Abstract
Traditionally, alloimmunization to transfused blood products has focused exclusively upon recipient antibodies recognizing donor alloantigens present on the cell surface. Accordingly, the immunological sequelae of alloimmunization have been antibody mediated effects (i.e. hemolytic transfusion reactions, platelet refractoriness, anti-HLA and anti-HNA effects, etc.). However, in addition to the above sequelae, there is also a correlation between the number of antecedent transfusions in humans and the rate of bone marrow transplant (BMT) rejection - under reduced intensity conditioning with HLA matched or HLA identical marrow. BMT of this nature is the only existing cure for a series of non-malignant hematological diseases (e.g. sickle cell disease, thalassemias, etc.); however, rejection remains a clinical problem. It has been hypothesized that transfusion induces subsequent BMT rejection through immunization. Studies in animal models have observed the same effect and have demonstrated that transfusion induced BMT rejection can occur in response to alloimmunization. However, unlike traditional antibody responses, sensitization in this case results in cellular immune effects, involving populations such as T cell or NK cells. In this case, rejection occurs in the absence of alloantibodies, and would not be detected by existing immune-hematological methods. We review human and animal studies in light of the hypothesis that, for distinct clinical populations, enhanced rejection of BMT may be an unappreciated adverse consequence of transfusion which current blood bank methodologies are unable to detect.
Keywords: Transfusion, Transplantation, Bone Marrow Transplant, Rejection, Alloimmunization
Introduction
Bone marrow transplantation (BMT) is currently the only existing cure for a variety of disorders, for which blood transfusion support is an integral component in the treatment of the disease [1-8]. In general practice, rejection of a bone marrow transplant is now a rare event, due to the use of stringent myeloablative conditioning regimens that promote engraftment through suppression of immunity in the patient prior to transplantation. The toxic effects of stringent regimens are particularly useful in killing off neoplastic cells, thus directly treating the cancer. However, for bone marrow diseases that can be cured by BMT but that lack neoplasia (e.g. aplastic anemia, sickle cell disease, thalassemias, etc.), it is difficult to justify the use of stringent conditioning regimens, which increase morbidity and/or mortality. Accordingly, for BMT to be used as a safe cure for non-malignant indications, transplantation protocols must be developed that facilitate engraftment without the toxic profiles associated with traditional conditioning regimens used for BMT to treat malignant disease. The development of such “reduced intensity” conditioning protocols have resulted in greater levels of subsequent BMT rejection. This review focusses on recent work testing the hypothesis that transfusion may play a role in inducing such BMT rejection by priming the recipient immune system against minor histocompatibility antigens (mHA) shared on the transfused product on the donor bone marrow.
Decreasing the Immunological Barrier to Transplantation
In an attempt to decrease antigenic barriers in BMT, much like the approach for solid organ transplants, matching of Human Leukocyte Antigens (HLA) between donor and recipient has been carried out. In the event of an HLA matched sibling, one can achieve an essentially HLA identical graft [8]. If an HLA-identical sibling donor is unavailable (as in most cases), an HLA-matched, unrelated donor can be utilized [8]; however, in this case the HLAs are seldom identical at all loci and some degree of mismatch inevitably exists.
The incidence of graft rejection is significantly decreased when using HLA-matched or HLA-identical donors. However, even using HLA identical donors does not eliminate immune barriers to transplantation. Except in the case of identical twins, even siblings that are HLA identical will still have multiple amino acid polymorphisms throughout the genome. When a peptide containing a polymorphism can be presented by an MHC molecule, the allopeptide/MHC complex constitutes a minor histocompatibility antigen (mHA)†. For the purposes of the current review, the definition of an mHA will be that which is predominant in the field of transplantation; in particular, a variant peptide on an MHC variant shared between donor and recipient.
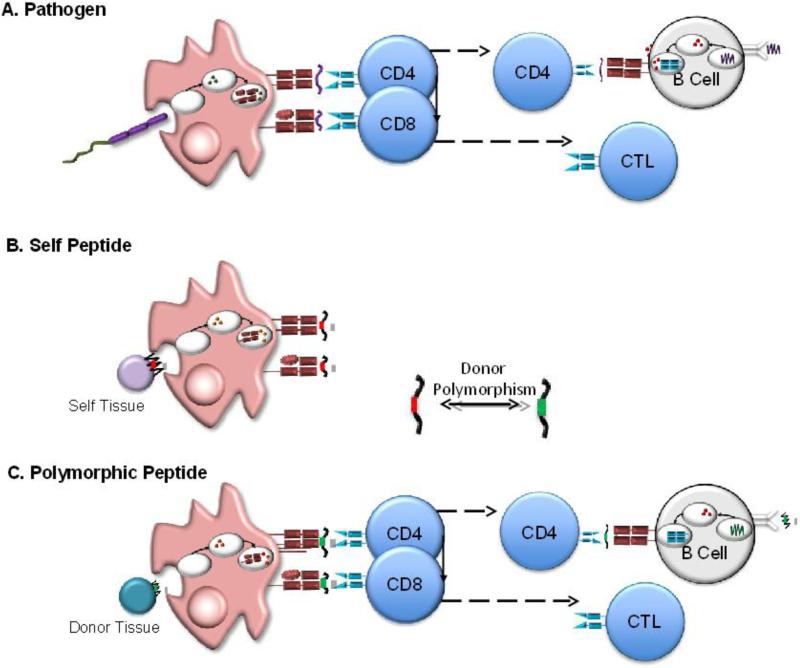
From a biochemical point of view, an mHA is very much like a peptide from a pathogen, in that it is a foreign peptide presented on self-MHC, albeit typically with smaller deviation from self-sequence than normal (see Figure 1). Thus, it is not surprising that mHAs are known to function as antigens for cellular immunity [9-11].
Figure 1. Molecular Nature of mHAs Consisting of Peptide/MHC complexes.
(A) Immune Response to Pathogens. Pathogens are consumed by antigen presenting cells, proteins from infectious microbes are processed into peptides, and the peptides are presented by MHC I and MHC II. T cell receptors from CD4 and CD8 T cells recognize peptide/MHCII and peptide/MHCI complexes, respectively. (B, C) Molecular Basis of mHA alloantigens. Donor polymorphisms (in this case single amino acid changes- indicated by red and green boxes) result in variant peptides presented by MHC. The depicted cartoons are not intended to indicate that the same peptide is presented by MHC I and MHC II.
