC units. Finally, as compared with FVB RBC units, B6 RBC units had significantly higher levels of byproducts of GSH synthesis, including 2-hydroxybutyrate, 2-aminobutyrate, and ophthalmate (Figure 2). Interestingly, accumulation of ophthalmate was previously reported to reflect GSH consumption in an effort to ameliorate oxidative stress20. Taken together, these results suggest that there may be greater flux through the GSH pathway in B6 RBC units, and that RBC storage quality correlates positively with an enhanced ability to handle oxidative stress.
Figure 2.
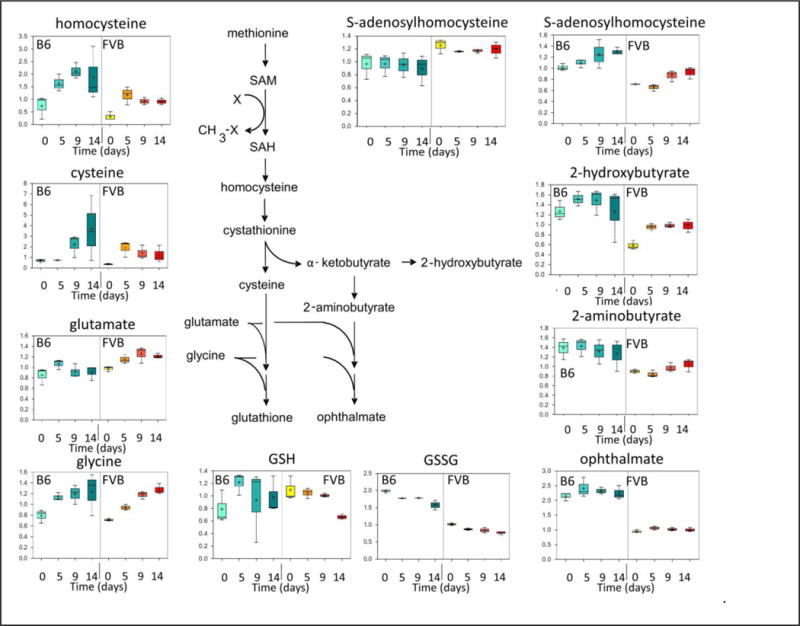
Metabolomic Analysis of Components of the Glutathione Synthesis and Degradation Pathway During Storage of Murine RBC Units. The same specimens described in Figure 1, Panels C–E, were analyzed for components in the glutathione synthesis pathway. These data represent the combined results from 3 separate experiments.
Evaluation of Glycolysis and Purine Metabolism During RBC Storage
When glycolytic pathways were analyzed (Figure 3A), the results were similar to what has been repeatedly reported regarding refrigerated storage of human RBCs6. In particular, glucose and 2,3-DPG levels progressively declined, whereas lactate levels progressively increased in stored RBC units from both mouse strains. In contrast, pyruvate levels increase and remain elevated during storage of B6, as compared to FVB, RBC units.
Figure 3.
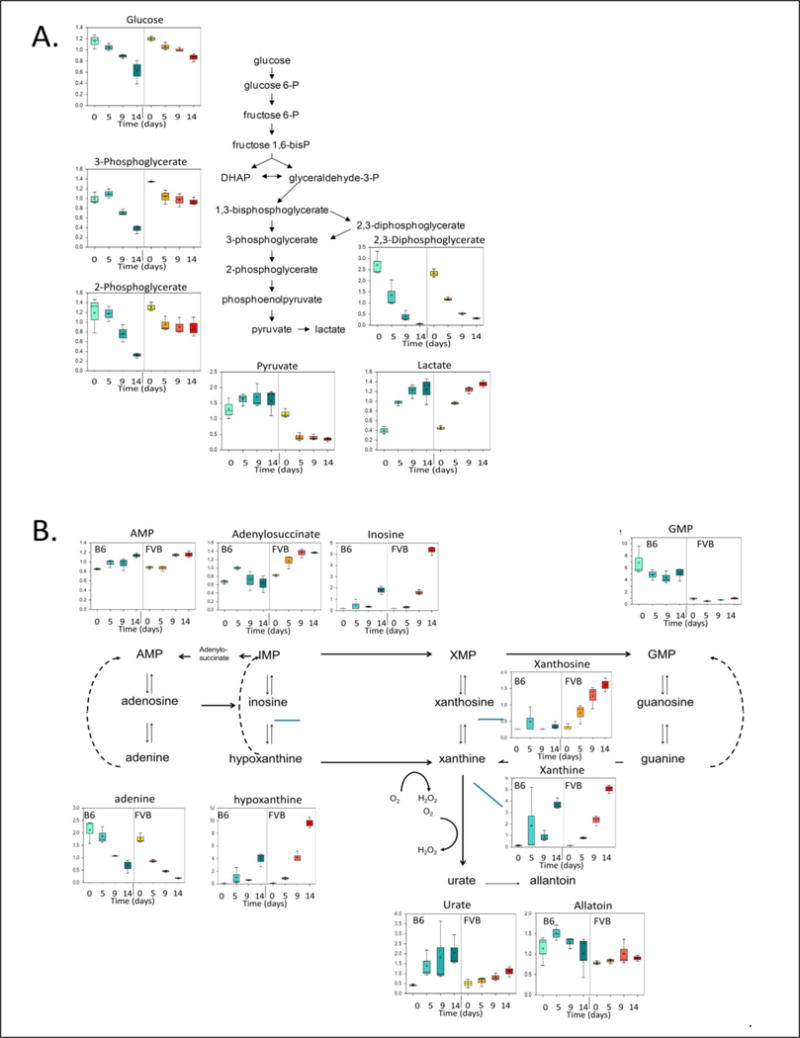
Metabolomic Analysis of Components of the Glycolytic and Purine Metabolic Pathways During Storage of Murine RBC Units. The same specimens described in Figure 1, Panels C–E, were analyzed for components of the glycolytic (Panel A) and purine metabolic (Panel B). These data represent the combined results from 3 separate experiments.
Because adenine is a component of various RBC storage solutions (e.g. CPDA-1), its initial levels in RBC units are high due to this supplementation. During refrigerated storage, adenine progressively declined, and AMP levels slowly increased, similarly in both B6 and FVB RBC units (Figure 3B). Inosine, into which adenine can be converted, increased steadily over storage time in RBC units from both mouse strains, but