Abstract
HLA-matched bone marrow transplantation (BMT) is a cure for nonmalignant hematological disorders; however, rejection rates are high and correlate with the number of antecedent transfusions. Recently, using murine models, we reported that minor antigens (mHAs) in transfused leukoreduced RBC or platelet units induce rejection of subsequent BMT. To study RBCs as an immunogen, we utilized transgenic donors that express a model mHA selectively on RBCs (HOD mouse). Transfusion of HOD blood did not induce BMT rejection of marrow that shared mHAs with the HOD RBCs. Likewise, no endogenous anti-HOD CD8+ T cell response was detected with antigen-specific tetramer reagents. Adoptively transferred OT-I T cells rapidly expanded after HOD blood transfusion; however, only a semi-effector phenotype was observed, (TNF-α and IFN-γ secretion, but essentially no Granzyme B). After initial expansion, OT-I T cells contracted rapidly to very low levels. A similar trend was observed by in vivo CTL assay, with only transient lytic activity. Together, these data indicate that RBCs may not be the component of RBC units that induces BMT rejection, and suggest that contaminating platelets or leukocytes may be responsible.
Keywords: Allosensitization, alloantigens, alloreactive T cells, bone marrow transplantation, transfusion
Introduction
When successful, bone marrow transplantation (BMT) is a cure for nonmalignant hematologic disorders such as sickle cell disease, aplastic anemia, Diamond-Blackfan anemia, and Fanconi anemia (1–8). For such disorders, where no malignancy is present, it is difficult to justify the use of myeloablative conditioning regimens, which can lead to morbidity and mortality. Accordingly, it is highly desirable to develop reduced-intensity conditioning regimens that minimize toxic side effects. Unfortunately, current reduced-intensity regimens result in significant rates of BMT rejection in patients with nonmalignant hematologic disorders (9–12).
As the frequency of rejection correlates with the number of transfusions given prior to BMT in humans (13–15) and in canine models (16–19), it has been hypothesized that minor antigens (mHAs) on donor blood induce immunity to mHAs expressed on subsequently transplanted marrow. In support of this hypothesis, we have previously reported that transfusion of stringently leukoreduced RBCs induces rejection of MHC matched:mHA mismatched BMT under reduced-intensity conditions (20). In other settings, it has been reported that BMT rejection across mHA barriers is mediated by CD8+ cytotoxic T lymphocytes (21–25). Consistent with this, we also reported that mHAs on transfused RBCs underwent cross-presentation and induced early expansion of antigen-specific CD8+ T cells (20, 26).
In aggregate, the above findings led us to propose that mHAs on RBCs themselves induce anti-mHA immunity leading to BMT rejection. However, leukoreduced RBC units are a complex product with residual leukocytes and variable numbers of platelets. We have recently reported that like RBCs, transfusion of mHA mismatched platelet products can induce BMT rejection (27). Although it is possible that both platelets and RBCs can induce BMT rejection, we cannot rule out that BMT rejection due to transfusion of RBC units was due to contaminating platelets, and vice-versa, or that trace leukocytes may be playing a role. Herein, we report that transfusion of blood expressing an mHA on RBCs (but not leukocytes or platelets) does not induce rejection of BMT expressing the same mHA. Moreover, it induces only a transient expansion of mHA specific CD8+ T cells with a semi-effector phenotype, followed by a rapid contraction to low levels. These findings suggest that the RBCs themselves may not be the component of RBC units that are responsible for inducing BMT rejection. Together, these studies provide a novel further characterization of the properties of transfused RBCs as an immunogen in the context of cross-priming into the MHC I pathway and effects upon CD8+ T cell responses.
Materials and Methods
Mice
C57BL/6 and B6.PL-Thy1a (B6.Thy1.1) mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and housed in Emory University Department of Animal Resources (DAR) facilities. Mice were 8 to 12 weeks of age, and all procedures were performed according to protocols approved by the Emory IACUC. The HOD (20) and mOVA (21) transgenic mice were previously described. FVB, HOD and mOVA mice were bred by the Emory DAR Animal Husbandry service.
Model of mHA transfusion and BMT
mOVA mice express full length ovalbumin on the cell surface under a ubiquitous promoter (beta actin) and are on a C57BL/6 background (H-2b MHC haplotype). Thus, transplant of mOVA bone marrow into C57BL/6 mice represents an MHC identical (H-2b) transplant with mOVA representing a mHA. HOD mice are on an FVB background (H-2q) and thus wild-type FVB mice (H-2q) are used as the appropriate negative control RBC. Because the HOD mice express the C terminal half of ovalbumin specifically on RBCs (and not leukocytes or platelets), then transfusion of HOD RBCs into C57BL/6 mice represents exposure to a minor antigen that is subsequently expressed by MHC matched donor bone marrow (mOVA marrow).
Viruses
The wild type mouse polyoma virus (PyV) strain A2 and recombinant PyV.OVA-I were prepared in baby mouse kidney cells, as previously described (28). Recombinant PyV.OVA-I was generated through the insertion of the SIINFEKL coding sequence in frame at a unique BlpI restriction site in the coding region of middle T antigen (29). Mice were injected subcutaneously in hind footpads with 1×106 PFU of virus.
Synthetic peptides
The SIINFEKL and LT359-368Abu (SAVKNYSKL) peptides were used. The LT359-368Abu peptide, in which the cysteine residue at position 7 was replaced with the analog residue α-aminobutyric acid is referred to as LT359 peptide (30).