Rejection Despite HLA identical BMT with Pharmacological Conditioning
Although development of reduced intensity conditioning regimens and matching of HLA represents substantial progress, up to 15% of patients still reject the graft. Indeed, recent trials of BMT to cure sickle cell disease reported high rejection rates in recipients of 6/7 HLA matched unrelated donors [12]. As a general principle, memory T and B cells are substantially less sensitive to elimination through pharmacological conditioning than are naïve cells. Thus, one popular hypothesis is that the patients who reject BMT have established immunity against donor mHAs prior to conditioning or BMT. Several sources of previous immunization are possible. First, pregnancy has been described to result in immunization to mHAs derived from fetal tissues. Second, graft rejection due to cross-reactivity between pathogen specific cellular immunity and an allopeptide in an HLA (or heterologous immunity) has been shown to occur in humans and animals [13-16]. Finally, since the vast majority of patients awaiting BMT for bone marrow disease are supported with transfusions prior to transplant, immunization to mHAs on transfused products has been hypothesized as a likely source of immunity to mHAs.
Of the above hypotheses, previous immunization from transfusion has been most rigorously investigated. Correlative studies have reported that chronically transfused patients reject HLA-matched bone marrow transplants at a greater frequency than minimally or un-transfused patients [17-22]. This association has been argued to support the hypothesis that blood transfusions sensitize patients against a subsequent transplantation [17, 19-25]. However, alternative explanations of the correlation between increased transfusions and BMT rejection exist. Multiple blood transfusions might simply correlate with patients who have a more advanced disease that results in a greater perturbation of the bone marrow microenvironment, which may lead to a less supportive compartment for transplanted marrow (e.g. an indication bias due to disease severity). Consistent with this interpretation, bone marrow injury from perivascular fibrosis of blood vessels in the marrow compartment and replacement of hematopoietic stem cells with adipose tissue has been observed in more severe non-malignant bone marrow failure disorders [4, 26]. Alternatively, the increased transfusions required for severe disease may be causal, but through non-immune mediated complications that disrupt the bone marrow microenvironment. For example, iron overload in sickle cell disease patients receiving chronic RBC transfusion has been noted to correlate with the failure to engraft a subsequent HLA-matched bone marrow transplant [27, 28]. Each of these interpretations is equally consistent with the observation that increased transfusion correlates with increased BMT rejection, and the hypotheses are not mutually exclusive. It is thus necessary to examine findings from reductionist systems that can separate the variables, in order to assess the individual hypotheses.
Sources of Immunization to mHAs by Transfused Cells
Studying the effect of transfusion on BMT rejection in healthy animals allows an experimental exclusion of issues relating to underlying hematological disease of the marrow. In addition, the use of animals allows BMT in MHC identical donors and recipients, removing the ambiguity that rejection in HLA “matched” humans may just be due to incomplete matching, which is inevitably present with the exception of HLA identical siblings. As early as the 1950s and 1960s, it was appreciated that transfusion of blood into a healthy experimental animal could predispose to rejection of a subsequent BMT [29-32]. However, these studies crossed major MHC barriers. Subsequently, and in a more defined fashion, blood transfusion was observed to induce bone marrow transplant rejection in DLA identical canines (equivalent to human HLA-identical bone marrow) [33-36]. Parallel to clinical observations in humans, the number of transfusions was noted to correlate with a significant increase in the frequency of BMT rejection [37]. Because the donors and recipients were matched at DLA or MHC loci (canine and murine studies, respectively) but genetically mismatched at other polymorphic loci, alloimmunization against mHAs expressed on cells contained in an antecedent transfusion have been posited to be responsible for rejection.
The majority of blood transfusions in humans consist of fractionated products that are often leukoreduced. Work in both canine and murine systems have found that similar to transfusion of whole blood, transfusion of leukoreduced RBC units can induce rejection of an MHC matched or MHC identical bone marrow transplant [36, 37]. However, the canine studies showed significantly decreased rates of rejection using leukoreduced RBCs or platelets, suggesting that “only some (and perhaps none) of the non-DLA antigens responsible for rejection reside on platelets and red blood cells” [36, 38]. These data indicated that either leukocytes were alone responsible and the extent of leukoreduction was insufficient, or that non-leukocyte components are capable of inducing rejection, albeit as a weaker immunogen.
Residual leukocytes in blood products have traditionally been accepted as the source of alloimmunization with regards to anti-HLA alloantibodies, and leukoreduction substantially decreases frequency of anti-HLA alloimmunization in humans. However, the incidence of transfusion associated BMT rejection in humans is not decreased when leukoreduced blood is used compared to nonleukoreduced blood [18, 39]. Consistent with interpretations of animal data above, the current observation gives rise to two competing hypotheses: 1) leukocytes are the main cellular alloimmunity to mHAs, but residual leukocytes that persist after leukoreduction are sufficient to induce alloimmunization that leads to graft rejection, and 2) bone marrow transplant rejection is due to the induction of cellular immunity to mHAs expressed on non-leukocyte populations (e.g. the RBCs themselves or residual platelets). Of course, these hypotheses are not mutually exclusive, and both pathways may be playing a role. It is also very important to note that literature relevant to this field has evolved over the past several decades, and the efficiency of leukoreduction technologies has substantially improved for subsequent studies compared to earlier publications.
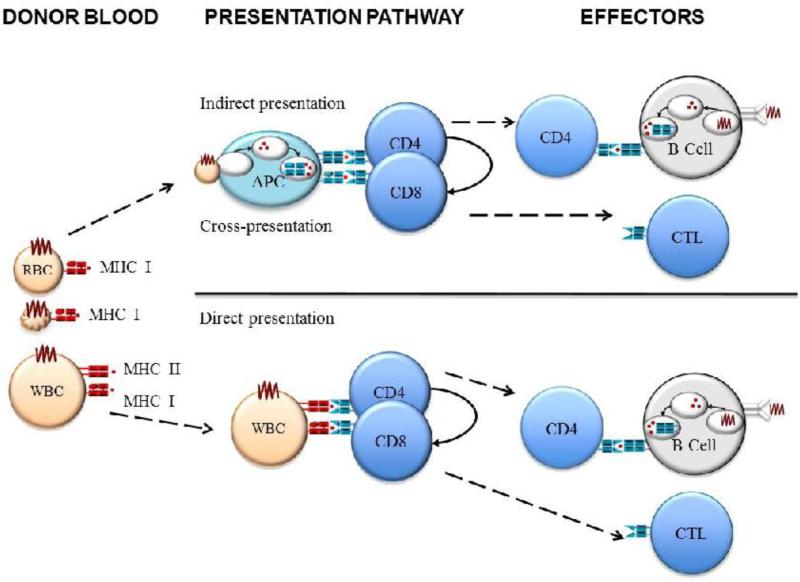
Induction of cellular immunity to mHAs on donor leukocytes could occur through either direct or indirect pathways, although for the direct pathway to occur and lead to BMT rejection, the blood donor would have to share an MHC variant with the recipient and the bone marrow donor (and the relevant donor mHA peptides) (see Figure 2). This is not to say that an MHC mismatch would not induce cellular immunity, but as an mHA is defined as a variant peptide in the same MHC, then there would have to be some MHC match between donor and recipient for an mHA response to occur (although T cells may recognize the same peptide/MHC complex with highly similar MHCs). In contrast, immunization by RBCs or platelets would presumably occur by the indirect pathway, as neither RBCs nor platelets have been observed to function as professional antigen presenting cells (see Figure 2). Of note, it has been speculated that in some settings, platelets may have the capacity to function as professional antigen presenting cells [40].
