Abstract
States of glucocorticoid excess are associated with defects in chondrocyte function. Most prominently there is a reduction in linear growth but delayed healing of fractures that require endochondral ossification to also occur. In contrast, little is known about the role of endogenous glucocorticoids in chondrocyte function. As glucocorticoids exert their cellular actions through the glucocorticoid receptor (GR), we aimed to elucidate the role of endogenous glucocorticoids in chondrocyte function in vivo through characterization of tamoxifen-inducible chondrocyte-specific GR knockout (chGRKO) mice in which the GR was deleted at various post-natal ages. Knee joint architecture, cartilage structure, growth plates, intervertebral discs, long bone length and bone micro-architecture were similar in chGRKO and control mice at all ages. Analysis of fracture healing in chGRKO and control mice demonstrated that in metaphyseal fractures, chGRKO mice formed a larger cartilaginous callus at 1 and 2 week post-surgery, as well as a smaller amount of well-mineralized bony callus at the fracture site 4 week post-surgery, when compared to control mice. In contrast, chondrocyte-specific GR knockout did not affect diaphyseal fracture healing. We conclude that endogenous GC signaling in chondrocytes plays an important role during metaphyseal fracture healing but is not essential for normal long bone growth.
Keywords: Glucocorticoids, Chondrocyte, Metaphyseal fracture, Diaphyseal fracture, Growth, Cartilage
Introduction
Glucocorticoids (GCs) have been widely used in the management of inflammatory diseases including rheumatoid arthritis (RA), asthma and inflammatory bowel disease [1,2]. It is well established that at pharmacological doses GCs have detrimental effects on bone, muscle and cartilage [3]. Both systemic GC therapy and endogenous GC excess (e.g. in the context of Cushing's disease) can cause growth retardation in children and adolescents [4,5]. An increase in fracture risk and poor fracture healing are also well-recognized adverse effects of long-term therapeutic GC use [6–8].
Long bone is formed by endochondral ossification [9]. In this process, mesenchymal cells (MSCs) firstly undergo differentiation into chondrocytes, which then differentiate into osteoblasts that form bone. Longitudinal growth depends on the tempo of differentiation of chondrocytes into osteoblasts, which also affects the mineral density of bone formed during endochondral ossification [10,11]. We have previously demonstrated that osteoblast function is physiologically regulated by endogenous GCs [12–16]. The role of endogenous GC signaling in chondrocyte-dependent processes such as longitudinal bone growth, formation of long-bone microarchitecture and fracture healing has not previously been explored. The glucocorticoid receptor (GR) is detected in proliferative, mature and hypertrophic chondrocytes in both human and rat growth plates [17,18]. Whereas chondrocyte specific GR target genes had been identified by expression profiling [19], its specific role in the regulation of chondrocyte function remains unclear. The aim of this study was therefore to investigate the role of endogenous GCs in cartilage and bone developments in normal physiology, and during fracture healing. To address this aim, we generated tamoxifen-inducible cartilage-specific GR knockout mice (Col2a1-CreERT2; GRflox/flox), which allow precise temporal control of GR deletion within chondrocytes [20,21].
Materials and methods
Generation of transgenic mice
Col2a1-CreERT2 transgenic mice were generated as described previously [21,22]. GRflox/flox transgenic mice were backcrossed to the C57BL/6 background for 10 generations as previously described [23,24]. To generate chondrocyte-specific GR knockout mice, Col2a1-CreERT2 transgenic mice were bred with GRflox/flox transgenic mice. Before being bred with GRflox/flox mice, Col2a1-CreERT2 mice were cross-bred with Rosa26R reporter mice to confirm the ability of the transgene to efficiently target chondrocytes, as described in previous studies [20,25]. Briefly, 2 week-old Col2a1-CreERT2;R26R mice were intra-peritoneally injected with tamoxifen for 5 consecutive days (1 mg/mouse/day) and harvested 8 weeks after injection. Cre-recombination efficiency was evaluated by X-Gal staining. The Col2a1-CreERT2;GRflox/flox mice were then generated and used in experiments as chondrocyte-specific GRKO mice (referred to as chGRKO mice). Efficient deletion in cartilage was demonstrated by detecting the deleted allele using PCR, while their Cre negative littermates, Cre−/−;GRflox/flox mice served as controls (referred to as CTR mice).
All mice were on the C57BL/6 background and mouse genotyping was determined by PCR using DNA extracted from mouse toe clips. Cre positivity was tested by using CreF (5′-ATCCGAAAAGAAAACGTT GA-3′) and CreR (5′-ATCCAGGTTACGGA-TATAGT-3′) primers. RosaFv (5′-GCGAAGAGTTTGTCCTCAACC-3′) and RosaRv (5′-AAAGTCGCTCTG AGTTGTTAT-3′) primers were used for Rosa26 reporter testing, while GR1 (5′-GGCATGCACATTACTGGCCTTCT-3′) and GR8 (5′-GTGTAGCA GCCAGCTTACAGGA-3′) primers were used for GRflox genotyping [23]. After tamoxifen injection, exon 3 of the GR gene is expected to be deleted in GRKO mice, the loss of which can be detected using GR1 and GR4 (5′-GTGTAGCAGCCAGCTTACAGGA-3′) primers as described [16].
Induction of GR knockout at various developmental stages
In order to investigate post-natal cartilage and bone development, GR deletion was induced at three different post-natal ages, namely at 2, 4 and 10 weeks of age. Both CTR mice and chGRKO littermates were injected i.p. with tamoxifen for 5 consecutive days (1 mg/mouse for 2 week-old mice; 1 mg/10 g of body weight for 4 week-old mice), followed by weekly body weight measurements for 8 weeks (referred to as 2 + 8 and 4 + 8 week-old chGRKO and CTR mice). In contrast, the 10 week-old mice were utilized for investigating cartilage changes after GR deletion. To allow endogenous GCs to have a long-term impact on cartilage maintenance, 10 week-old CTR and chGRKO mice were left for 24 weeks after i.p. tamoxifen injection (1 mg/10 g body weight, 5 days). These animals are referred to as 10 + 24 week-old chGRKO and CTR mice.
Animals were harvested 8 or 24 weeks after tamoxifen injection and tissues were analyzed as described further below.
Fracture models
The fracture models employed in the current study were performed at two different sites: the tibial metaphysis and the mid-tibial diaphysis (Supplementary Fig. 1). Both models include a unilateral (right-sided) open transverse tibial fracture followed by intramedullary needle fixation. Ten-week-old chGRKO and CTR mice were anesthetized by i.p. injection with 0.1 mL xylazine and 0.75 mL ketamine in 10 mL saline (0.12 mL/10 g bodyweight). Surgery was then performed under aseptic conditions. A 1.2 cm skin incision was made along the antero-medial surface of the tibia. At the medial side of the patellar ligament, a 27G syringe needle was inserted into the bone marrow cavity through the tibial plateau. The needle was then removed, and an electric saw was used to transect the tibia, followed by reinsertion of the needle. Care was taken to protect the soft tissues and preserve the periosteum and fibula. Both chGRKO and CTR mice were i.p. injected with tamoxifen on days 1, 3, and 5 post fracture surgery as described previously [26].
Groups of chGRKO and CTR mice were sacrificed at weeks 1, 2, 3 and 4 postoperatively and bone samples were obtained from the operation side. During the procedure, callus tissue was carefully protected and the intramedullary needle was removed. Samples harvested were analyzed by X-ray, micro-CT, histology and mechanical testing.
X-ray and micro-CT analysis
X-ray images of live mice were taken using a Faxitron imager (MX 20/DX 50, Wheeling, USA) immediately after surgery and 1, 2, 3 and 4 week post-surgery.