with higher levels in FVB RBC units. Both hypoxanthine and xanthine increased significantly over time, with higher endpoint levels in FVB, as compared to B6, RBC units; these results suggest that a substantial quantity of inosine can be metabolized in a way that prevents subsequent ATP synthesis. Urate levels were also higher in B6, as compared to FVB, RBC units, and levels of allantoin (the main breakdown product of urate) remained relatively stable in both B6 and FVB RBC units.
Accumulation of Eicosanoids During RBC storage
The RBC membrane is a rich source of phospholipids, and the oxidation and/or enzymatic processing of these can produce arachidonic acid, which, in turn, can be converted into prostaglandins and/or leukotrienes (the latter, together, are termed eicosanoids). Eicosanoids have potent and widespread effects on inflammation, vascular tone, vascular permeability, and platelet activation21–23. Interestingly, arachidonic acid (or, equivalently, arachidonate) accumulated progressively during storage of both B6 and FVB RBC units (Figure 4). Although baseline levels were significantly higher in FVB RBC units, peak levels at Day 14 were equivalent in both strains. The source of arachidonic acid in this setting is likely to be phospholipase-induced release of fatty acids from the sn-2 position of inositol-containing phospholipids. However, unlike the similar levels of arachidonic acid, there was a substantial increase in lysophospholipid by-products (i.e. 1-palmitoylglycerophosphoinositol and 1-stearoylglycerophosphoinositol) in FVB, but not B6, RBC units. There was also a dramatic accumulation of eicosanoids during storage of FVB, but not B6, RBC units. The latter included prostaglandin E2 and several leukotriene precursors (i.e. 5-HETE, 12-HETE, and 15-HETE). In addition, in FVB RBC units, the levels of linoleic acid metabolites (i.e. 13-HODE and 9-HODE) significantly increased during storage; in contrast, these remained low in B6 RBC units. Together, these data suggest that there is increased generation and metabolism of arachidonic acid in FVB RBC units, with concomitant increases in the synthesis of multiple eicosanoids during storage of these units.
Figure 4.