Figure 2. Indirect and Direct Pathways of Antigen Presentation.
In the indirect pathway (top) donor antigens are processed and presented by recipient antigen presenting cells (APC). In the direct pathway, donor APCs, with their own peptides presented on MHC, stimulate recipient T cells.
The Role of RBCs in Inducing mHA Based BMT Rejection
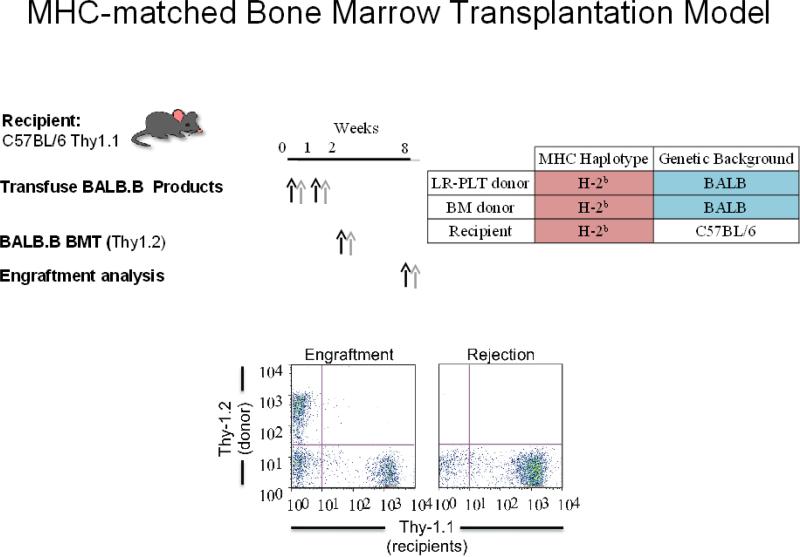
It is currently unclear to what extent RBCs can themselves serve as a source of alloimmunization in transfusion-induced BMT rejection. A series of murine models have been engineered to specifically ask this question. In mice, the MHC locus is designated as “H-2”. One of the strengths of murine systems is that there are numerous distinct strains of mice that have both been bred to homogeneity and have been separate from each other for decades (in the case of C57BL/6 mice, for a century). Two such strains are C57BL/6 (B6) and BALB/c mice. Each strain has a distinct set of MHC genes; the haplotype defined by the set of MHC genes is designated by a superscript. B6 mice carry the H-2b haplotype and BALB/c mice carry the H-2d haplotype. These two strains have also been used to generate MHC congenic mice at the H-2 locus. BALB.B mice have the identical genetic composition as BALB/c mice, except that they carry the B6 MHC haplotype (H-2b). Thus, BALB.B and B6 mice are MHC identical but have genome wide variation in other genes, giving rise to a large number of amino acid polymorphisms. When peptides containing these polymorphisms are presented by MHC I or MHC II they constitute mHA differences between BALB.B and B6. Thus, the BALB.B→B6 strain combination can serve as a model of MHC identical/mHA mismatched transfusion and BMT (see Figure 3).
Figure 3. Description of the Murine BALB.B→B6 System.
BALB.B (donor) blood and bone marrow is transfused and transplanted, respectively, into B6 recipients. Both strains have identical MHC (H-2b), but have divergent genetic backgrounds. Donors carry a Thy1.1 variant whereas recipients encode Thy1.2; thus, Thy alleles are used for flow cytometric based analysis of BMT engraftment.
It has been reported that transfusion of peripheral blood from BALB.B donors into B6 recipients, followed by a BALB.B→B6 BMT (under reduced intensity conditioning) leads to BMT rejection [37]; in contrast, engraftment is observed in both recipients that are not transfused prior to BMT and in control mice that are transfused with syngeneic blood prior to BMT. Thus, rejection is not due to a non-specific effect of the transfusion procedure. The BALB.B→B6 rejection occurs to the same extent if the RBCs are leukoreduced using standard filters routinely used on RBCs, which have a similar degree of leukoreduction on mouse RBCs as on human RBCs [37], raising the possibility that leukocytes are not required for transfusion induced BMT rejection. Of course, leukoreduction is not leukodepletion, and some leukocytes persist. Moreover, platelets are present in the murine RBC units. Hence, these data are suggestive but not demonstrative of RBC effects.
To formally test if peptides on RBCs can enter the indirect pathway, be processed and presented by recipient APCs, and lead to T cell activation and expansion, two separate approaches were used. Model antigens were either chemically crosslinked to wild type RBCs or specifically expressed just on RBCs through the generation of a transgenic animal with RBC specific gene regulatory elements [37, 41]. Transfusion of either antigen-crosslinked or transgenic RBCs into wild-type B6 recipients resulted in activation and expansion of T cells specific for a peptide from the model RBC antigen presented in the MHC of the recipient strain (B6) [37, 41]. These data demonstrate strong evidence that deviations in non-MHC protein sequence on RBCs can serve as an mHA that induces T cell activation upon transfusion of the RBCs.
The above data pointed to a system in which RBCs themselves are sufficient to induce BMT rejection across mHA barriers. However, in further testing this hypothesis, a surprising observation was made; when a model antigen is restricted to RBC expression (in this case a transgenic donor), transfusion of the RBCs does not induced BMT rejection when marrow expressing the transgene is subsequently transplanted [42]. This is not because the transgene on RBCs doesn't enter the immune system, because recipient antigen-specific T cells proliferate in response to transfusion of the transgenic RBCs [42]. Nor is the lack of rejection due to inability of the transgene to serve as a rejection vector, since immunization of wild type mice with a stronger immunogenic vector (Listeria expressing the model antigen) does induce BMT rejection [42]. At first consideration, these findings appear to justify a rejection of the hypothesis that mHAs on RBCs can induce BMT rejection, and this may very well be the case. However, several caveats exist regarding this interpretation. A substantial caveat is that there are multiple mHAs that differ between donor and recipient in the BALB.B →B6 system; however, the model antigen system limits this difference to just the model antigen being studied. Thus, it is possible that rejection in the BALB.B→B6 system, but not the model RBC antigen system, may simply be an issue of the extent of antigenic difference.