Micro-CT analyses of tibiae were performed using Skyscan 1172 (Bruker SkyScan, Kontich, Belgium) as previously described [27]. The tibiae were placed into the sample holder, surrounded by phosphate buffered saline (PBS) with low-density foam to firmly position the specimen. Scanning was performed at 60 keV, 167 µA, 1475 ms without using a filter. In total, approximately 1300 projections were collected at a resolution of 7.59 µm per pixel. Reconstruction of sections was performed using GPU Accelerated NRecon software. After reconstruction, data were analyzed using CTAn software (CTAn1.8, Skyscan). The region of interest (ROI) was selected by using automated algorithms from the original reconstructed images. For the tibiae examined for the mouse characterization study, the 1-mm-long ROI started from 0.5 mm proximal to the metaphyseal growth plate. For the facture study, 100 slices above and below the fracture line were selected as the ROI (in total 1.518-mm-long). Morphologic measurements of the ROI were performed and the following 3D parameters were obtained: bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp).
Bone density and mineralization of bone during fracture healing were examined using CTAn software. The mineral density of the callus was presented as a grayscale index from 0 to 255 in a positive correlative analysis. In the metaphyseal fracture study, tissue was classified as low-density bone when the grayscale value exceeded ‘75’ but was below ‘110’; grayscale values of greater than ‘110’ signified high-density bone. Due to a change in the X-ray source between the scanning of samples, different thresholds were employed for the analysis of the diaphyseal fracture group. For this part of the study, the threshold value for high-density bone remained at ‘110’ whereas a grayscale value above ‘60’ but below ‘110’ was classified as low-density bone.
Histologic staining and immunohistochemistry
Bone samples were fixed in 4% paraformaldehyde (PFA, Merck KGaA, Darmstadt, Germany) and decalcified in 10% EDTA. Serial longitudinal 4 µm-thick sections were cut and stained with hematoxylin and eosin (H&E), Safranin O/fast green and Toluidine blue for standard examinations. Since cartilage was stained red, while other tissues including bone and muscle were stained green by safranin O/fast green staining, the red area was measured by Image J software (National Institutes of Health, USA) and recorded as the area of cartilaginous callus.
The efficiency of GR deletion in the chondrocytes of chGRKO when compared with CTR mice was determined by immunohistochemistry. Immunolocalization of GR was performed using anti-GR rabbit polyclonal antibody (1:400; sc-1004, Santa Cruz Biotechnology), after heat-induced antigen retrieval in citrate buffer. The signals were detected using biotinylated goat anti-rabbit secondary antibodie (Vectastain ABC-Peroxidase Kits, Vector Laboratories, Burlingame, CA, USA) and DAB substrate (Vector Laboratories). Sections were counterstained with Harris Hematoxylin.
Mechanical testing
Bone specimens were assessed using a three-point bending test on an Instron 5944, with data collected using BlueHill 3 software. Bones were stored at −80 °C prior to testing. Tibiae were placed so that the medial side faced downwards, with the upper fixture contacting the mid diaphysis, with a support span of 8 mm. Samples were preloaded at 0.25 mm/min until 1 N was reached, at which point the rate increased to 0.5 mm/min until failure.
Statistical analysis
Unless stated otherwise, data is presented as mean ± standard error of the mean (SEM). Comparisons between two groups of normally distributed variables were performed using a student's T-test. For comparisons between more than two groups a One-Way-ANOVA with subsequent Bonferroni-adjusted post-hoc analysis was performed. When applicable, repeated-measures ANOVA (‘Split-Plot-ANOVA’) were used to determine statistical significance within and between groups over time. Significance was accepted where p < 0.05.
Results
Assessment of tamoxifen-induced Cre-recombinase in Col2a1-CreERT2; R26R transgenic mice
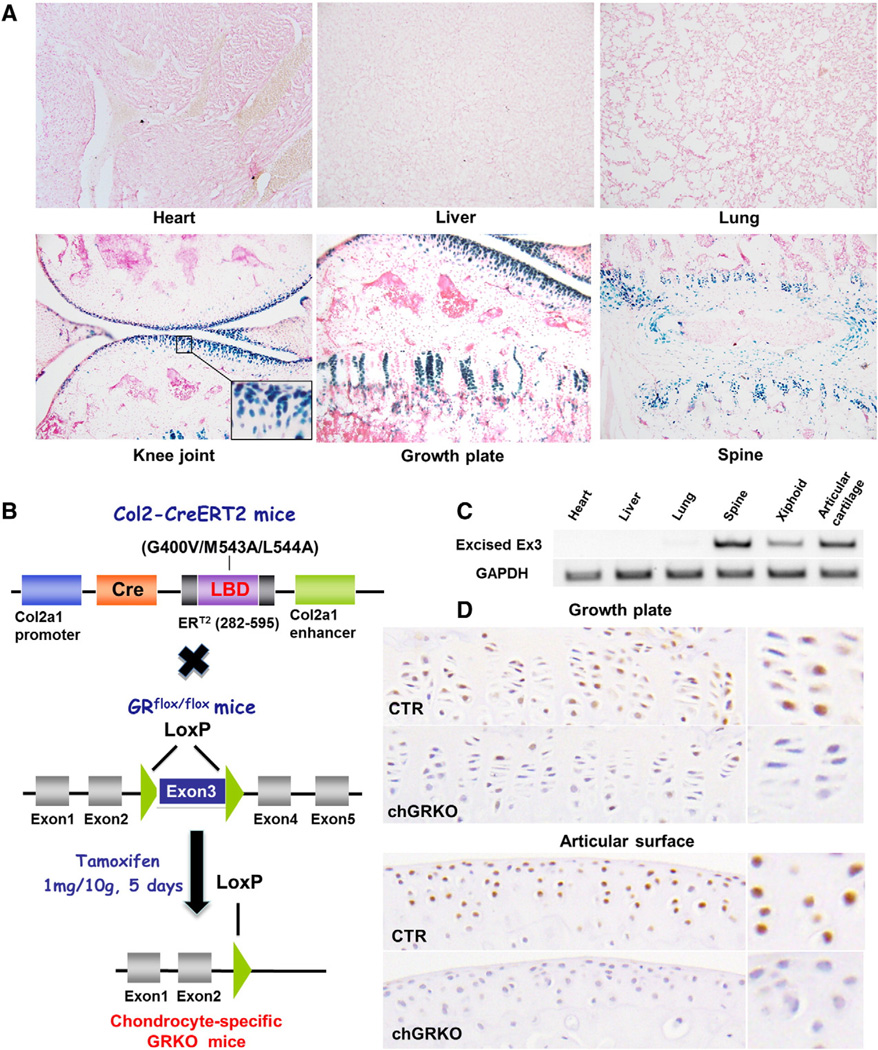
To assess the activity and specificity of tamoxifen-induced Cre re-combination in vivo, Col2a1-CreERT2 transgenic mice were crossed with Rosa26R reporter mice (R26R strain), which were then treated with tamoxifen at the age of 2 weeks (daily injection for 5 consecutive days) and sacrificed 8 weeks later. As shown in Fig. 1A, in Col2a1-CreERT2;R26R transgenic mice, X-Gal positivity was observed only in chondrocytes of the mice treated with tamoxifen to activate Cre-recombinase. Cryo-sections showed the highest percentage of X-Gal positive chondrocytes at the articular surface of knee joint cartilage (over 90%) with no transgene expression seen in subchondral bone tissue. Chondrocytes located in the growth plate also exhibited distinct staining, although a lower proportion of cells were affected (approximately 60%). In the intervertebral discs, the efficiency of tamoxifen-induced Cre-recombination was around 80%, with no X-Gal positive staining in the nucleus pulposus of the intervertebral discs. Although cells in the nucleus pulposus produce type II collagen, over 80% of its protein component is proteoglycans [20]. Another explanation for the low Cre-expression in the nucleus pulposus could be low penetration by tamoxifen. There was no positive X-Gal staining seen in non-cartilaginous tissues, such as heart, liver or lung (Fig. 1A). These results demonstrated that Cre expression was restricted to chondrocytes without any off-target expression.