Blood collection and transfusion
Mouse blood was obtained by retro-orbital bleeding into ACD solution A (BD, Franklin Lakes, NJ) and then washed 3 times with phosphate buffered saline (PBS). Each transfusion consisted of 100 μl of packed RBCs diluted with 400 μl PBS injected in the tail vein.
Bone Marrow Transplantation
B6.Thy1.1 recipients were conditioned by total body irradiation with 650 cGy. The next day, 5×106 whole bone marrow cells were prepared from the femur and tibia of donor mOVA mice and injected intravenously into the recipients. T cell engraftment was measured 6 weeks after transplant by flow cytometry using the Thy1.1 (recipient) and Thy1.2 (donor cells) congenic markers.
Flow cytometry
All monoclonal antibodies were purchased from BD Biosciences (San Jose, CA) except where noted. Intracellular staining was performed using the Cytofix/Cytoperm kit (BD Biosciences) with a PE conjugated Granzyme B specific MAb (Clone GB12, Invitrogen). PE conjugated Annexin-V and 7AAD staining was performed using the PE Annexin V Apoptosis Detection Kit I (BD Biosciences). Staining for surface OVA expression on mOVA bone marrow was performed with polyclonal anti-OVA (obtained by immunization of mice with OVA/CFA) followed by conjugated goat anti-mouse Ig. All data were acquired on a FACSort flow cytometer (Beckton Dickinson, San Jose, CA) and analyzed with FlowJo software (TreeStar, Ashland, OR).
Intracellular Cytokine Staining
Splenocytes from recipient mice were incubated with or without SIINFEKL peptide at a 10 μM concentration for 5 hours at 37°C in the presence of GolgiPlug with Brefeldine A (BD Biosciences, San Jose, CA) used according to manufacturer’s recommendations. OT-I T cells (CD8+-Kb-SIINFEKL+) were stained with antibodies against intracellular cytokines (IFNγ, TNFα).
In vivo cytotoxicity assay
In vivo cytotoxicity assays were performed according to a method adapted from Barber D, et al. (31). RBC lysed splenocytes from B6 mice were pulsed with 10μM SIINFEKL peptide, LT359 peptide or no peptide for 30 min at 37°C. Excess peptide was removed by washing with PBS. Each cell population was then labeled differently: no peptide cells with 3μM CFDA-SE (CFSE, Invitrogen); SIINFEKL pulsed cells with 0.25μM CFSE; and LT359 pulsed cells with 2μM FarRed DDAO-SE (FarRed, Invitrogen). 5×106 cells of each peptide pulsed populations were mixed and injected into the experimental mice. 12 to 15 hours after injection, spleens from recipient mice were harvested and the remaining labeled target cells were analyzed by flow cytometry. Percent specific lysis was calculated as follows: [1 − (#peptide-pulsed events / #unpulsed events) / (#peptide-pulsed events naive mouse / #unpulsed events naive mouse)] × 100.
Results
Transfusion with HOD blood does not induce BMT rejection
To test the hypothesis that mHAs on the RBCs themselves are capable of inducing BMT rejection, we engineered a model system utilizing HOD and mOVA donor mice. HOD mice express a fusion protein containing OVA specifically on RBCs, with no detectable expression on leukocytes or platelets (20). Because the HOD antigen is only expressed on erythroid lineages, it is not possible to study BMT rejection through rejection of HOD transplants. However, mOVA mice express OVA under an actin promoter, with wide tissue distribution (21). Thus, transfusion of HOD RBCs followed by BMT with mOVA marrow was used to model exposure to the same mHA on an antecedent transfusion and a subsequent BMT (see Figure 1A for experimental design and strain specifics). Surface OVA expression was detected on CD34+ bone marrow cells from mOVA mice by staining with polyclonal anti-sera to the whole OVA protein, suggesting that mOVA could serve as a target antigen for BMT rejection (Figure 1B). Thus, by transfusing recipients with RBC units from HOD mice, and then subsequently performing a BMT with mOVA donors, the immunological effects of exposure to an mHA on RBCs and its subsequent effects on BMT can be assessed. Because HOD mice are on an FVB background, wild-type FVB mice not expressing any OVA peptides were used as a negative control.
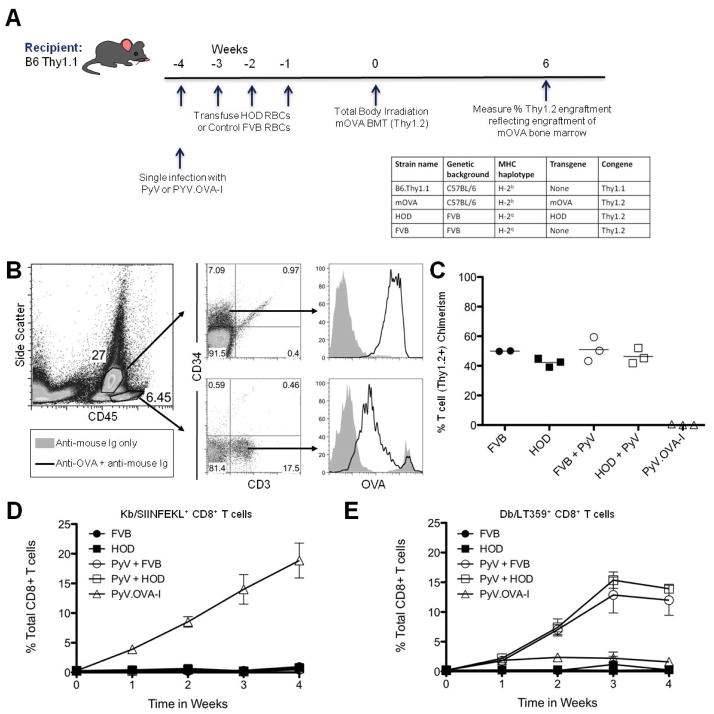
Figure 1. Multiple transfusions with HOD blood do not induce rejection of mOVA BMT or mHA specific CD8+ T cells.