An additional consideration is that the lack of RBC induced BMT rejection may be context dependent. Because the RBC units were collected under sterile conditions, it was posited that the transfused RBC units were not immunogenic. As a result of lacking ‘danger signals’ (e.g. microbial particles) capable of activating innate immunity, which is required for the activation of optimal adaptive immune responses to a variety of antigens. However, engraftment of an MHC-matched bone marrow transplant has been found to occur in RBC specific mHA-mismatched transfused recipients despite systemic chronic infection with polyomavirus [42]. Although administration of Poly (I:C) (an activator of innate immunity) prior to transfusion has been shown to significantly increase humoral alloimmunity to a RBC specific mHA [43], it has been demonstrated that Poly (I:C) treatment prior to transfusion of RBC specific mHA-mismatched blood does not result in rejection of a subsequent BMT sharing the same mHA [42]. Together, these data argue against RBCs as a source of mHAs in transfusion induced BMT rejection.
It has also been hypothesized that BMT rejection did not occur in RBC specific mHA-mismatched transfused recipients not just because the RBCs were not sufficiently immunogenic, but because RBCs themselves are tolerogenic. After transfusion of RBCs expressing a model mHA, mHA specific CD8+ T cells were not detected in the peripheral blood or spleen of transplanted recipients [42]. This could have been due to a low CD8+ T cell precursor frequency, as expansion of adoptively transferred antigen specific CD8+ T cells were demonstrated to occur in response to transfusion of the RBC specific mHA-mismatched blood [37, 42]. However, expansion of the CD8+ T cells in response to the RBC specific mHA was shown to be short lived and followed by a rapid contraction phase, which was found to correlate with a significant enhancement in apoptotic cells [42]. Moreover, endogenous CD8+ T cells were never detected despite repeat exposure to mHA expressing RBCs. This was not due to the inability of endogenous CD8+ T cells to activate and expand, as infection with a virus expressing the mHA induced a robust response [42].
In aggregate, the data thus far provide substantial support for the notion that transfusion of RBC units can induce BMT rejection and that mHAs on the RBCs themselves enter and activate the recipient immune system. However, more detailed analysis suggests that mHAs on RBCs are not sufficient to induce rejection, and may induce tolerance under some conditions. These findings provide the rational basis for hypotheses that focus on implicating non-RBC components of RBC units as the source for immunization that leads to BMT rejection.
Are Residual Leukocytes in Donor Units Required to Mediate Transfusion Induced BMT Rejection?
Outside the scope of RBC antigens, humoral alloresponses to RBC transfusions (prior to leukoreduction technologies) included induction of alloantibodies to HLA antigens in approximately 8% of transfused patients [44]. Likewise, but more prevalent, non-leukocyte reduced platelet transfusions have been reported to result in a frequency of humoral immunity to HLA antigens in up to 45 - 70% of transfused patients [45, 46]. Roughly 13 - 30% of these HLA sensitized patients have been found to have refractoriness to subsequent transfusions [45, 46].
Leukocytes and platelets both express HLA antigens. Removal of leukocytes from platelet units substantially decreases induction of anti-MHC antibodies in mice, dogs, and humans, thus indicating that leukocytes appear to be more immunogenic than platelets [45, 47-49]. It is currently unclear if the induction of anti-HLA antibodies by leukoreduced platelet units is due to the immunogenicity of the residual leukocytes that escape leukoreduction or due to the ability of platelets themselves to induce alloantibodies. There are data to support both hypotheses, and data to argue against both hypotheses [47, 50-54]. Thus, at the current time, this issue remains a matter of dispute.
T cell mediated immunity to mHAs presented by MHC is not detected by any of the routine clinical laboratory assays. Thus, there are essentially no data regarding the frequency with which transfused blood induces mHA based alloimmunity at the T cell level. Although the frequency with which RBCs, platelets, and leukocytes induce alloantibodies may parallel immunization rates to mHAs presented by MHC, there is no reason that such needs to be the case.
Using animal models, the role of leukocytes in transfusion induced BMT rejection was tested by utilizing a system in which the mHA is restricted to leukocytes. The male antigen (H-Y antigen) is an mHA consisting of polymorphisms in the nuclear proteins, Smcy and Uty [55]. Mature RBCs and platelets in circulation are anucleated, and thus RBCs and platelets likely do not carry the H-Y mHA. It has been reported that transfusion of whole blood from male into female B6 mice induces rejection of a subsequent male-to-female BMT [37]. Normal engraftment occurs in control mice that are either untransfused or transfused with female blood [37]. However, the incidence of rejection was found to be significantly diminished when the blood was filter leukoreduced. These findings suggest that the extent of leukocyte decrease by filter leukoreduction is sufficient to prevent transfusion-induced BMT rejection by leukocytes as an immunogen. Taken together with data from the BALB.B →B6 system, in which transfusion of blood filter reduced by the same method induces BMT rejection, this finding also provides a rational basis to question the concept that residual leukocytes in blood units are the sole immunogen responsible for alloimmunization in the case of BMT rejection. However, one must consider that the antigen barrier in the H-Y system may be substantially lower than in the BALB.B →B6 system, and thus residual leukocytes may still be responsible in the BALB.B →B6 model.
Transfusions of Leukoreduced Platelet Units Are Capable of Inducing BMT Rejection
To test the capacity of platelets to cause transfusion induced BMT rejection, a clinically modeled platelet transfusion system has been generated using the same BALB.B→B6 model system described above. In this case, the donor and recipient are MHC identical, but differ at non-MHC loci, resulting in multiple mHAs between strains. Donor murine platelets were isolated using differential centrifugation, and collecting platelet rich plasma (PRP) [56]. The PRP was then passed over a leukoreduction filter. The final product had no detectable leukocytes and only trace numbers of RBCs. Platelets isolated in this fashion show visible swirling, aggregate normally in response to collagen, and have a normal post-transfusion recovery and survival. Platelets isolated in this fashion potently lead to transfusion induced BMT rejection [56], with a degree and kinetics very similar to the BMT rejection observed in animals transfused with RBC units [37]. However, this only means that “platelet units” induced BMT rejection across mHA barriers. As with RBC units, due to the presence of residual leukocytes that may be responsible, one can neither conclude nor exclude that it is the platelet itself that is the immunogen.