Fig. 1.
Chondrocyte-specific Cre activity of Col2a1-CreERT2;R26+/+ and chGRKO mice. A, positive X-GAL staining was only detected in the cartilage of Col2a1-CreERT2;R26+/+ mice, and not in other tissues; B, construct of chGRKO mice generated by cross-breeding Col2-CreERT2 mice with GRflox/flox mice. The LoxP sites flanking exon3 of the GR gene resulted in the termination of gene transcription; C, the product of GR knockout was assessed by PCR from multiple tissues including heart, liver, lung, spine, xiphoid and knee joint of 10 + 8 week-old chGRKO mice. Positive expression was detected in spine and long bone, but not in other tissues by PCR using GR1 and GR4 primers. Expression of the glyceraldehyde phosphate dehydrogenase gene (GAPDH) was used as internal control; D, immunohistochemistry staining displayed decreased GR-positive chondrocytes in both the growth plate and articular surface of chGRKO mice compared to CTR mice.
To confirm the specificity of tamoxifen-induced Cre recombination in the chGRKO mouse model, the deletion of GR was examined by RT-PCR in multiple tissues including heart, liver, lung, spine, xiphoid and knee joint in 10 + 8-week-old chGRKO mice. In GRflox/flox mice, exon 3 of the GR gene is flanked by LoxP sites as shown in Fig. 1B [23]. When cross-bred with Col2-CreERT2 mice and treated with tamoxifen, exon 3 of chGRKO mice becomes deleted which creates a stop codon (TAG) impacting on GR translation. The knockout product of GR was detected by GR1 and GR4 primers as previously described [23]. The positive expression was detected in spine, xiphoid and knee joint tissues but not in heart, liver, and lung tissues (Fig. 1C).
The efficiency of GR deletion in the chondrocytes of chGRKO mice was consistent with Cre activity detected in Col2a1-CreERT2;R26R transgenic mice. Immunohistochemistry staining revealed that in growth plates, ~85% chondrocytes were GR positive in the CTR mice, while this percentage was decreased to ~25% in the chGRKO mice (Fig. 1D). As for the articular cartilage, there was barely GR-positive chondrocytes detected in chGRKO mice while the CTR mice exhibited approximately 90% GR-positive chondrocytes (Fig. 1D).
Evaluation of GR knockout impact on cartilage and postnatal bone development
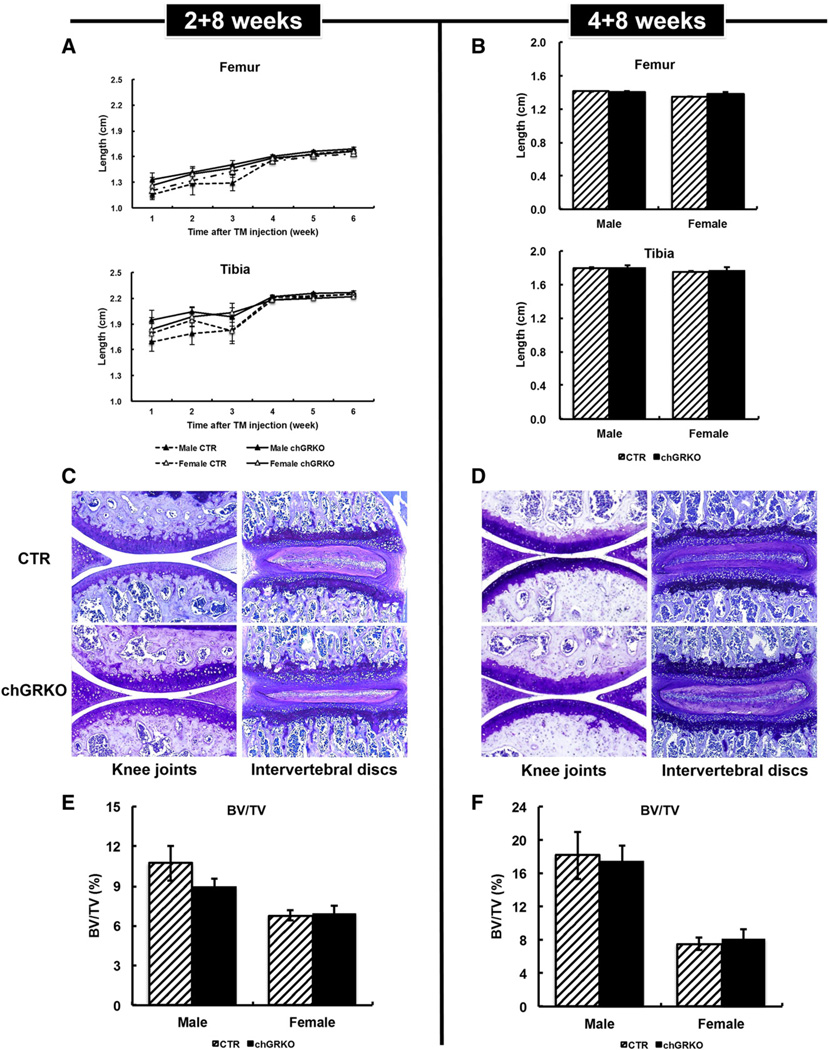
Two and four-week-old chGRKO and CTR mice displayed normal body weight gain during the 8 weeks following tamoxifen injection, with no significant difference between chGRKO and CTR mice (data not shown). Femur and tibia lengths increased with age in both genotype and age groups with no apparent difference between CTR and chGRKO mice, or between genders at any time points (Figs. 2A, B). Toluidine blue staining of knee joint and vertebral sections revealed regular articular and spinal cartilage in all mice of both age groups (see Figs. 2C, D for representative images). While micro-CT analysis of tibiae revealed greater BV/TV in male compared to female mice, chGRKO and CTR mice exhibited similar BV/TV and trabecular bone architecture (including Tb.Th, Tb.Sp and Tb.N) (Figs. 2E, F and data not shown).
Fig. 2.
GR knockout showed no apparent effect on 2+8 and 4+8 week-old mice. A, weekly X-ray images of femurs and tibiae displayed no difference between 2+8 week-old chGRKO and CTR mice; B, 4+8 week-old chGRKO and CTR mice did not exhibit any difference in femur and tibia length; C and D, toluidine blue staining showed normal chondrocytes and cartilage in both knee joint and vertebra of both 2 + 8 (C) and 4 + 8 week-old chGRKO and CTR mice (D); E and F, micro-CT analysis of tibiae indicated similar bone volume in 2 + 8 and 4 + 8 week-old chGRKO and CTR mice.