Bone marrow from mOVA mice was stained for OVA expression with polyclonal anti-OVA antiserum and secondary antibody (or secondary along as a control) and evaluated on CD45dim-CD34+ hematopoietic stem cells and CD45hi-CD3+ T cells (B). B6.Thy1.1 mice were given with four weekly transfusions of HOD or control wild-type FVB blood (with no HOD expression) followed by BMT from mOVA donors (Thy1.2). Some mice were infected with PyV or PyV.OVA-I as indicated. Engraftment was assessed 6 weeks after BMT (C). Peripheral blood of animals from each group was stained with anti-CD8 and Kb/SIINFEKL tetramer or Db/LT359 tetramer at the indicated timepoints (D + E). Symbols for panel C, D: FVB ●, HOD ■, PyV + FVB ○, PyV + HOD □, PyV.OVA-I △. Data shown are representative of 3 separate experiments with 2 to 3 mice per group. Error bars represent the standard deviation for the data point.
Four transfusions of RBCs were given, as this was our previously reported regimen that caused BMT rejection in response to RBC unit transfusion in wild-type mice with undefined mHAs (20). However, despite four transfusions with HOD blood, BMT rejection was not observed (Figure 1C). Moreover, the percent chimerism in HOD blood recipients was equivalent to control mice receiving wild-type FVB RBCs. This negative finding was not due to the inability of mHAs from OVA to serve as a vector for rejection of mOVA bone marrow, as control mice infected with polyomavirus that expresses the SIINFEKL peptide from OVA (PyV.OVA-I) had robust BMT rejection.
As RBC units are collected under sterile conditions, the RBCs do not contain any microbial products, and thus transfusion introduces a foreign antigen in the absence of an obvious activator of innate immunity. As innate immune activation is required for full responses to many antigens, additional mice were infected with wild-type polyomavirus (PyV), followed by transfusion and subsequent BMT. No rejection was induced by HOD transfusion despite systemic infection with PyV (Figure 1C). Additional activators of innate immunity were also studied, to control for potential idiosyncratic properties of PyV. Poly (I:C) is a toll-like receptor 3 agonist that induces viral-like inflammation (32); and we have previously reported that poly (I:C) injection substantially increases humoral responses to transfused RBCs (33). However, in the current situation, administration of poly (I:C) did not modify the outcome of the BMT (data not shown).
To assess the extent of immunization in the CD8+ T cell compartment, recipient mice were bled weekly and cells were stained with Kb-SIINFEKL tetramer, which represents an immunodominant CD8+ T cell epitope for OVA. Despite 4 transfusions with HOD blood, no SIINFEKL specific CD8+ T cells were observed in the absence or presence of PyV infection (Figure 1D). This was not due to lack of capacity to respond, or due to poor tetramer reagents, as a robust SIINFEKL tetramer specific population was observed in control mice infected with PyV.OVA-I (Figure 1D). Since circulating lymphocytes may not reflect the biology in the lymphatics, representative mice were sacrificed and splenocytes were also analyzed; SIINFEKL specific T cells were likewise undetectable in the spleen (data not shown). Likewise, failure of PyV to enhance the response to HOD RBCs was not due to the virus failing to infect, as strong tetramer responses to the immunodominant peptide from PyV (Db-LT359) was observed (Figure 1E). Together, these data indicate that an mHA on transfused RBCs neither induces BMT rejection nor detectable CD8+ T cell expansion, either on its own or in the context of inflammation induced by a viral infection.
CD8+ T cells expand and contract rapidly upon cross-priming by RBC associated mHAs
We have previously reported that RBC associated antigens can be cross-presented to adoptively transferred CD8+ T cells and as a consequence induce early proliferation of the CD8+ T cells (20). However, in the current study, we did not detect expansion of endogenous SIINFEKL specific CD8+ T cells upon transfusion with HOD blood (in the absence of adoptive transfer). Our initial analysis of adoptively transferred cells focused only at early time points (20); thus, both of the above observations could be explained by the hypothesis that CD8+ T cells specific for RBC mHAs undergo initial proliferation followed by a subsequent deletion. In the absence of adoptively transferred CD8+ T cells, such an expansion and contraction would likely be undetectable due to the low precursor frequency of CD8+ T cells specific to a given antigen in naïve mice. To circumvent this limitation, we performed detailed kinetics analysis of OT-I splenocytes adoptively transferred into B6 mice followed by transfusion with HOD blood. As in the previous experiments, some of the mice were also infected with PyV to activate innate immunity. Positive control mice were infected with PyV.OVA-I.