Efferent and Afferent Pathways of Cellular Immune Responses to Transfused Platelets
Depletion of recipient CD4+ T cells after transfusions but prior to transplantation has been reported to prevent transfusion induced BMT rejection across mHA barriers [57]. On the contrary, CD4+ T cell depletion after rejection of an initial BMT but prior to rejection of a re-transplantation had no effect upon rejection of the second BMT [57]. Thus, CD4+ T cells appear to be required for the initiation of the immune response, but are not required as a final effector cell. In contrast, depletion of CD8+ T cells at any point (prior to transfusion, prior to BMT, or prior to re-transplant) prevents BMT rejection [57]. Moreover, these findings suggest that in addition to allogeneic transfusions, transplanted bone marrow is in itself an additional immunizing event, which (in the presence of CD4+ helper T cells) stimulates the terminal differentiation of primed CD8+ T cells to full effectors capable of mediating graft rejection.
In vivo cytolytic T cell assays (CTL assays) showed that in vivo kill correlated with BMT rejection and depletion studies [57], leading to the interpretation that CD8+ T cells are a necessary effector of BMT rejection. The molecular mechanisms utilized by CD8+ T cells to reject the BMT across mHA disparities and mediate clearance of donor targets in vivo has yet to be identified, though currently hypothesized mechanisms include Fas-FasL pathways, perforingranzyme, and/or toxic cytokine release (e.g. IFN or TNF ).
The basis for CD4+ T cell requirement early in transfusion-induced BMT rejection is unclear. In different rejection systems, CD4+ T cells have been shown to be capable of providing T cell help, directly acting as cytolytic effectors, and/or bi-functional activities [11, 58-62]. CD4+ T cell help can be acquired through licensing antigen presenting cells, direct actions on CD8+ T cells, and/or an indirect effect of background IL-2 production. Similar to CD8+ T cell cytolytic mechanisms, cytolytic functions of CD4+ T cells have also been hypothesized to occur through Fas-FasL pathways, perforin-granzyme mechanisms, and/or toxic cytokine release (e.g. IFN or TNF ).
The data observed thus far for MHC identical/mHA mismatched BMT rejection (in the BALB.B →B6 system) elucidate a central role for T cells. In addition, it has been demonstrated that antibodies are not required for rejection. In particular: 1) mHA reactive antibodies are not detected in transfused recipients prior to BMT or after rejection [56] and 2) transfusions still induce rejection of an MHC-matched bone marrow transplant in animals deficient in B cells (due to a mutation in the μ immunoglobulin chain) [57]. Because the mHAs shared between the platelet and bone marrow donors in these studies are unknown, it is possible that the current model systems are biased to the induction of cellular alloimmune responses and that the mHAs do not represent extracelluar epitopes. Accordingly, there may be a role for antibodies in some mHA based rejection in other systems where mHAs also induce extracellular antibody epitopes.
Potential Clinical Interventions for Transfusion Induced BMT Rejection
In an attempt to eliminate alloimmunization to blood transfusions, leukocyte reduction methodologies have been clinically implemented. Indeed, the incidence of humoral immunization to allogeneic HLA antigens and subsequent refractoriness is diminished [44-46, 63-66]. However, even with leukoreduction, residual leukocytes remain that may have immunizing effects. Alternatively, in the event that mHAs on non-leukocyte populations are capable of inducing BMT rejection though indirect antigen processing pathways (e.g. platelet mHAs), then it is unlikely that leukocyte reduction will have an effect. Resolution of this issue is of critical importance. If residual leukocytes are alone responsible for BMT rejection, then all that is required is better leukoreduction technologies. In contrast, if non-leukocyte populations can induce BMT rejection, then no manner of leukoreduction alone will solve the problem.
One potential approach to inhibit alloimmunization to blood transfusions in general is to avoid chronic transfusion. However, this strategy requires the introduction of transplantation as a potential cure earlier on in the disease and preceding aggressive transfusion support. As the severity of many diseases is unclear at its onset, and because BMT carries risks, this is unlikely to be a reasonable approach. Moreover, because many patients lack HLA-identical sibling donors and acquisition of donors overall is difficult, it is typically not logistically feasible to rapidly transplant with an HLA identical donor.
As an alternative to limiting transfusion, donors and recipients could in theory be matched for mHA disparities through genotyping or immunological screening for compatibility. Genotyping methodologies are presently available, though are of limited use in this particular context, because mHAs that induce BMT rejection are not defined. The number of polymorphic mHAs that may play a role in transfusion-induced BMT rejection are unknown, but may be large. As it is currently quite challenging to match transfusion recipients even for the well described alloantigens that induce antibody responses, adding additional mHAs to the list of “things to be matched” is not likely to be a feasible approach, even if the mHAs were identified and tests for their presence developed.
As avoiding exposure to mHAs does not seem feasible, either through limited transfusions or mHA matching, alternate approaches must be considered. One such approach is to inhibit the onset of alloimmunity through pharmacological immunosuppression. Because any increase in risk of disease from pathogens or opportunistic infections would not be acceptable in the relevant patient populations; traditional immunosuppressants would not be candidates. However, one potential immunomodulatory treatment that has previously been demonstrated to be efficient in prolonging solid organ graft survival is the blockade of T cell costimulation, which is essential in propagating and augmenting T cell responses [67, 68]. There are a number of costimulatory pathways critical to optimizing cellular responses, including CD28/B7, CD40/CD40L, OX40/OX40L, LFA-1/ICAM, LFA-3/CD2, ICOS/ICOSL, 4-1BB/4-1BBL, LIGHT/HVEM, CD27/CD70, and CD30/CD30L. In the absence of costimulation but recognition of the mHA:MHC complex, T cells can undergo tolerance either in the form of anergy or clonal deletion. Thus, pharmacological approaches to negatively regulate cellular immunity have been developed with a focus on blocking costimulation.
The CD28/B7 costimulatory pathway is currently known as the most critical for T cell activation, differentiation, and clonal expansion [67, 69, 70]. Cytotoxic T lymphocyte antigen 4 (CTLA4) is a coinhibitory protein upregulated on activated T cells to dampen CD28/B7 costimulation and modulate T cell responses through inhibition of IL-2, cytokine production, and impediment in the progression of the cell cycle at G1 to S phase [67, 71]. CTLA4 is structurally homologous to CD28 and competitively binds to B7 costimulatory ligands; CTLA4 has a higher avidity to the B7 ligands. CTLA4-Ig is a recombinant fusion protein that combines the extracellular domain of human CTLA4 with a modified human IgG1 (CTLA4-Ig), and has been specifically developed to block the CD28/B7 pathway.