Evaluation of GR knockout effects in 10 + 24 week-old mice
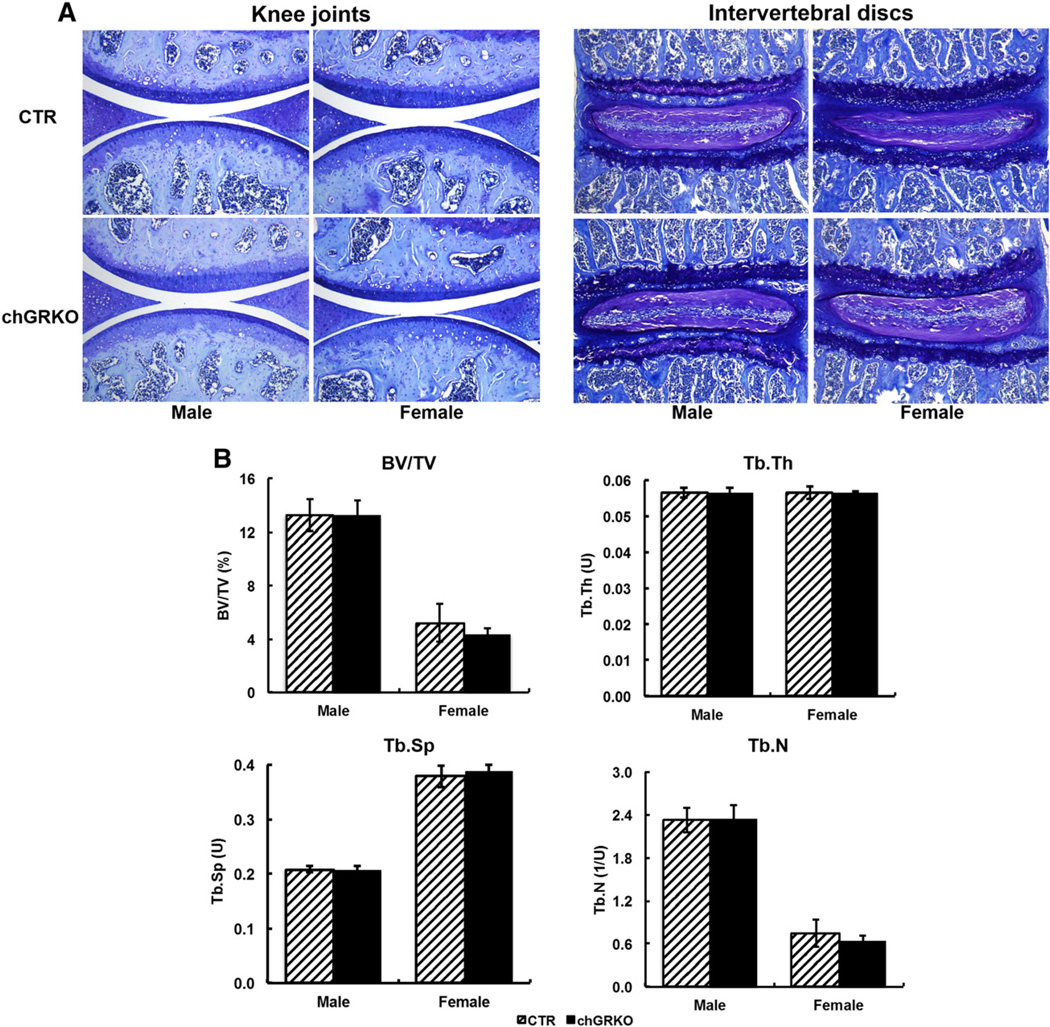
Tamoxifen is known to have effects on estrogen receptor signaling, which could potentially affect endogenous estrogen levels and thus bone metabolism and structure. Although no bone-specific effects have ever been reported in tamoxifen-inducible transgenic mouse models, we analyzed sexually mature mice for potential phenotypic bone changes. Micro-CT confirmed that there was no change in bone volume or trabecular bone microarchitecture resulting from tamoxifen treatment (Supplementary Fig. 2). Ten-week-old chGRKO and CTR mice were also characterized 24 weeks after 5 day tamoxifen injection. Instead of the bone modeling and cartilage development seen in young mice, bone remodeling and cartilage degeneration are dominant in adult mice. Analysis of chondrocytemorphology revealed thinner articular cartilage and less trabecular bone in the tibiae of 10+24week-old mice compared with the previous 2 age groups. However, neither the knee joint nor the spine showed any difference in chondrocyte morphology among the four groups (Fig. 3A). Micro-CT analysis also revealed that chGRKO and CTR mice were similar in BV/TV, Tb.Th, Tb.Sp and Tb.N.
Fig. 3.
GR knockout displayed no apparent effects on 10 + 24 week-old mice. A, 24 weeks after tamoxifen injection, toluidine blue staining displayed similar chondrocyte and cartilage morphologies in knee joint and vertebra of both chGRKO and CTR mice; B, micro-CT data did not indicate differences in BV/TV, Tb.Th, Tb.Sp, or Tb.N between chGRKO and CTR mice.
The above characterization of chGRKO mice indicates that endogenous GC signaling in chondrocytes is not essential for postnatal cartilage development and long bone growth. The similar phenotype also indicated that these mice could be used to investigate the role of endogenous GC action on chondrocytes during fracture healing.
Metaphyseal fracture study
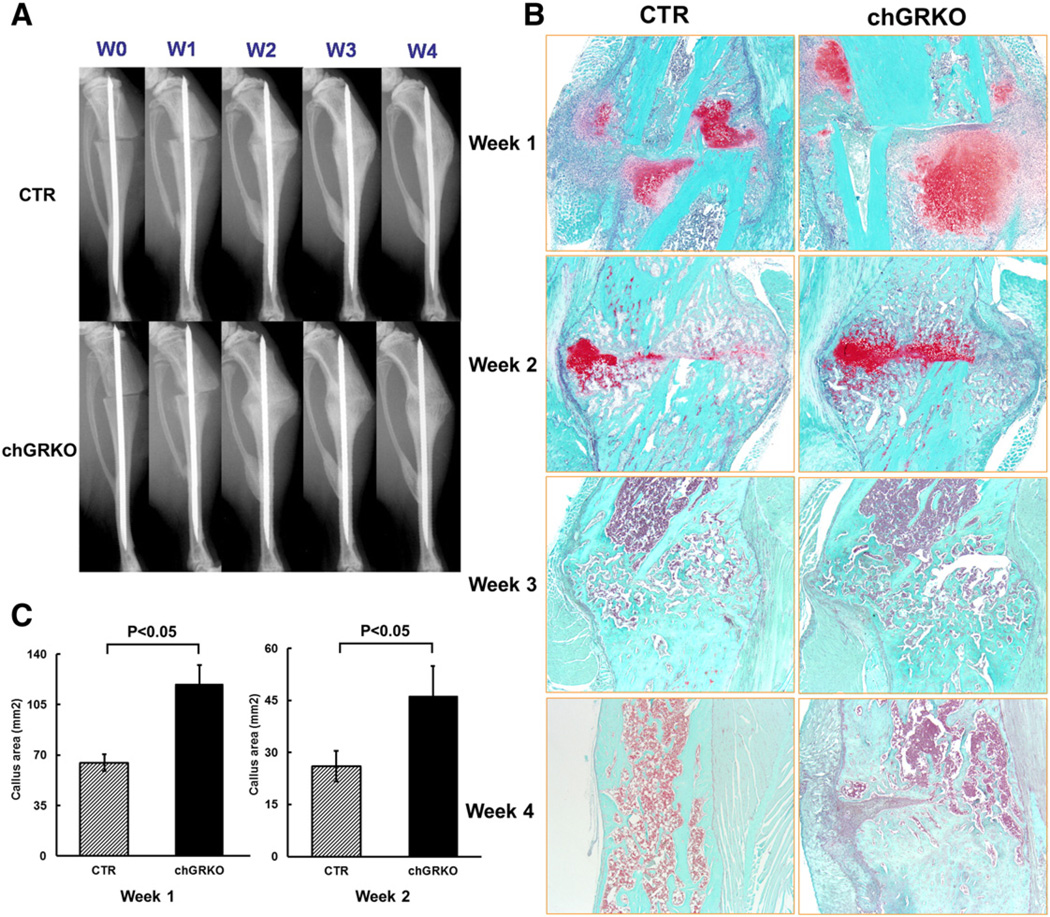
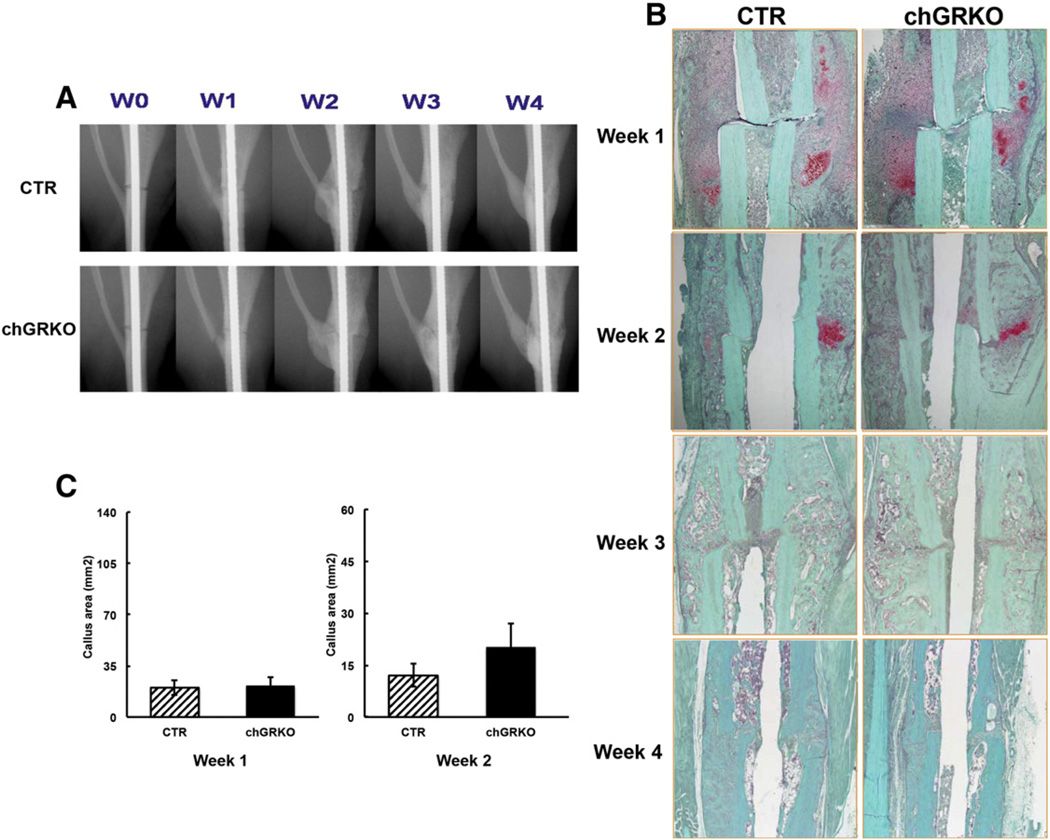
After fracture surgery, both chGRKO and CTR mice were i.p. injected with tamoxifen on days 1, 3, and 5 to induce Cre activity and GR knock-out as described previously [26]. The successful chondrocyte-specific deletion of GR at the fracture sites was shown by RT-PCR in callus tissue of chGRKO mice 1, 2 and 3 week post-surgery (Supplementary Fig. 3). The progression of defect repair was examined via X-ray imaging of all tibial defects at weeks 0, 1, 2, 3 and 4 following surgery. X-ray images displayed similar fracture pattern in CTR and chGRKO mice with a consistent transverse gap at the tibial metaphysis (Fig. 