Consistent with our previous findings (20), OT-I T cells displayed a 5-fold expansion in the spleen of recipient mice after transfusion with HOD blood 4 days after transfusion (Figure 2A). However, numbers rapidly declined by 8 days post-transfusion and remained at very low levels out to 14 days. Activation of innate immunity by infection with wild-type PyV enhanced the initial response to about a 20-fold expansion, but did not prevent the rapid contraction to very low levels by 8 days post-transfusion. The failure of OT-I T cells to establish a persistent response after HOD transfusion was not due to their inability to do so, as positive control mice infected with PyV.OVA-I had only moderate post-peak contraction and settled into a stable populations, as is typical during viral infection. The observed responses were antigen-specific, as no significant expansion was seen in mice transfused with wild-type control FVB blood regardless of infection with PyV (figure 2A, upper panel). As BMT rejection occurs in the bone marrow compartment, analysis of bone marrow was also carried out. No increase in OT-I T cells was seen from 4–14 days compared to baseline levels (No transfusion). Thus, lack of accumulation of OT-I T cells was not due to trafficking to the bone marrow and away from the spleen.
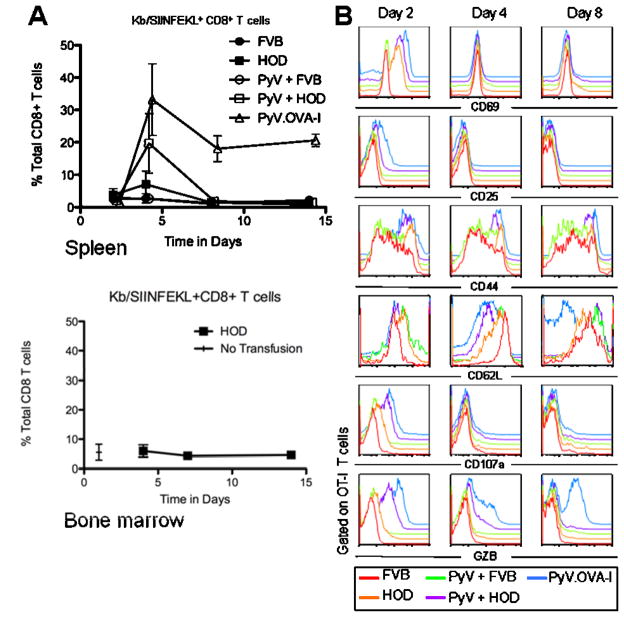
Figure 2. Transfusion with HOD blood induces transient activation and expansion of adoptively transferred OT-I T cells followed by profound contraction.
Mice received adoptive transfer of OT-I CD8 T cells followed by transfusion with HOD or FVB blood and/or infection with PyV or PyV-OVA-I as indicated. Spleens were harvested and Kb/SIINFEKL+CD8+ T cells were enumerated in the spleen and bone marrow (A). Cells from spleen were also stained with the indicated antibodies (B). Data shown is the compounded results of 3 separate experiments with 2 to 3 mice per group; a representative plot is shown for bone marrow and this has been performed 4 times with similar results. Symbols: FVB ●, HOD ■, PyV + FVB ○, PyV + HOD □, PyV.OVA-I △. Error bars represent the standard deviation for the data point
CD8+ T cells responding to RBC mHAs display partial activation and effector molecule expression
To assess the phenotype of CD8+ T cells responding to RBC antigens, OT-I T cells were stained for several markers known to correlate to activation and effector function. FVB transfusion and PvV-OVA-I infection were used as negative and positive controls, respectively. OT-I T cells displayed an increased expression of both CD69 and CD44 in response to HOD blood, indicating cellular activation. As is typical for these molecules, CD69 expression was transient whereas CD44 remained elevated (figure 2B). Unlike positive control cells stimulated with PyV-OVA-I, transfusion of HOD blood induced only minimal increases in CD25 at early time points and only a slight decrease in CD62L.
Analysis of molecules associated with effector function demonstrated that only a small induction of CD107a was found in the OT-I T cells of mice transfused with HOD at day 2 when compared to FVB control. In contrast, PyV.OVA-I infected mice displayed robust staining for CD107a at day 2. Similarly, only very slight levels of Granzyme B expression were observed in OT-I T cells responding to HOD transfusion whereas high levels were induced by PyV-OVA-I. Interestingly, mice infected with PyV immediately before transfusion with HOD blood showed increased expression of both CD107a and Granzyme B, compared to either PyV or HOD blood alone, suggesting a synergistic effect of viral infection on the CD8+ T cell differentiation in response to RBC associated mHAs.