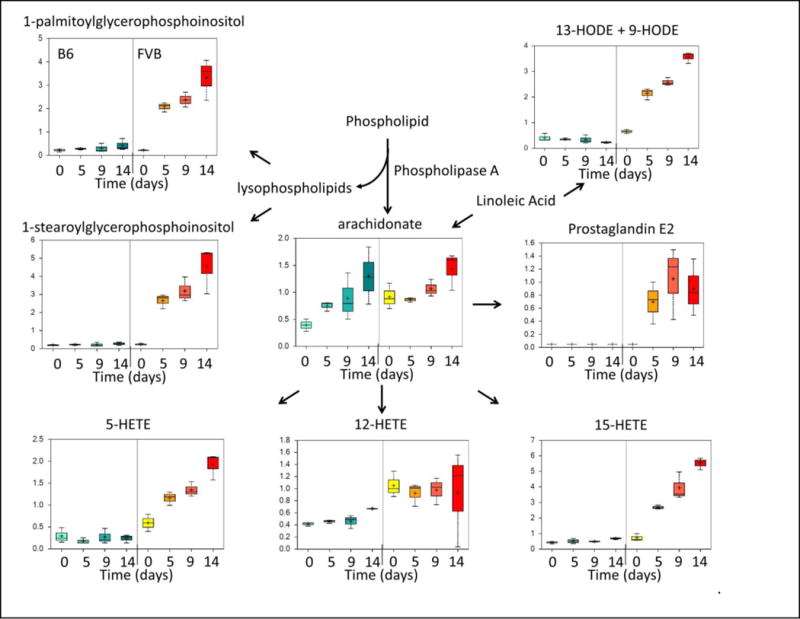
Arachidonic Acid and Eicosanoid Levels in Stored Murine RBC Units, as Measured by Metabolomic Analysis. The same specimens described in Figure 1, Panels C–E were analyzed for determining levels of arachidonic acid and various eicosanoids. These data represent the combined results from 3 separate experiments.
Pyrimidine Metabolism During RBC storage
Assessment of pyrimidine metabolism demonstrated that FVB RBC units had higher levels of cytidine at baseline, as compared to those obtained from B6 mice (Figure 5A). In addition, during storage, cytidine levels increased to a significantly greater extent in FVB, as compared to B6, RBC units. Levels of N4-acetylcytidine (a cytidine metabolite) were also approximately 8-fold higher, at all time points measured, in FVB compared to B6 RBC units (Figure 5A). Finally, the levels of one downstream metabolite, uracil, were significantly greater in FVB than B6 RBC units at end of storage; however, no differences were observed in the levels another metabolite, 5-methylcytidine.
Figure 5.
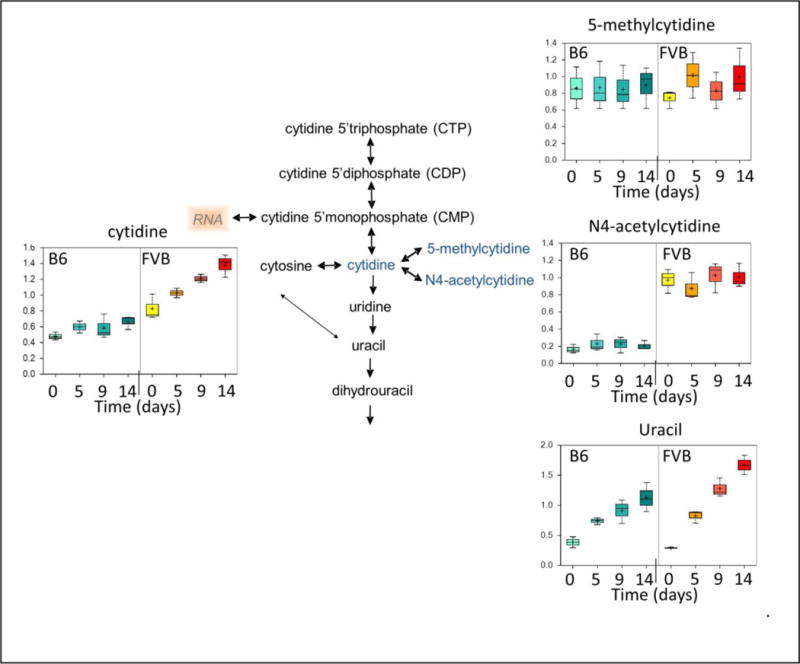
Pyrimidine Metabolism in Murine RBC Units with Differential Storage Biology. The same murine specimens described Figure 1 were analyzed for cytidine metabolism (cytidine, 5-methylcytidine, and N4-acetylcytidine). These data represent the combined results from 3 separate experiments.