CTLA4-Ig is currently a U.S. Food and Drug Administration (FDA) approved pharmacological reagent for treatment of psoriasis, rheumatoid arthritis, and renal transplantation [72, 73]. Although not without mild side effects associated with immunosuppression, CTLA4-Ig is not known to cause serious problems associated with stronger immunosuppressants. Thus, CTLA4-Ig is a potential promising therapeutic target for immunomodulation during transplantation and/or transfusions. It has recently been demonstrated in a murine model, that a single dose of CTLA4-Ig at the time of an initial transfusion prevents rejection of an MHC-matched bone marrow transplant, with the inhibition of alloimmunity to mHAs correlating to engraftment [74]. Conversely, if alloimmunity to mHAs on transfused platelet units is established prior to BMT, it has been shown that CTLA4-Ig at the time of transplantation is not efficient in blocking subsequent rejection [74]. These observations are consistent with reports that costimulatory blockade resistance can occur as a result of alloreactive T cell memory responses, which in general have a lower activation threshold and thus require less costimulation [15, 69]. This very lack of effect upon memory T cells is one explanation for why CTLA4-Ig does not result in substantial opportunistic infections. These data argue that administration of CTLA4-Ig at the time that transfusion therapy was initiated, would be required.
Conclusions
Currently, BMT is the only effective curative treatment for congenital and acquired non-malignant bone marrow failure disorders including aplastic anemia, β-thalassemia, sickle cell anemia, Fanconi anemia, and others [1-8]. Because neoplasia is absent in these syndromes, it is difficult to justify the toxic effects associated with myeloablative stringent pre-BMT conditioning regimens that are otherwise utilized in malignant settings. Rather, patients with non-malignant bone marrow failures typically receive an HLA-matched (or identical) bone marrow transplant under non-myeloablative reduced intensity conditions [3, 5, 75]. However, under reduced intensity conditions, engraftment of an HLA-matched bone marrow transplant is associated with a higher frequency of rejection [1, 18, 76]. Because the BMT is matched at HLA loci, this raises the possibility that the vector mediating rejection in these patients is immunity to mHAs. Of course, “HLA matched” is not “HLA identical”; however, in cases where HLA identical transplantation does occur (e.g. in HLA matched human siblings or animal models), then mHAs are the most likely vectors mediating rejection.
It is clinically well appreciated that the probability of rejection is related to the number of transfusions a patient receives prior to transplantation; the incidence of rejection increases with transfusions [17, 19-22]. Although the noted correlation may not be causal, as an increase in transfusion support might simply reflect more advanced disease and the resultant perturbation of the bone marrow microenvironment [4, 26-28]. In an animal model, transfusion of units of leukoreduced RBCs or platelets induces rejection of a bone marrow transplant in which the bone marrow shares mHAs with the transfused blood product [36, 37, 56]. Because these animals lack an underlying bone marrow failure disorder, and thereby had no damage to the bone marrow compartment, the most likely explanation for the failure to engraft is the antecedent transfusions. The fact that engraftment occurs if T cell are depleted in mice, which otherwise reject, is a strong indication of an immune mediated rejection. Of course, these studies do not rule out the simultaneous contribution of an underlying bone marrow disease in the failure to engraft in humans. Nonetheless, the human correlations are consistent with an interpretation that transfusion induces BMT rejection and the animal data thus far have demonstrated that transfusion of RBC or platelet units induces rejection of an MHC-matched/mHA mismatched bone marrow transplant through induction of cellular immunity against donor marrow. Ongoing human and animal trials will be required to determine to what extent recipient immunity is responsible for post-transfusion rejection of BMT in humans, and if present, what cellular immunogens are responsible (e.g. residual leukocytes or other cells or components) and what interventions can be taken to mitigate these effects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: JCZ has sponsored research agreements with Immucor Inc., and Terumo. JCZ is a consultant for Haemonetics. SRP has no conflicts to declare.
The term “minor antigen” has a dual meaning in transfusion medicine. In the context of immunohematology a minor antigen is a non-HLA associated epitope against which alloantibodies are formed. For cellular immunology, minor antigens are variant peptides presented by the same MHC molecule, but are a target for allospecific T cells and not an alloantibody recognized epitope.
References
- 1.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41:109–117. doi: 10.1038/sj.bmt.1705943. [DOI] [PubMed] [Google Scholar]
- 2.Camitta BM, Thomas ED, Nathan DG, Gale RP, Kopecky KJ, Rappeport JM, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53:504–514. [PubMed] [Google Scholar]
- 3.Horan JT, Liesveld JL, Fenton P, Blumberg N, Walters MC. Hematopoietic stem cell transplantation for multiply transfused patients with sickle cell disease and thalassemia after low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte globulin. Bone Marrow Transplant. 2005;35:171–177. doi: 10.1038/sj.bmt.1704745. [DOI] [PubMed] [Google Scholar]
- 4.Or R, Aker M, Shapira MY, Resnick I, Bitan M, Samuel S, et al. Allogeneic stem cell transplantation for the treatment of diseases associated with a deficiency in bone marrow products. Springer Semin Immunopathol. 2004;26:133–142. doi: 10.1007/s00281-004-0169-z. [DOI] [PubMed] [Google Scholar]
- 5.Resnick IB, Shapira MY, Slavin S. Nonmyeloablative stem cell transplantation and cell therapy for malignant and non-malignant diseases. Transpl Immunol. 2005;14:207–219. doi: 10.1016/j.trim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Thomas ED, Storb R, Fefer A, Slichter SJ, Bryant JI, Buckner CD, et al. Aplastic anaemia treated by marrow transplantation. Lancet. 1972;1:284–289. doi: 10.1016/s0140-6736(72)90292-9. [DOI] [PubMed] [Google Scholar]
- 7.Wagner JE, Eapen M, MacMillan ML, Harris RE, Pasquini R, Boulad F, et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109:2256–2262. doi: 10.1182/blood-2006-07-036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walters MC, Patience M, Leisenring W, Eckman JR, Buchanan GR, Rogers ZR, et al. Barriers to bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 1996;2:100–104. [PubMed] [Google Scholar]