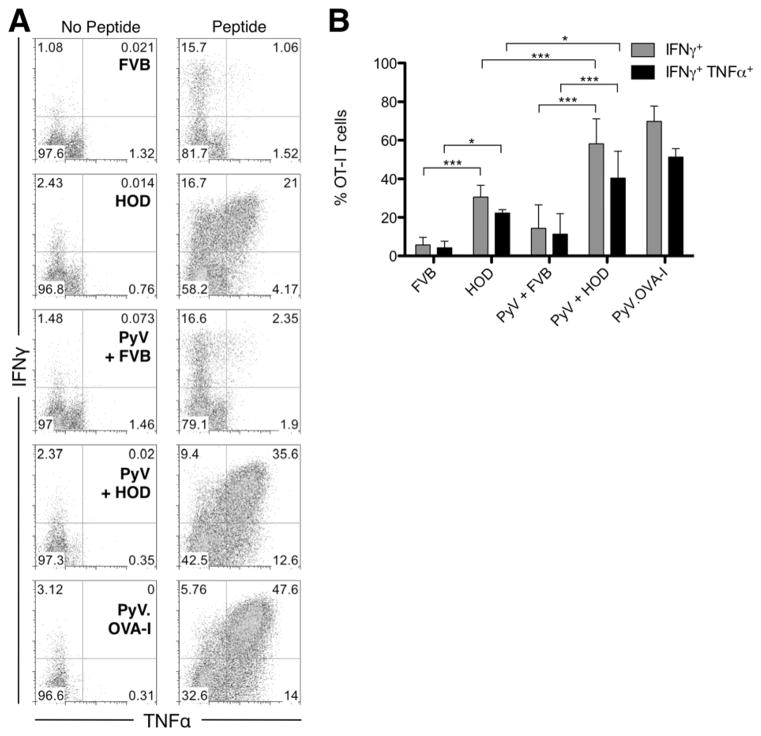
In addition, we measured the frequency of cytokine secreting OT-I T cells upon stimulation with SIINFEKL peptide ex vivo at day 4, which corresponds to the peak of the response in the OT-I T cells. Representative data plots are shown in figure 3A of intracellular cytokine staining. Transfusion of HOD blood induced expression of both IFNγ and TNFα in a significant number of OT-I T cells, and about 20% of the OT-I T cells simultaneously secreted IFNγ as well as TNFα. This effect was significantly enhanced when the mice were also infected with PyV. This effect was specific, as no increase was seen in mice receiving control FVB blood or PyV in the absence of HOD transfusion (figure 3B).
Figure 3. Transfusion with HOD blood induces expression of IFNγ and TNFα in responding OT-I cells.
IFNγ and TNFα secretion was measured by intracellular cytokine staining after ex vivo peptide restimulation of splenocytes from recipient mice 4 days post transfusion. Representative flow cytometry plots of the staining with or without SIINFEKL peptide restimulation for the different experimental groups are shown. Plots are gated on CD8+ Kb/SIINFEKL tetramer+ events. Combined data shown are the compounded results of 3 separate experiments with 2 mice per group (B). Statistical analysis was performed with two-way ANOVA analysis with Bonferroni post-tests, * = p<0.05, *** = p<0.001. Error bars represent the standard deviation for the data point.
CD8+ T cells that become activate in the absence of some effector molecule induction (e.g. Granzyme B) have been associated with regulatory functions. As Treg are defined largely by the expression of Foxp3, we thus performed intracellular staining on the OT-I T cells for Foxp3 expression. In the mice transfused with HOD blood we did not observe any expression of Foxp3 when compared to the FVB control group (data not shown). A discrete Foxp3+ population was observed in control CD4+ T cells, and not seen with isotype matched control, indicating that the assay was functioning.
Together, the above data demonstrate that whereas CD8+ T cells exposed to their cognate antigen expressed by a viral infection express the full array of effector molecules, exposure to the same antigen on RBCs results development of a partial effector phenotype with expression of effector cytokines, but decreased changes in surface molecules and only minimal signs of degranulation or expression of Granzyme B.
Transient lytic activity is observed in CD8+ T cells responding to mHAs on RBCs
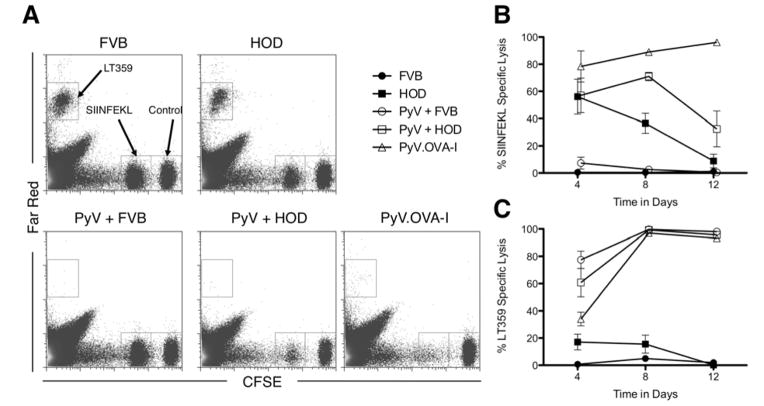
Because only partial effector molecule expression was observed, we tested the lytic activity of CD8+ T cells stimulated by mHAs on RBCs. We adapted a previously described in vivo CTL assay (31) to the HOD antigen where SIINFEKL or LT359 peptide pulsed spleen cells were used as targets. Representative plots are shown in figure 4A. OT-I T cells were transferred into naïve mice followed by transfusion with HOD RBCs and/or infection with PyV, as above. Four days after transfusion and/or infection, mice transfused with HOD blood displayed a relatively strong lytic activity specific for the SIINFEKL epitope (figure 4B). Consistent with the contraction of CD8+ T cells seen in mice transfused with HOD RBCs, the lytic activity rapidly decreases and becomes undetectable at day 12. Similar to the enhancement of effector molecule expression, transfusion of HOD blood coincident with PyV infection prolongs the window of lytic activity temporarily, followed by a rapid decline. As a control for infection with PyV.OVA-I virus we measured lytic activity against the LT359 epitope (the immunodominant epitope for this virus). We observed a strong and robust lytic activity against LT359 at later time points in all the groups that were infected with PyV virus (figure 4C).
Figure 4. Transfusion with HOD blood induces transient cytolytic activity against OVA in adoptively transferred OT-I T cells.