DISCUSSION
The data presented herein demonstrate that different inbred strains of mice (i.e. B6 and FVB) have significant differences in RBC storage quality (defined as post-transfusion RBC recovery), which is the gold standard for evaluating the storage of human RBC donor units. Because post-transfusion RBC clearance depends on the storage quality of the RBCs themselves, as well as on the activity of the recipient’s mononuclear phagocyte system, we used one mouse strain as the universal transfusion recipient for these studies (i.e. GFP transgenic mice on a B6 background). This experimental design decreases concerns regarding differences in RBC clearance biology in the recipient, allowing an exclusive focus on the storage of the donor RBC units. Although this approach could raise the concern that the B6 transfusions were syngeneic, whereas the FVB transfusions were allogeneic, the 24-hr RBC recovery assay is complete long before adaptive immunity is induced. In addition, B6 mice have no “naturally-occurring” antibodies that recognize FVB RBC antigens as tested by flow cytometry, both in vitro and in vivo (data not shown). Moreover, there is no difference when B6xFVB F1 recipients are used, the storage quality of B6 RBCs is significantly better than FVB RBCs (data not shown). Finally, the described accumulation of oxidative damage (Figure 1) and eicosanoids (Figure 4) occurs in vitro during storage prior to transfusion; thus, these effects are unrelated to potential allogenicity in vivo. Taken together, these data describe strain-specific variations in donor RBC storage quality, and suggest that this variation is caused, at least in part, by genetic determinants.
The current results with stored mouse RBC units suggest that oxidative damage increases with storage duration, particularly for FVB mice (Figure 1C). In addition, levels of two endogenous anti-oxidants (i.e. alpha-tocopherol and ergothioneine) are maintained at higher levels in B6, as compared to FVB, stored RBCs units (Figure 1E). Although the results for the GSH biosynthetic pathway are more complex (Figure 1D and Figure 2), and future kinetic studies will be required, one explanation that is consistent with the current data posits that synthesis and flux through this pathway is greater in stored B6 RBC units, thereby allowing these units to handle oxidative stress more effectively than those obtained from FVB mice. In addition, the findings presented in Figures 1 and 2 are consistent with a previous report of oxidative stress-induced hemolysis in inbred mouse strains; in this setting, B6 RBCs exhibited decreased H2O2-induced injury, as compared to RBCs obtained from other strains; however, FVB mice were not studied24. Finally, an important role for oxidative stress and glutathione in the biology of RBC storage has been described previously, particularly with regard to human RBC storage25–27.
Recently, eicosanoids have been reported to accumulate during storage of human RBC units. The same molecular species as we observed in FVB RBC units were reported in human units (i.e. Arachidonic acid, 5-HETE, 12-HETE, and 15-HETE)13 ; however, prostaglandin increase was not noted. To the best of our knowledge, the current report is the first demonstration of substantial donor variation (in mice) regarding the accumulation of eicosanoids during RBC storage, and also the first indication that this variation may have a genetic basis in any species. In FVB RBC units, the maintenance of arachidonic acid levels during storage, in the context of increasing levels of lysophospholipids and eicosanoids, suggests a rapid flux through this pathway (in terms of arachidonic acid generation and its subsequent conversion into downstream products). In contrast, although arachidonic acid levels increase during storage of B6 RBC units, there is little generation of lysophospholipids or conversion to eicosanoids. Taken together, these results suggest the testable hypothesis that FVB RBC units have higher phospholipase, cyclooxygenase, and lipoxygenase activities, as compared to B6 RBC units. It is worth noting that C57BL/6 mice are deficient in a secreted phospholipase A2 (sPLA2), which may explain increased arachidonic acid generation in FVB mice. However, this would not explain the increased conversion into prostaglandins and leukotrienes, which would require other differences in cyclooxygenase and lipoxygenase activities. Of particular note is that analysis of human RBC units have not detected cellular or soluble phospholipase activity (cPLA2 and sPLA2 were not detected)28. Rather, an alternate source of phospholipase has been implicated, in particular, peroxiredoxin-628. The extent to which similar findings hold in the murine system remain to be determined.