- 9.Goulmy E. Human minor histocompatibility antigens. Curr Opin Immunol. 1996;8:75–81. doi: 10.1016/s0952-7915(96)80108-7. [DOI] [PubMed] [Google Scholar]
- 10.Roopenian DC. What are minor histocompatibility loci? A new look at an old question. Immunol Today. 1992;13:7–10. doi: 10.1016/0167-5699(92)90197-F. [DOI] [PubMed] [Google Scholar]
- 11.Simpson E, Scott D, James E, Lombardi G, Cwynarski K, Dazzi F, et al. Minor H antigens: genes and peptides. Transpl Immunol. 2002;10:115–123. doi: 10.1016/s0966-3274(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 12.Kamani NR, Walters MC, Carter S, Aquino V, Brochstein JA, Chaudhury S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: results of one cohort from the phase II study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biol Blood Marrow Transplant. 2012;18:1265–1272. doi: 10.1016/j.bbmt.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford ML, Kirk AD, Larsen CP. Donor-reactive T-cell stimulation history and precursor frequency: barriers to tolerance induction. Transplantation. 2009;87:S69–74. doi: 10.1097/TP.0b013e3181a2a701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15:405–410. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. 1989;73:606–613. [PubMed] [Google Scholar]
- 18.Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeg HJ, Self S, Storb R, Doney K, Appelbaum FR, Witherspoon RP, et al. Decreased incidence of marrow graft rejection in patients with severe aplastic anemia: changing impact of risk factors. Blood. 1986;68:1363–1368. [PubMed] [Google Scholar]
- 20.Gluckman E, Horowitz MM, Champlin RE, Hows JM, Bacigalupo A, Biggs JC, et al. Bone marrow transplantation for severe aplastic anemia: influence of conditioning and graft-versus-host disease prophylaxis regimens on outcome. Blood. 1992;79:269–275. [PubMed] [Google Scholar]
- 21.Storb R, Thomas ED, Buckner CD, Clift RA, Deeg HJ, Fefer A, et al. Marrow transplantation in thirty “untransfused” patients with severe aplastic anemia. Ann Intern Med. 1980;92:30–36. doi: 10.7326/0003-4819-92-1-30. [DOI] [PubMed] [Google Scholar]
- 22.Stucki A, Leisenring W, Sandmaier BM, Sanders J, Anasetti C, Storb R. Decreased rejection and improved survival of first and second marrow transplants for severe aplastic anemia (a 26-year retrospective analysis) Blood. 1998;92:2742–2749. [PubMed] [Google Scholar]
- 23.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 24.Nydegger UE, Riedler GF, Flegel WA. Histoblood groups other than HLA in organ transplantation. Transplant Proc. 2007;39:64–68. doi: 10.1016/j.transproceed.2006.10.222. [DOI] [PubMed] [Google Scholar]
- 25.Nydegger UE, Tevaearai H, Berdat P, Rieben R, Carrel T, Mohacsi P, et al. Histo-blood group antigens as allo- and autoantigens. Ann N Y Acad Sci. 2005;1050:40–51. doi: 10.1196/annals.1313.006. [DOI] [PubMed] [Google Scholar]
- 26.Mankad VN, Williams JP, Harpen MD, Manci E, Longenecker G, Moore RB, et al. Magnetic resonance imaging of bone marrow in sickle cell disease: clinical, hematologic, and pathologic correlations. Blood. 1990;75:274–283. [PubMed] [Google Scholar]
- 27.Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C, et al. Bone marrow transplantation in patients with thalassemia. N Engl J Med. 1990;322:417–421. doi: 10.1056/NEJM199002153220701. [DOI] [PubMed] [Google Scholar]
- 28.Storey JA, Connor RF, Lewis ZT, Hurd D, Pomper G, Keung YK, et al. The transplant iron score as a predictor of stem cell transplant survival. J Hematol Oncol. 2009;2:44. doi: 10.1186/1756-8722-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes DWH, Loutit JF. What is the recovery factor in spleen. Nucleonics. 1954;12:68–71. [Google Scholar]
- 30.Dacosta H, Amiel JL, Mathe G. Immunization against Grafts of Allogenic Hematopoietic Cells by Prior Blood Transfusion [in French] Vox Sang. 1964;9:420–430. doi: 10.1111/j.1423-0410.1964.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 31.Loutit JF, Micklem HS. Active and passive immunity to transplantation of foreign bone marrow in lethally irradiated mice. Br J Exp Pathol. 1961;42:577–586. [PMC free article] [PubMed] [Google Scholar]
- 32.Van Putten LM, Van Bekkum DW, de Vries MJ, Balner H. The effect of preceding blood transfusions on the fate of homologous bone marrow grafts in lethally irradiated monkeys. Blood. 1967;30:749–757. [PubMed] [Google Scholar]
- 33.Storb R, Epstein RB, Rudolph RH, Thomas ED. The effect of prior transfusion on marrow grafts between histocompatible canine siblings. J Immunol. 1970;105:627–633. [PubMed] [Google Scholar]
- 34.Storb R, Rudolph RH, Graham TC, Thomas ED. The influence of transfusions from unrelated donors upon marrow grafts between histocompatible canine siblings. J Immunol. 1971;107:409–413. [PubMed] [Google Scholar]
- 35.Storb R, Kolb HJ, Graham TC, Kane PJ, Thomas ED. The effect of prior blood transfusions on hemopoietic grafts from histoincompatible canine littermates. Transplantation. 1972;14:248–252. doi: 10.1097/00007890-197208000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Storb R, Weiden PL, Deeg HJ, Graham TC, Atkinson K, Slichter SJ, et al. Rejection of marrow from DLA-identical canine littermates given transfusions before grafting: antigens involved are expressed on leukocytes and skin epithelial cells but not on platelets and red blood cells. Blood. 1979;54:477–484. [PubMed] [Google Scholar]
- 37.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114:2315–2322. doi: 10.1182/blood-2009-04-214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storb R, Deeg HJ, Weiden PL, Graham TC, Atkinson KA, Slichter SJ, et al. Marrow graft rejection in DLA-identical canine littermates: antigens involved are expressed on leukocytes and skin epithelial cells but probably not on platelets and red blood cells. Transplant Proc. 1979;11:504–506. [PubMed] [Google Scholar]
- 39.Stern M, Passweg JR, Locasciulli A, Socie G, Schrezenmeier H, Bekassy AN, et al. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation. 2006;82:218–226. doi: 10.1097/01.tp.0000226156.99206.d1. [DOI] [PubMed] [Google Scholar]
- 40.Chapman LM, Aggrey AA, Field DJ, Srivastava K, Ture S, Yui K, et al. Platelets present antigen in the context of MHC class I. J Immunol. 2012;189:916–923. doi: 10.4049/jimmunol.1200580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimring JC, Hair GA, Deshpande SS, Horan JT. Immunization to minor histocompatibility antigens on transfused RBCs through crosspriming into recipient MHC class I pathways. Blood. 2006;107:187–189. doi: 10.1182/blood-2005-07-3059. [DOI] [PubMed] [Google Scholar]