Cytolytic activity against SIINFEKL or LT359 peptide pulsed targets was measured in the spleen using an in vivo cytolytic assay. Representative flow cytometry plots for the different experimental groups are shown (A). Combined data at the indicated timepoints are shown (B+C). Data shown are the compounded results of 3 separate experiments with 2 mice per group. Symbols: FVB ●, HOD ■, PyV + FVB ○, PyV + HOD □, PyV.OVA-I △. Error bars represent the standard error of the mean for the data point.
Differential apoptosis is not alone responsible of altered outcomes in CD8+ T cell populations
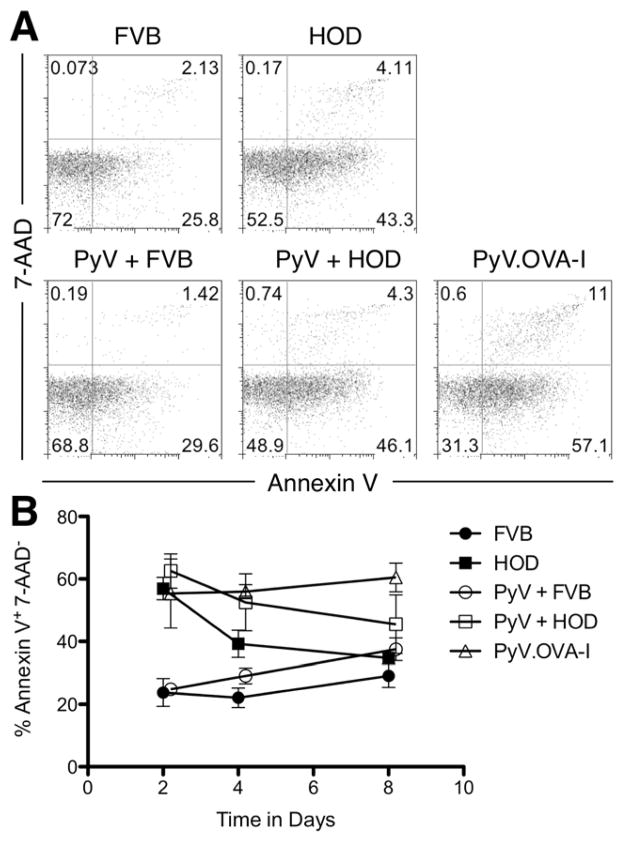
We hypothesized that the rapid contraction phase seen in CD8+ T cells responding to mHAs on RBCs was due to increased induction of apoptosis, analogous to activation induced cell death. To test this hypothesis, we assayed levels of phophatidylserine on the cell surface of OT-I T cells by staining with Annexin V. Cell viability was determined by co-staining with 7-AAD. A representative flow panel is shown in figure 5A. A baseline level of apoptosis was seen in control mice receiving FVB blood, and this was only slightly increased by infection with wild-type PyV. In contrast, there was a significant increase in Annexin V positive cells after transfusion with HOD RBCs, with no significant enhancement by coincident infection with PyV (Figure 5B). Interestingly, the highest levels of apoptosis are seen in mice infected with PyV.OVA-I. Kinetic analysis demonstrates that the percentages of apoptotic cells are progressively declining in mice that received HOD RBCs (with or without PyV). In contrast, percentages of apoptotic cells are increasing both in control mice that did not receive antigen (FVB or FVB+PyV) and mice that received PyV-OVA. Given that activated CD8+ T cells are simultaneously undergoing proliferation and apoptosis, these data must be considered in the context of cellular expansion. We conclude that some CD8+ T cells are induced to undergo apoptosis when exposed to antigen either on RBCs or a virus, and that this apoptosis likely contributes to the contraction phase in response to either stimulus. However, taken together with enumeration data (see figure 2A), net deletion likely predominates in response to RBCs whereas persistence predominates in response to PyV-OVA I. As even a greater percentage of cells undergo apoptosis in the latter case, we conclude that deletion of CD8+ T cells after HOD transfusion is not likely due to a net increase in apoptosis. However, we also cannot rule out effects of trafficking to other tissues, as these experiments do not enumerate all OT-I T cells in the entire animal.
Figure 5. Measurement of apoptosis in OT-I T cells stimulated with HOD RBCs.
Apoptosis in live adoptively transferred OT-I cells was measured by staining splenocytes of recipient mice with Kb/SIINFEKL tetramer, 7-AAD, and Annexin V. Representative flow cytometry plots gated on the CD8+ KbSIINFEKL tetramer+ events for the different experimental groups at day 4 after transfusion/infection are shown (A). Data shown are the compounded results of 2 separate experiments with 2 mice per group. Symbols: FVB ●, HOD ■, PyV + FVB ○, PyV + HOD □, PyV.OVA-I △. Error bars represent the standard error of the mean for the data point (B).
Discussion
In a previous report, we demonstrated that transfusion of RBC units induced rejection of subsequent BMT across mHA barriers. In the current report, we now demonstrate that when the system is reduced to a single mHA system shared by RBC donor and BM donor, the transfusion of RBC units prior to transplant is insufficient to induce BMT rejection. There are several hypotheses that are consistent with the observed data. First, it may be an issue of antigenic difference and dose, as in the previous report showing rejection, the mice were fully mismatched for mHAs at multiple loci (BALB.B → C57BL/6). Second is the fact that while the RBC units in our previous report were stringently leukoreduced, residual leukocytes still remained. Thus, if contaminating leukocytes are responsible, then genetically restricting the mHA to RBCs in the current study may remove immunization. Third, contaminating platelets in the leukoreduced RBC units in our previous report may have been responsible; indeed, we have recently reported that leukoreduced platelet units can also induce BMT rejection (27). The current data question whether RBCs are immunogenic, and in fact demonstrate that at least under these conditions the assays performed do not detect an immune response, thus raising the issue that contaminating leukocytes or platelets may be responsible for transfusion induced BMT rejection. The current data do not directly test the roles of leukocytes, platelets, or other components of the unit, nor do the data exclude that RBC mHAs may contribute to rejection; however, the current data do serve to reject the hypothesis that mHAs on RBCs are alone sufficient to induce rejection.