4A). At week 1, minimal displacement of the fractured tibiae and newly formed fractures at the fibula were detected in both chGRKO and CTR mice. By week 2, formation of soft callus was observed, which bridged the fracture gap. By week 3 a blurred gap with more consolidated bridging was observed and almost completed bridging was seen at the last time point (week 4). More extensive soft callus was observed in chGRKO mice at week 3 and week 4, suggesting delayed healing compared to CTR mice.
Fig. 4.
X-ray and histological evaluations of the images of metaphyseal fracture healing. A, X-ray images of tibial fractures of the metaphyseal fracture study exhibited fracture healing in chGRKO and CTR mice; B, representative histological images of tibial fractures of chGRKO and CTR mice from week 1 to week 4. Safranin O/fast green staining exhibited larger cartilaginous callus in chGRKO mice than CTR mice at weeks 1 and 2, while tibiae harvested at weeks 3 and 4 only demonstrated woven bone; C, histomorphometric analysis confirmed that chGRKO mice displayed greater cartilaginous callus area 1 and 2 week post-surgery when compared with CTR mice.
In agreement with X-ray imaging, histologic analysis of the bones confirmed gradual fracture healing. Sections stained with safranin O/fast green demonstrated that cartilaginous callus was formed across the fracture site 1 week after fracture surgery (Fig. 4B, stained red). Cartilaginous callus area was significantly greater in chGRKO compared to CTR mice (Fig. 4C). Two weeks after surgery, the majority of the cartilaginous callus in both chGRKO and CTR mice was replaced by bony callus, however, the remaining cartilaginous callus area was still larger in chGRKO than in CTR mice (Fig. 4C). Three and four weeks after fracture surgery, the cartilaginous callus had been replaced by bony callus. Representative histologic images showed that while CTR mice showed normal bone remodeling and healing, this was delayed in chGRKO mice.
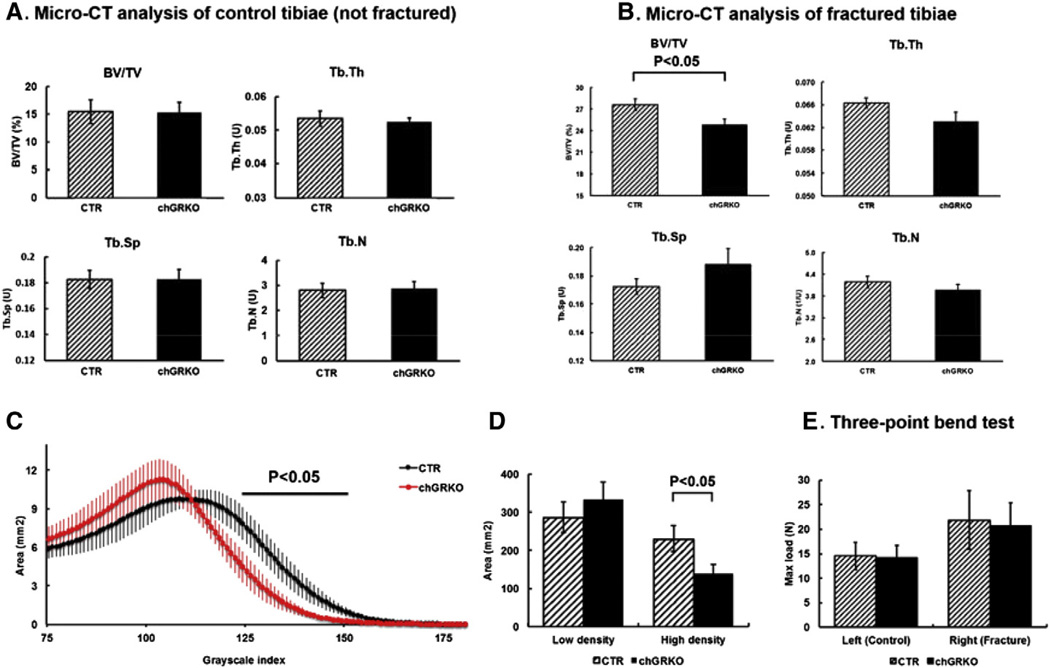
To evaluate the quality of healed bone, fractured and contralateral (non-fractured) tibiae were harvested at week 4 and subjected to micro-CT scanning and analysis. As shown in Fig. 5A, non-fractured tibiae showed similar BV/TV, Tb.Th, Tb.N and Tb.Sp in both chGRKO mice and CTR littermates. In contrast, in the fractured tibiae, BV/TV was significantly lower in the callus area of chGRKO mice compared with CTR mice, indicating less bone formed at the fracture site of chGRKO mice. Consistently, the data also demonstrated a trend towards decreased Tb.Th and Tb.N, and increased Tb.Sp in chGRKO mice compared to CTR mice (Fig. 5B).