- 42.Desmarets M, Mylvaganam G, Waller EK, Josephson CD, Pack C, Lukacher AE, et al. Minor antigens on transfused RBCs crossprime CD8 T cells but do not induce full effector function. Am J Transplant. 2011;11:1825–1834. doi: 10.1111/j.1600-6143.2011.03730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD, et al. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 44.Blumberg N, Heal JM, Gettings KF. WBC reduction of RBC transfusions is associated with a decreased incidence of RBC alloimmunization. Transfusion. 2003;43:945–952. doi: 10.1046/j.1537-2995.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- 45.The Trial to Reduce Alloimmunization to Platelets Study Group Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337:1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 46.Laundy GJ, Bradley BA, Rees BM, Younie M, Hows JM. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814–825. doi: 10.1111/j.1537-2995.2004.03387.x. [DOI] [PubMed] [Google Scholar]
- 47.Claas FH, Smeenk RJ, Schmidt R, van Steenbrugge GJ, Eernisse JG. Alloimmunization against the MHC antigens after platelet transfusions is due to contaminating leukocytes in the platelet suspension. Exp Hematol. 1981;9:84–89. [PubMed] [Google Scholar]
- 48.Slichter SJ, Fish D, Abrams VK, Gaur L, Nelson K, Bolgiano D. Evaluation of different methods of leukoreduction of donor platelets to prevent alloimmune platelet refractoriness and induce tolerance in a canine transfusion model. Blood. 2005;105:847–854. doi: 10.1182/blood-2003-08-2942. [DOI] [PubMed] [Google Scholar]
- 49.Vamvakas EC. Meta-analysis of randomized controlled trials of the efficacy of white cell reduction in preventing HLA-alloimmunization and refractoriness to random-donor platelet transfusions. Transfus Med Rev. 1998;12:258–270. doi: 10.1016/s0887-7963(98)80002-3. [DOI] [PubMed] [Google Scholar]
- 50.Frangoulis B, Besluau D, Chopin M, Degos L, Pla M. Immune response to H-2 class I antigens on platelets. I. Immunogenicity of platelet class I antigens. Tissue Antigens. 1988;32:46–54. doi: 10.1111/j.1399-0039.1988.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 51.Frangoulis B, Chopin M, Besluau D, Degos L, Pla M. Immune response to H-2 class I antigens on platelets. II. Specific decrease of H-2 class I-specific antibody response induced by treatment with allogeneic platelets. Tissue Antigens. 1988;32:78–86. doi: 10.1111/j.1399-0039.1988.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 52.Kao KJ, del Rosario ML. Role of class-II major histocompatibility complex (MHC)-antigen-positive donor leukocytes in transfusion-induced alloimmunization to donor class-I MHC antigens. Blood. 1998;92:690–694. [PubMed] [Google Scholar]
- 53.Semple JW, Speck ER, Cosgrave D, Lazarus AH, Blanchette VS, Freedman J. Extreme leukoreduction of major histocompatibility complex class II positive B cells enhances allogeneic platelet immunity. Blood. 1999;93:713–720. [PubMed] [Google Scholar]
- 54.Semple JW, Speck ER, Milev YP, Blanchette V, Freedman J. Indirect allorecognition of platelets by T helper cells during platelet transfusions correlates with anti-major histocompatibility complex antibody and cytotoxic T lymphocyte formation. Blood. 1995;86:805–812. [PubMed] [Google Scholar]
- 55.Roopenian D, Choi EY, Brown A. The immunogenomics of minor histocompatibility antigens. Immunol Rev. 2002;190:86–94. doi: 10.1034/j.1600-065x.2002.19007.x. [DOI] [PubMed] [Google Scholar]
- 56.Patel SR, Cadwell CM, Medford A, Zimring JC. Transfusion of minor histocompatibility antigen-mismatched platelets induces rejection of bone marrow transplants in mice. J Clin Invest. 2009;119:2787–2794. doi: 10.1172/JCI39590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel SR, Smith NH, Kapp L, Zimring JC. Mechanisms of alloimmunization and subsequent bone marrow transplantation rejection induced by platelet transfusion in a murine model. Am J Transplant. 2012;12:1102–1112. doi: 10.1111/j.1600-6143.2011.03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmerman ZF, Levy RB. MiHA reactive CD4 and CD8 T-cells effect resistance to hematopoietic engraftment following reduced intensity conditioning. Am J Transplant. 2006;6:2089–2098. doi: 10.1111/j.1600-6143.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 59.Falkenburg JH, van de Corput L, Marijt EW, Willemze R. Minor histocompatibility antigens in human stem cell transplantation. Exp Hematol. 2003;31:743–751. doi: 10.1016/s0301-472x(03)00190-5. [DOI] [PubMed] [Google Scholar]
- 60.Youssef AR, Otley C, Mathieson PW, Smith RM. Role of CD4+ and CD8+ T cells in murine skin and heart allograft rejection across different antigenic desparities. Transpl Immunol. 2004;13:297–304. doi: 10.1016/j.trim.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 62.Ryu SJ, Jung KM, Yoo HS, Kim TW, Kim S, Chang J, et al. Cognate CD4 help is essential for the reactivation and expansion of CD8 memory T cells directed against the hematopoietic cell-specific dominant minor histocompatibility antigen, H60. Blood. 2009;113:4273–4280. doi: 10.1182/blood-2008-09-181263. [DOI] [PubMed] [Google Scholar]
- 63.Hendrickson JE, Roback JD. Platelet Transfusion Refractory Patients. In: Hillyer CD, Shaz BH, Zimring JC, Abshire TC, editors. Transfusion Medicine and Hemostasis. Elsevier; Burlington, MA: 2009. pp. 283–286. [Google Scholar]
- 64.Kiefel V, Konig C, Kroll H, Santoso S. Platelet alloantibodies in transfused patients. Transfusion. 2001;41:766–770. doi: 10.1046/j.1537-2995.2001.41060766.x. [DOI] [PubMed] [Google Scholar]
- 65.Taaning E, Simonsen AC, Hjelms E, Svejgaard A, Morling N. Platelet alloimmunization after transfusion. A prospective study in 117 heart surgery patients. Vox Sang. 1997;72:238–241. doi: 10.1046/j.1423-0410.1997.7240238.x. [DOI] [PubMed] [Google Scholar]
- 66.Zimring JC. Recent developments and future directions of alloimmunization to transfused blood products. Clin Lab Med. 2010;30:467–473. doi: 10.1016/j.cll.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 68.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 69.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229:294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vincenti F. Costimulation blockade in autoimmunity and transplantation. J Allergy Clin Immunol. 2008;121:299–306. doi: 10.1016/j.jaci.2008.01.002. quiz 307-298. [DOI] [PubMed] [Google Scholar]
- 71.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 72.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 73.Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyo J, Neumayer HH, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91:976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 74.Gilson CR, Patel SR, Zimring JC. CTLA4-Ig prevents alloantibody production and BMT rejection in response to platelet transfusions in mice. Transfusion. 2012;52:2209–2219. doi: 10.1111/j.1537-2995.2011.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Srinivasan R, Takahashi Y, McCoy JP, Espinoza-Delgado I, Dorrance C, Igarashi T, et al. Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol. 2006;133:305–314. doi: 10.1111/j.1365-2141.2006.06019.x. [DOI] [PubMed] [Google Scholar]
- 76.Viollier R, Socie G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41:45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]