Whether RBCs themselves or contaminating leukocytes/platelets are required is a central issue to future blood product preparation. In the event that it is RBCs, but the extent of mHA mismatch is critical, then even partial matching for RBC mHAs may decrease effects on subsequent BMT rejection. However, if contaminating leukocytes or platelets are responsible, then more stringent depletion protocols may prevent the induction of BMT rejection.
Within the current report, the failure of transfusion of HOD RBCs to induce rejection of mOVA marrow is consistent with both the lack of observed endogenous tetramer positive cells and the lack of an increase in steady state numbers of adoptively transferred CD8+ OT-I T cells. These data support a model in which mHAs on RBCs are not immunogenic. We cannot unequivocally rule out that the CD8+ T cells traffic to non-lymphoid tissues (e.g. lungs or liver), but if such is the case, then they would likely have little effect upon BMT rejection, as our data do demonstrate lack of accumulation in the bone marrow. The current data do not distinguish lack of immunogenicity from induction of tolerance; however, for the purposes of understanding the immune vectors in transfused RBC units, these data do question if RBCs are alone sufficient to induce CD8+ T cell based immunity.
It must be acknowledged that the OT-I TCR has especially high affinity for the Kb-SIINFEKL complex, and that the observed biology may be biased towards behavior of high affinity CD8+ T cells. Indeed, it has been shown in some systems that OT-I T cells are deleted by encountering presented SIINFEKL but expand in response to lower affinity peptides (35). However, as the biology using adoptively transferred OT-I T cells (deletion of reactive cells) reflects the observed patterns of lack of BMT rejection in mice not receiving adoptive transfer, there is no basis to reject the OT-I findings as inconsistent with biology involving a range of TCR affinities.
In summary, the current report demonstrates that a model mHA with restricted expression to RBCs undergoes cross-presentation, but neither induces stable differentiation of CD8+ T cells into effectors nor induces BMT rejection. Transfusion of RBC products by us and others, in both mice and dogs, have been shown to induce subsequent BMT rejection. However, RBC products are never 100% pure, and it has not been technically feasible to rule out the contribution of contaminating leukocytes and/or platelets. Using a genetic approach to restrict an mHA to RBCs, these studies bring into question whether it is the RBCs themselves that are responsible or if contaminating leukocytes and/or platelets are responsible. Although these studies do not rule out that mHAs on RBCs play a role in inducing BMT rejection, they suggest that such mHAs are not alone sufficient to do so.
Acknowledgments
Funding sources: This work was funded by NIH grants R21HL086312 and R01HL092977 awarded to J.C.Z.
Abbreviations
- RBCs
Red Blood Cells
- BMT
Bone Marrow Transplant
- mHAs
minor histocompatibility antigens
- PyV
polyoma virus
- 7-ADD
7-amino-actinomycin D
Footnotes
Disclosures:
Conflicts of Interest: No commercial organization was involved in these studies. The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation
References
- 1.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117(4):850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41(2):109–117. doi: 10.1038/sj.bmt.1705943. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman E, Wagner JE. Hematopoietic stem cell transplantation in childhood inherited bone marrow failure syndrome. Bone Marrow Transplant. 2008;41(2):127–132. doi: 10.1038/sj.bmt.1705960. [DOI] [PubMed] [Google Scholar]
- 4.Vlachos A, Ball S, Dahl N, Alter BP, Sheth S, Ramenghi U, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142(6):859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georges GE, Storb R. Stem cell transplantation for aplastic anemia. Int J Hematol. 2002;75(2):141–146. doi: 10.1007/BF02982018. [DOI] [PubMed] [Google Scholar]
- 6.Fleitz J, Rumelhart S, Goldman F, Ambruso D, Sokol RJ, Pacini D, et al. Successful allogeneic hematopoietic stem cell transplantation (HSCT) for Shwachman-Diamond syndrome. Bone Marrow Transplant. 2002;29(1):75–79. doi: 10.1038/sj.bmt.1703321. [DOI] [PubMed] [Google Scholar]
- 7.Roy V, Perez WS, Eapen M, Marsh JC, Pasquini M, Pasquini R, et al. Bone marrow transplantation for diamond-blackfan anemia. Biol Blood Marrow Transplant. 2005;11(8):600–608. doi: 10.1016/j.bbmt.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Schrier SL, Angelucci E. New strategies in the treatment of the thalassemias. Annu Rev Med. 2005;56:157–171. doi: 10.1146/annurev.med.56.082103.104718. [DOI] [PubMed] [Google Scholar]
- 9.Iannone R, Casella JF, Fuchs EJ, Chen AR, Jones RJ, Woolfrey A, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant. 2003;9(8):519–528. doi: 10.1016/s1083-8791(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsohn DA, Duerst R, Tse W, Kletzel M. Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet. 2004;364(9429):156–162. doi: 10.1016/S0140-6736(04)16628-2. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamurti L. Hematopoietic cell transplantation: a curative option for sickle cell disease. Pediatr Hematol Oncol. 2007;24(8):569–575. doi: 10.1080/08880010701640531. [DOI] [PubMed] [Google Scholar]