Fig. 5.
Micro-CT and mechanical analysis of tibiae 4 weeks after the metaphyseal fracture surgery. A, no difference was observed in control tibiae (left side) between chGRKO and CTR mice in BV/TV, Tb.Th, Tb.Sp or Tb.N; B, in fractured tibiae (right side), BV/TV was significantly decreased in chGRKO mice compared with CTR mice. There was also a trend towards decreased Tb.Th and Tb.N, and increased Tb.Sp observed in chGRKO mice, which is consistent with the decreased BV/TV, although no statistically significant difference was detected; C, distributions of the mineral density for repaired bone of the chGRKO and CTR mice. The threshold value for the binarization of the micro-CT images (75) corresponds to the lowest value in the diagram. chGRKO mice exhibited a different distribution pattern from CTR mice; D, The healed callus was classified as low-density bone when the grayscale value exceeded ‘75’ but was below ‘110’; grayscale values of greater than ‘110’ signified high-density bone. chGRKO mice showed no apparent difference in low-density bony callus but a significant decrease of high-density bony callus in the fracture site compared with CTR mice; E, three-point bend testing demonstrated higher max load in the control tibiae compared to fractured tibiae, with no difference between CTR and chGRKO mice.
As a consequence of ossification, remodeling and mineralization during the fracture healing process, the repaired bone consists of bone formed at different time points and thus with different mineral content [28,29]. Using the mineral density distribution obtained by micro-CT analysis [30,31], a higher density indicates better-mineralized bony callus while a lower density represents less-mineralized bony callus. As shown in Fig. 5C, chGRKO mice exhibited a distribution pattern different from CTR mice. chGRKO mice had a reduction in the size of tissue with high density compared to CTR mice (Fig. 5D). This indicated that there was a smaller amount of well-mineralized bony callus at the fracture site and bone repair was delayed in chGRKO mice, findings consistent with the results of the histologic analyses obtained at week 3 and week 4.
Mechanical bone strength was assessed by three-point bending of fractured and non-fractured tibiae. Control tibiae of CTR and chGRKO mice displayed similar strength with maximum loads of 14.6 N (CTR) and 14.3 N (chGRKO). In contrast, maximum loads applied to the fractured tibiae were 20.8 N for chGRKO and 21.9 N for CTR mice, with no significant difference between the two groups.
Diaphyseal fracture study
In both CTR and chGRKO mice, healing following diaphyseal fracture was similar to that observed after metaphyseal fracture. However, X-ray analysis revealed that both CTR and chGRKO mice developed smaller calluses at the diaphyseal fracture site compared to metaphyseal fracture (Fig. 6A). In agreement with X-ray imaging, histological images of the diaphyseal fractures for both CTR and chGRKO mice revealed less displacement of fractured bone at all four time points when compared to the metaphysis (Fig. 6B). In addition, the size of the cartilaginous callus formed in mice following diaphyseal fracture was significantly smaller when compared to the metaphyseal fracture (Supplementary Fig. 4). Interestingly, histomorphometry analysis revealed no difference in cartilaginous callus sizes formed at weeks 1 and 2 post diaphyseal fracture in CTR and chGRKO mice (Fig. 6C).
Fig. 6.
X-ray and histological evaluations of the images of diaphyseal fracture healing. A, Representative X-ray images of tibial fractures over the course of the diaphyseal fracture study; B, Representative histological images of tibial fractures displayed less displacement of fractured bone at all four time points than corresponding metaphyseal fractures. In addition, chGRKO mice demonstrated similar fracture repair compared to the CTR mice; C, histomorphometric analysis at weeks 1 and 2 revealed similar cartilaginous callus area in chGRKO and CTR mice.
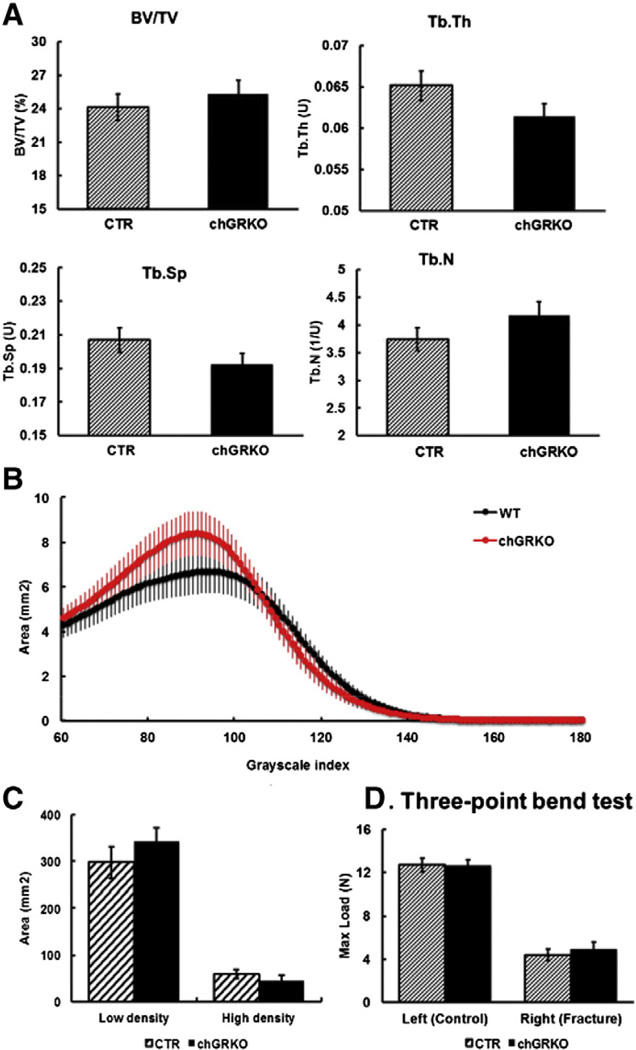
The quality of the newly formed bone 4 weeks after diaphyseal fractures was quantified by micro-CT analysis. As shown in Fig. 7A, bony microarchitecture of the fractured tibiae was similar in chGRKO and CTR mice. The mineral density distribution also showed no significant difference between fractured diaphysis of chGRKO and CTR mice either for low-density or high-density bony callus (Figs. 7B, C).
Fig. 7.
Micro-CT and mechanical analysis of tibiae after the diaphyseal fracture surgery. A, No difference was observed in fractured tibiae (right side) between chGRKO and CTR mice on BV/TV, Tb.Th, Tb.Sp or Tb.N; B–C, distributions of the mineral density for repaired bone of the chGRKO and CTR mice. The threshold value for the binarization of the micro-CT images (60) corresponds to the lowest value in the diagram. The density distribution of bony callus in chGRKO mice showed no significant difference compared to CTR mice; D, Max load of three-point bend testing did not demonstrate any difference between CTR and chGRKO tibiae.