While the above changes have been noted in “stored RBC units”, neither the location (e.g. cellular or supernatant) nor the source (e.g. the RBCs themselves, residual leukocytes, or contaminating platelets) is determined by the current methodologies. Although leukoreduction results in a unit with essentially undetectable leukocytes, it seems likely that some remain. Moreover, platelets are far more numerous in mice than in humans, and while leukoreduction does decrease murine platelets in RBC units, significant numbers of detectable platelets persist (data not shown). Accordingly, while RBCs may turn out to be the source of eicosanoids, it would be premature and potentially incorrect to draw any inferences that the RBCs are the source of eicosanoids from the current studies. Nevertheless, regardless of the cellular source, the observation of dramatic differences between genetically distinct strains persists.
Long-term dietary and/or environmental factors could affect RBC storage quality. Indeed, the results of the classical human studies of Dern et al. could be due to heritable factors or long-term dietary behavior and/or environmental exposure29. In the current study, control over variation in acquired factors which may influence RBC storage was attempted in a controlled laboratory environment. All animals were females of the same age, were provided the same commercial mouse chow ad libitum and were housed in the same vivarium with identical sources of bedding, water, and caging.
We developed this mouse model of RBC storage to begin to understand whether genetic determinants affect RBC storage quality with a long-term goal of improving our understanding of human RBC storage. The latter assumes that there will be relevant correlations between observations made concerning mouse RBC storage with analogous studies of human RBC storage. In this regard, there are several important caveats and considerations. First, given the complexity and diversity of the biochemical pathways at hand, it is likely that multiple different pathways may affect RBC storage biology. Second, we evaluated only two inbred mouse strains, although there are multiple independent strains with known differences in hematological phenotype30. Third, genetic (and dietary and environmental) diversity will be even higher in the outbred human population. Thus, extending these approaches to different mouse strains, along with a wide-spread population-based analysis of humans, will be required in order to capture the variety of different mechanisms and genetics that may affect RBC storage. The current findings represent a beginning to this type of analysis, both as a proof-of-principle and as an opportunity for hypothesis generation. Future methodological studies will also be required, for example, to determine whether the findings presented herein result from metabolic changes in the stored RBCs themselves, in the supernatant, or both. Finally, these metabolomic results identify static concentrations of individual analytes; although it is possible to hypothesize about the flux through potential pathways, kinetic metabolic studies will be required to further test these hypotheses. In addition to metabolomics, proteomic analysis may be very useful to identify alterations in enzymes that may be logically predicted to contribute to the metabolic changes observed in this report.
One long-term practical consequence of these findings, and of the results from subsequent studies that may extend these findings, is to develop rapid and robust measures and metrics by which human RBC storage quality may be assessed prior to release of the units from the Blood Bank. In addition, identifying relevant measures and mechanisms may provide metrics for assisting in developing improved RBC storage conditions and systems.
Acknowledgments
These studies were funded in part by NIH R01 HL092977
Footnotes
Conflicts of Interest: James Zimring and John Roback have filed provisional patent applications to cover intellectual property contained in this report. No other authors have a conflict of interest.