- 12.Viollier R, Socie G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41(1):45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 13.Champlin RE, Nimer SD, Ireland P, Oette DH, Golde DW. Treatment of refractory aplastic anemia with recombinant human granulocyte-macrophage-colony-stimulating factor. Blood. 1989;73(3):694–699. [PubMed] [Google Scholar]
- 14.Deeg HJ, Self S, Storb R, Doney K, Appelbaum FR, Witherspoon RP, et al. Decreased incidence of marrow graft rejection in patients with severe aplastic anemia: changing impact of risk factors. Blood. 1986;68(6):1363–1368. [PubMed] [Google Scholar]
- 15.Gluckman E, Horowitz MM, Champlin RE, Hows JM, Bacigalupo A, Biggs JC, et al. Bone marrow transplantation for severe aplastic anemia: influence of conditioning and graft-versus-host disease prophylaxis regimens on outcome. Blood. 1992;79(1):269–275. [PubMed] [Google Scholar]
- 16.Storb R, Kolb HJ, Graham TC, Kane PJ, Thomas ED. The effect of prior blood transfusions on hemopoietic grafts from histoincompatible canine littermates. Transplantation. 1972;14(2):248–252. doi: 10.1097/00007890-197208000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Storb R, Rudolph RH, Graham TC, Thomas ED. The influence of transfusions from unrelated donors upon marrow grafts between histocompatible canine siblings. J Immunol. 1971;107(2):409–413. [PubMed] [Google Scholar]
- 18.Storb R, Epstein RB, Rudolph RH, Thomas ED. The effect of prior transfusion on marrow grafts between histocompatible canine siblings. J Immunol. 1970;105(3):627–633. [PubMed] [Google Scholar]
- 19.Storb R, Deeg HJ. Failure of allogeneic canine marrow grafts after total-body irradiation. Allogeneic “resistance” versus transfusion-induced sensitization. Transplantation. 1986;42(6):571–580. doi: 10.1097/00007890-198612000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114(11):2315–2322. doi: 10.1182/blood-2009-04-214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3(11):1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 22.Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008;181(8):5313–5322. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marijt WA, Kernan NA, Diaz-Barrientos T, Veenhof WF, O’Reilly RJ, Willemze R, et al. Multiple minor histocompatibility antigen-specific cytotoxic T lymphocyte clones can be generated during graft rejection after HLA-identical bone marrow transplantation. Bone Marrow Transplant. 1995;16(1):125–132. [PubMed] [Google Scholar]
- 24.Zimmerman Z, Shatry A, Deyev V, Podack E, Mammolenti M, Blazar BR, et al. Effector cells derived from host CD8 memory T cells mediate rapid resistance against minor histocompatibility antigen-mismatched allogeneic marrow grafts without participation of perforin, Fas ligand, and the simultaneous inhibition of 3 tumor necrosis factor family effector pathways. Biol Blood Marrow Transplant. 2005;11(8):576–586. doi: 10.1016/j.bbmt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman ZF, Levy RB. MiHA reactive CD4 and CD8 T-cells effect resistance to hematopoietic engraftment following reduced intensity conditioning. Am J Transplant. 2006;6(9):2089–2098. doi: 10.1111/j.1600-6143.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 26.Zimring JC, Hair GA, Deshpande SS, Horan JT. Immunization to minor histocompatibility antigens on transfused RBCs through crosspriming into recipient MHC class I pathways. Blood. 2006;107(1):187–189. doi: 10.1182/blood-2005-07-3059. [DOI] [PubMed] [Google Scholar]
- 27.Patel SR, Cadwell CM, Medford A, Zimring JC. Transfusion of minor histocompatibility antigen-mismatched platelets induces rejection of bone marrow transplants in mice. J Clin Invest. 2009;119(9):2787–2794. doi: 10.1172/JCI39590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukacher AE, Wilson CS. Resistance to polyoma virus-induced tumors correlates with CTL recognition of an immunodominant H-2Dk-restricted epitope in the middle T protein. J Immunol. 1998;160(4):1724–1734. [PubMed] [Google Scholar]
- 29.Andrews NP, Pack CD, Lukacher AE. Generation of antiviral major histocompatibility complex class I-restricted T cells in the absence of CD8 coreceptors. J Virol. 2008;82(10):4697–4705. doi: 10.1128/JVI.02698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews NP, Pack CD, Vezys V, Barber GN, Lukacher AE. Early virus-associated bystander events affect the fitness of the CD8 T cell response to persistent virus infection. J Immunol. 2007;178(11):7267–7275. doi: 10.4049/jimmunol.178.11.7267. [DOI] [PubMed] [Google Scholar]
- 31.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171(1):27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 33.Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD, et al. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46(9):1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 34.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19(3):315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Kuniyasu Y, Qamar A, Sheikh SZ, Jhandier MN, Hakim W, Mehal WZ. Blocking intrahepatic deletion of activated CD8+ T cells by an altered peptide ligand. Cell Immunol. 2005;238(1):31–37. doi: 10.1016/j.cellimm.2005.12.006. [DOI] [PubMed] [Google Scholar]