Healed tibiae were harvested at week 3 for mechanical testing to prevent a potential catch-up effect that could eliminate any difference between chGRKO and CTR mice. As shown in Fig. 7D, maximal loads in the control tibiae were similar to those in the metaphyseal fracture group, while fractured tibiae showed a dramatic reduction compared to controls, with no difference between chGRKO and CTR mice.
Discussion
In the current study, we found no apparent effect of chondrocytic disruption of GC signaling on linear growth and bone architecture in growing and young adult mice. However, the data demonstrated that the deletion of GR in chondrocytes delayed metaphyseal fracture healing.
Tamoxifen-induced Cre recombination was shown to occur specifically in chondrocytes of Col2a1-CreERT2;R26R transgenic mice. The ~90% recombination efficiency of articular chondrocytes demonstrated the effectiveness of the tamoxifen-inducible Cre–LoxP system. On the other hand, the chondrocytes of the growth plate demonstrated comparatively lower recombination efficiency (~60%), which is still a level comparable to other studies of the growth plate [20,22]. In addition, the recombination efficiency was assessed 8 weeks after tamoxifen injection, indicating that Cre activity could be sustained long enough for further, subsequent studies.
Characterization of chGRKO mice and their CTR littermates at three different ages revealed no apparent effects of GR knockout on normal cartilage and bone development. Thus, long bone length of younger, 2 and 4week-oldmicewas similar in chGRKO and CTR mice. Chondrocytes of the articular surface and intervertebral discs appeared normal on histology, and trabecular bone architecture was similar in chGRKO mice compared with CTR mice in all age groups. As chondrocytes are responsible for endochondral ossification and are intimately involved in both long bone growth and trabecular bone formation [9], it is perhaps surprising that GR knockout in chondrocytes does not affect long bone growth or trabecular architecture. Glucocorticoids have been implicated in the regulation of chondrogenesis and osteoblast differentiation, as well as maintaining homeostasis in cartilage and bone on a physiological level [19]. Endogenous GCs are important in the maintenance of cellular homeostasis through their binding to the intracellular GR, which is upregulated during chondrocyte maturation [18]. However, global GR deletion results in postnatal lethality due to attenuated lung development [32] but these mice exhibit normal growth plate development at birth [16]. In addition, mesenchymal GR conditional knockout mice with deletion of the GR in chondrocytes, osteoblasts, myoblasts and fibroblasts also display early lethality, delayed lung development and abdominal wall closure defects in the presence of a normal skeletal phenotype and normal growth plate development [24]. Collectively, the phenotype of these chGRKO mouse lines indicates that a role of GR in endochondral ossification or cartilage development is not essential, at least at the embryonic stage.
This is obviously different under pathological conditions such as fracture and fracture healing. Despite the well-known development of osteoporosis and an increased fracture risk resulting from long-term systemic GC treatment [6], only a few animal studies have been conducted to investigate the effects of pharmacological and supra-pharmacological doses of GCs on fracture healing [7,33,34]. Even less is known about the role of endogenous GCs in bone healing. The current study demonstrates that deletion of the GR in chondrocytes results in delayed healing of long bone metaphyseal but not diaphyseal fractures. In the metaphyseal fracture study, histology showed that compared to CTR mice, chGRKO mice formed a larger cartilaginous callus and the resulting bone was less well remodeled. In addition micro-CT analysis demonstrated a decrease in bone volume and a more detailed analysis of density distribution revealed a decrease in the amount of high-density bone in chGRKO mice compared with CTR mice. All of these results indicated a delay in fracture healing at the metaphyseal fracture site. In contrast, GR knockout in the chondrocytes did not have any apparent effects on diaphyseal fracture healing, suggesting that endogenous GC signaling in chondrocytes is essential to normal fracture healing at the metaphysis but not the diaphysis of the tibia. The mechanisms underlying this difference are unclear. One possible explanation could be a greater dependence of metaphyseal fracture healing on endochondral ossification compared to the diaphyseal fracture site [9]. In order to determine the dependency of fracture healing on the presence of cartilage across the two fracture sites we compared the cartilaginous callus size in CTR mice of both fracture studies at weeks 1 and 2. We found that the cartilaginous callus formed across the fracture site was significantly smaller in diaphyseal fracture healing compared to metaphyseal fracture healing at both time points, potentially indicating a greater dependency of metaphyseal fracture healing on the presence of cartilage. Even though the mechanisms governing endogenous GC action in chondrocytes during fracture repair remain unclear, the findings of our study suggest that the molecular mechanisms underlying fracture repair are not only complex but also site-specific.
The three-point bending test was performed to test the mechanical strength of the fracture site at the experimental endpoint. Bending tests are useful for evaluating bone strength of small animals, such as rodents, whose bones are difficult to use in compression or traction tests [35]. The three point-bending test is a more practical and commonly used technique for mice [36,37] than the alternative four-point bending test, since the latter may introduce a new element of variability when the test object surfaces are curved as occurs in bone [35]. The mechanical testing results did not reflect the phenotypic differences between chGRKO and CTR mice following metaphyseal fractures. In addition, the fractured tibiae displayed an even higher maximum load compared with control tibiae, indicating that this test might be affected by the size of the bony callus. Given the importance of the fracture position in mechanical testing and bone size in bone strength this is not a surprising finding. In the current study, the analysis of mineralization distribution within the bony callus appeared to be a more suitable tool to assess fracture healing. Thus, tissues with different mineral densities (in proportion of the total tissue area) could be clearly distinguished by grayscale index distribution. This allowed for further analysis of the components of the callus rather than the total bone volume. In addition, this method is unaffected by the position of the fracture line or the size of the callus.
The delayed fracture healing caused by GR knockout in cartilage could be due to a defective interference of disrupted endogenous GC signaling by the inflammatory process. The acute inflammatory response is an important part of fracture healing which to a certain degree is maintained by inflammatory cytokines [38] and mesenchymal stem cells (MSCs) [39]. As a principle mediator of the anti-inflammatory action of GCs, knocking out the GR may imbalance the degree of inflammation and thereby interfere with fracture healing. Moreover, the inflammation caused by fracture could increase the expression of 11β-HSD1 locally [40], which may in turn affect the healing process.
In conclusion, chondrocyte-specific GR knockout is associated with delayed fracture healing in the metaphysis of the tibia has no apparent effect on normal cartilage and bone development. This finding points to a role of chondrocytic endogenous GC signaling in the normal endochondral fracture repair process.
Acknowledgments
The authors acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Australian Centre for Microscopy & Microanalysis at the University of Sydney. This work was supported by the National Health & Medical Research Council, Australia, Project Grant 632818 to HZ, MJS and MSC and DFG Immunobone (Tu220/6 to J.T.).
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bone.2014.08.016.
Footnotes
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Jinwen Tu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Tu, Zhou, Seibel, Cooper, Chen, Tuckermann.
Acquisition of data: Tu, Henneicke, Zhang, Stoner, Cheng, Schindeler.
Analysis and interpretation of data: Tu, Henneicke, Zhou, Seibel, Cooper.
Manuscript writing: Tu, Zhou, Cooper, Seibel.