References
- 1.van de Watering L. Red cell storage and prognosis. Vox Sang. 2011;100:36–45. doi: 10.1111/j.1423-0410.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 2.van de Watering L. Pitfalls in the current published observational literature on the effects of red blood cell storage. Transfusion. 2011;51:1847–1854. doi: 10.1111/j.1537-2995.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- 3.Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, Lachance C, Lee S, Walker CR, Hutton B, Ducharme R, Balchin K, Ramsay T, Ford JC, Kakadekar A, Ramesh K, Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 4.Lacroix J, Hebert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, Cook D, Marshall JC, McIntyre L, Turgeon AF. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev. 2011;25:197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, Triulzi D, Stowell CP. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. 2010;43:107–116. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–59. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Hess JR. Red cell storage. J Proteomics. 2010;73:368–373. doi: 10.1016/j.jprot.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, Sireci AN, Stephens HL, Stotler BA, Wojczyk BS, Zimring JC, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hod EA, Spitalnik SL. Harmful effects of transfusion of older stored red blood cells: iron and inflammation. Transfusion. 2011;51:881–885. doi: 10.1111/j.1537-2995.2011.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hod EA, Spitalnik SL. Stored red blood cell transfusions: Iron, inflammation, immunity, and infection. Transfus Clin Biol. 2012;19:84–89. doi: 10.1016/j.tracli.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kor DJ, Van Buskirk CM, Gajic O. Red blood cell storage lesion. Bosn J Basic Med Sci. 2009;9(Suppl 1):21–27. doi: 10.17305/bjbms.2009.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–2554. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tissot JD, Rubin O, Canellini G. Analysis and clinical relevance of microparticles from red blood cells. Curr Opin Hematol. 2010;17:571–577. doi: 10.1097/moh.0b013e32833ec217. [DOI] [PubMed] [Google Scholar]
- 15.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 16.Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominici M, Tadjali M, Kepes S, Allay ER, Boyd K, Ney PA, Horwitz E, Persons DA. Transgenic mice with pancellular enhanced green fluorescent protein expression in primitive hematopoietic cells and all blood cell progeny. Genesis. 2005;42:17–22. doi: 10.1002/gene.20121. [DOI] [PubMed] [Google Scholar]
- 18.Klein HG, Anstee DJ. Mollisoni’s Blood Transfusion in Clinical Medicine. Malden, Mass: Blackwell Publishing Inc; 2005. [Google Scholar]
- 19.Hoffmann-Fezer G, Maschke H, Zeitler HJ, Gais P, Heger W, Ellwart J, Thierfelder S. Direct in vivo biotinylation of erythrocytes as an assay for red cell survival studies. Ann Hematol. 1991;63:214–217. doi: 10.1007/BF01703446. [DOI] [PubMed] [Google Scholar]
- 20.Soga T, Baran R, Suematsu M, Ueno Y, Ikeda S, Sakurakawa T, Kakazu Y, Ishikawa T, Robert M, Nishioka T, Tomita M. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281:16768–16776. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 21.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33:304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Di Gennaro A, Haeggstrom JZ. The leukotrienes: immune-modulating lipid mediators of disease. Adv Immunol. 2012;116:51–92. doi: 10.1016/B978-0-12-394300-2.00002-8. [DOI] [PubMed] [Google Scholar]
- 23.Pratico D, Dogne JM. Vascular biology of eicosanoids and atherogenesis. Expert Rev Cardiovasc Ther. 2009;7:1079–1089. doi: 10.1586/erc.09.91. [DOI] [PubMed] [Google Scholar]
- 24.Kruckeberg WC, Doorenbos DI, Brown PO. Genetic differences in hemoglobin influence on erythrocyte oxidative stress hemolysis. Blood. 1987;70:909–914. [PubMed] [Google Scholar]
- 25.Dumaswala UJ, Wilson MJ, Wu YL, Wykle J, Zhuo L, Douglass LM, Daleke DL. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic Res. 2000;33:517–529. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- 26.Dumaswala UJ, Zhuo L, Jacobsen DW, Jain SK, Sukalski KA. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Radic Biol Med. 1999;27:1041–1049. doi: 10.1016/s0891-5849(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 27.Dumaswala UJ, Zhuo L, Mahajan S, Nair PN, Shertzer HG, Dibello P, Jacobsen DW. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol Cell Physiol. 2001;280:C867–C873. doi: 10.1152/ajpcell.2001.280.4.C867. [DOI] [PubMed] [Google Scholar]
- 28.Silliman CC. Lipids: Free Fatty Acids, Eicosanoids, and Lysophospholipids and the Pro-Inflammatory Effects of Transfusion ASH Meeting 2012. Scientific Program. 2012:SC1–48. [Google Scholar]
- 29.Dern RJ, Wiorkowski JJ. Studies on the preservation of human blood. IV. The hereditary component of pre- and poststorage erythrocyte adenosine triphosphate levels. J Lab Clin Med. 1969;73:1019–1029. [PubMed] [Google Scholar]
- 30.Mouse Phenome Database [monograph on the internet] 2012 Available from: http://phenome.jax.org/