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 2.Hench PS. Cortisone, hydrocortisone and corticotrophin; some facts and speculations with special reference to rheumatoid arthritis. Trans Assoc Life Insur Med Dir Am. 1951;35:5–33. [PubMed] [Google Scholar]
- 3.Hardy R, Cooper MS. Glucocorticoid-induced osteoporosis—a disorder of mesenchymal stromal cells? Front Endocrinol (Lausanne) 2011;2:24. doi: 10.3389/fendo.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorva RA, Turpeinen MT. Asthma, glucocorticoids and growth. Ann Med. 1994;26(4):309–314. doi: 10.3109/07853899409147907. [DOI] [PubMed] [Google Scholar]
- 5.Allen DB. Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin North Am. 1996;25(3):699–717. doi: 10.1016/s0889-8529(05)70348-0. [DOI] [PubMed] [Google Scholar]
- 6.Vapra A, Kanmer K. [Pathologic bone fractures in patients treated with corticosteroid preparations for long periods of time] Vopr Revm. 1967;7(3):80–82. [PubMed] [Google Scholar]
- 7.Luppen CA, Blake CA, Ammirati KM, Stevens ML, Seeherman HJ, Wozney JM, Bouxsein ML, et al. Recombinant human bone morphogenetic protein-2 enhances osteotomy healing in glucocorticoid-treated rabbits. J Bone Miner Res. 2002;17(2):301–310. doi: 10.1359/jbmr.2002.17.2.301. [DOI] [PubMed] [Google Scholar]
- 8.Doyon AR, Ferries IK, Li J. Glucocorticoid attenuates the anabolic effects of parathyroid hormone on fracture repair. Calcif Tissue Int. 2010;87(1):68–76. doi: 10.1007/s00223-010-9370-3. [DOI] [PubMed] [Google Scholar]
- 9.Kronenbe HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 10.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19(10 Suppl):S4–S6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 11.Webb JCJ. A review of fracture healing. Curr Orthop. 2000;14:457–463. [Google Scholar]
- 12.Zhou H, Mak W, Kalak R, Street J, Fong-Yee C, Zheng Y, et al. Glucocorticoid-dependent Wnt signaling by mature osteoblasts is a key regulator of cranial skeletal development in mice. Development. 2009;136(3):427–436. doi: 10.1242/dev.027706. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ. Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J Biol Chem. 2008;283(4):1936–1945. doi: 10.1074/jbc.M702687200. [DOI] [PubMed] [Google Scholar]
- 14.Weber AJ, Li G, Kalak R, Street J, Buttgereit F, Dunstan CR, et al. Osteoblast-targeted disruption of glucocorticoid signalling does not delay intramembranous bone healing. Steroids. 2010;75(3):282–286. doi: 10.1016/j.steroids.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Buttgereit F, Zhou H, Kalak R, Gaber T, Spies CM, Huscher D, et al. Transgenic disruption of glucocorticoid signaling in mature osteoblasts and osteocytes attenuates K/BxN mouse serum-induced arthritis in vivo. Arthritis Rheum. 2009;60(7):1998–2007. doi: 10.1002/art.24619. [DOI] [PubMed] [Google Scholar]
- 16.Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11(6):517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Silvestrini G, Mocetti P, Di Grezia R, Berni S, Bonucci E. Localization of the glucocorticoid receptor mRNA in cartilage and bone cells of the rat. An in situ hybridization study. Eur J Histochem. 2003;47(3):245–252. doi: 10.4081/834. [DOI] [PubMed] [Google Scholar]
- 18.James CG, Appleton CT, Ulici V, Underhill TM, Beier F. Microarray analyses of gene expression during chondrocyte differentiation identifies novel regulators of hypertrophy. Mol Biol Cell. 2005;16(11):5316–5333. doi: 10.1091/mbc.E05-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James CG, Ulici V, Tuckermann J, Underhill TM, Beier F. Expression profiling of dexamethasone-treated primary chondrocytes identifies targets of glucocorticoid signalling in endochondral bone development. BMC Genomics. 2007;8:205. doi: 10.1186/1471-2164-8-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O'Keefe RJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45(1):44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M, Chen M, Lichtler AC, O'Keefe RJ, Chen D. Tamoxifen-inducible Cre-recombination in articular chondrocytes of adult Col2a1-CreER(T2) transgenic mice. Osteoarthritis Cartilage. 2008;16(1):129–130. doi: 10.1016/j.joca.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23(1):99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 24.Li A, Hardy R, Stoner S, Tuckermann J, Seibel M, Zhou H. Deletion of mesenchymal glucocorticoid receptor attenuates embryonic lung development and abdominal wall closure. PLoS One. 2013;8(5):e63578. doi: 10.1371/journal.pone.0063578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci U S A. 1999;96(9):5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie C, Xue M, Wang Q, Schwarz EM, O'Keefe RJ, Zhang X. Tamoxifen-inducible CreER-mediated gene targeting in periosteum via bone-graft transplantation. J Bone Joint Surg Am. 2008;90(Suppl. 1):9–13. doi: 10.2106/JBJS.G.01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henneicke H, Herrmann M, Kalak R, Brennan-Speranza TC, Heinevetter U, Bertollo N, et al. Corticosterone selectively targets endo-cortical surfaces by an osteoblast-dependent mechanism. Bone. 2011;49(4):733–742. doi: 10.1016/j.bone.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 29.Fajardo RJ, Cory E, Patel ND, Nazarian A, Laib A, Manoharan RK, et al. Specimen size and porosity can introduce error into microCT-based tissue mineral density measurements. Bone. 2009;44(1):176–184. doi: 10.1016/j.bone.2008.08.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 31.Lukas C, Ruffoni D, Lambers FM, Schulte FA, Kuhn G, Kollmannsberger P, et al. Mineralization kinetics in murine trabecular bone quantified by time-lapsed in vivo micro-computed tomography. Bone. 2013;56(1):55–60. doi: 10.1016/j.bone.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9(13):1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 33.Waters RV, Gamradt SC, Asnis P, Vickery BH, Avnur Z, Hill E, et al. Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop Scand. 2000;71(3):316–321. doi: 10.1080/000164700317411951. [DOI] [PubMed] [Google Scholar]
- 34.Bostrom MP, Gamradt SC, Asnis P, Vickery BH, Hill E, Avnur E, et al. Parathyroid hormone-related protein analog RS-66271 is an effective therapy for impaired bone healing in rabbits on corticosteroid therapy. Bone. 2000;26(5):437–442. doi: 10.1016/S8756-3282(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 35.Beaupied H, Lespessailles E, Benhamou CL. Evaluation of macrostructural bone biomechanics. Joint Bone Spine. 2007;74(3):233–239. doi: 10.1016/j.jbspin.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Murnaghan M, McIlmurray L, Mushipe MT, Li G. Time for treating bone fracture using rhBMP-2: a randomised placebo controlled mouse fracture trial. J Orthop Res. 2005;23(3):625–631. doi: 10.1016/j.orthres.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Nyman JS, Moss HE, Gutierrez G, Mundy GR, Yang X, et al. Local low-dose lovastatin delivery improves the bone-healing defect caused by Nf1 loss of function in osteoblasts. J Bone Miner Res. 2010;25(7):1658–1667. doi: 10.1002/jbmr.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konstantinidis I, Papageorgiou SN, Kyrgidis A, Tzellos TG, Kouvelas D. Effect of non-steroidal anti-inflammatory drugs on bone turnover: an evidence-based review. Rev Recent Clin Trials. 2013;8(1):48–60. doi: 10.2174/1574887111308010008. [DOI] [PubMed] [Google Scholar]
- 39.Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raza K, Hardy R, Cooper MS. The 11beta-hydroxysteroid dehydrogenase enzymes–arbiters of the effects of glucocorticoids in synovium and bone. Rheumatology (Oxford) 2010;49(11):2016–2023. doi: 10.1093/rheumatology/keq212. [DOI] [PubMed] [Google Scholar]