Abstract
Background
Platelet transfusions can induce humoral and cellular alloimmunity. Anti-HLA antibodies can render patients refractory to subsequent transfusion, and both alloantibodies and cellular alloimmunity can contribute to subsequent bone marrow transplant rejection. Currently, there are no approved therapeutic interventions to prevent alloimmunization to platelet transfusions other than leukoreduction. Targeted blockade of T cell costimulation has shown great promise in inhibiting alloimmunity in the setting of transplantation, but has not been explored in the context of platelet transfusion.
Study Design and Methods
We tested the hypothesis that the costimulatory blockade reagent CTLA4-Ig would prevent alloreactivity against major and minor alloantigens on transfused platelets. BALB/c (H-2d) mice and C57BL/6 (H-2b) mice were used as platelet donors and transfusion recipients, respectively. Alloantibodies were measured by indirect immunofluorescence using BALB/c platelets and splenocytes as targets. Bone marrow transplants were carried out under reduced intensity conditioning using BALB/b (H-2b) donors and C57BL/6 (H-2b) recipients to model HLA identical transplants. Experimental groups were given CTLA4-Ig (before or after platelet transfusion) with control groups receiving isotype matched antibody.
Results
CTLA4-Ig abrogated both humoral alloimmunization (anti-H-2d antibodies) and transfusion induced bone marrow transplant rejection. Whereas a single dose of CTLA4-Ig at time of transfusion prevented alloimmunization to subsequent platelet transfusions, administration of CTLA4-Ig after initial platelet transfusion was ineffective. Delaying treatment until after platelet transfusion failed to prevent bone marrow transplant rejection.
Conclusions
These findings demonstrate a novel strategy using an FDA approved drug that has the potential to prevent the clinical sequela of alloimmunization to platelet transfusions.
Introduction
Platelet transfusion therapy can be a life-sustaining treatment for many patients with severe thrombocytopenia. However, alloimmunization is a potential sequelae of platelet transfusion with serious consequences for chronically transfused patients. Induction of alloantibodies, typically against HLA and/or human platelet antigens (HPAs), can lead to poor survival of transfused platelets expressing the offending antigens 1–3. In the case of alloimmunization against multiple specificities, patients can become increasingly refractory to transfused platelets. In severe instances, platelet transfusions may cease to be a viable treatment, leaving few options for maintaining hemostasis. Although leukoreduction of platelets has significantly decreased humoral alloimmunization, anti-HLA antibodies still form in at least 18% of transfused patients 4. Currently, there are no approved therapeutic interventions in humans to mitigate risk of alloimmunization other than leukoreduction.
A subset of thrombocytopenic patients suffer bone marrow disorders that can be cured by successful bone marrow transplantation (BMT). Stringent myeloablative conditioning regimens used during BMT for treatment of malignancy have made BMT rejection a very infrequent event, mostly due to destruction of the recipient immune system. However, in congenital or acquired BMT failure syndromes, in which no neoplasia is present, it is difficult to justify stringent conditioning due to the significant morbidity and mortality involved. Rather, BMT for non-malignant disease are typically carried out with HLA-matched BMT under reduced intensity conditions 5–7. However, under these conditions roughly 15% of transplanted patients reject the HLA-matched BMT 8–10. Because the BMT is largely matched at the MHC loci (or identical in the case of HLA matched siblings), the most likely immunological vector mediating rejection in these patients is alloreactivity to minor histocompatibility antigens (mHAs) expressed on the donor bone marrow. Recently, we have reported in a murine model that transfusion of leukoreduced platelets (LR-PLTs) induces BMT rejection if the LR-PLTs and bone marrow share mHAs 11. In this case, the vector of rejection is T cells and not antibodies (Patel, SR., et al manuscript in submission). Thus, in the context of refractoriness to platelet transfusion and transfusion induced BMT rejection, alloimmunization to platelet antigens (in either humoral or cellular compartments), has the potential to cause serious immunological sequelae.
One strategy that has demonstrated efficacy in preventing alloresponses in settings of experimental solid organ transplantation is the blockade of T cell costimulation. Activation and generation of an effective T cell response is generally accepted to require at least two distinct signals. Signal 1 is delivered via interaction of the T cell receptor (TCR) and the peptide:MHC complex. Although signal 1 is required for T cell activation, it is not alone sufficient. An additional second signal is required, consisting of costimulation from molecules on antigen presenting cells (APCs), canonically B7.1 and B7.2 on APCs ligating CD28 on responding T cells; although a multitude of costimulatory signals have now been described 12. T cells that receive signal 1 without signal 2 not only fail to differentiate into mature effector T cells, but can be rendered ineffective through induction of anergy, a regulatory-like phenotype, or possibly deletion 13.
Blockade of the CD28-B7.1/B7.2 signaling pathway can be achieved pharmacologically using a recombinant fusion protein that combines the extracellular domain of the human cytotoxic T-lymphocyte associated antigen 4 (CTLA4) with a modified constant region of human IgG1 (CTLA4-Ig). CTLA4 is a T cell surface receptor that competes with CD28 for binding to B7.1 and B7.2 costimulatory molecules as well as delivering inhibitory signals to the T cell 14. CTLA4-Ig functions by binding to and blocking B7.1/B7.2, thus depriving responding T cells from costimulation through the CD28-B7 axis 15–17. CTLA4-Ig, known commercially as Abatacept, is currently approved by the U.S. Food and Drug and Administration (FDA) for the treatment of psoriasis and rheumatoid arthritis 18. A second-generation derivative, known commercially as Belatacept, has recently received FDA approval for use in renal transplantation 19, 20. Because CTLA4-Ig has shown clinical success in inhibiting autoimmunity and alloimmunization, we hypothesized that the immunosuppressive effects of CTLA4-Ig would extend to the prevention of alloimmunization in response to LR-PLT transfusions.
Herein, we report that CTLA4-Ig prevents both humoral alloimmunization to MHC class I antigens (murine equivalent of HLA) and also prevents LR-PLT transfusion induced BMT rejection. We further demonstrate that administration of CTLA4-Ig prior to initiation of platelet transfusion therapy is required, as administration after platelet transfusion and before BMT does not prevent rejection. These observations demonstrate that blockade of the CD28-B7.1/B7.2 costimulatory pathway during the initial antigen exposure is alone sufficient to inhibit alloimmunization to transfused platelets in a murine model.
Materials and Methods
Mice
Female C57BL/6 (H-2b), BALB/c (H-2d), and BALB.B [C.B10-H-2b/LiMcdJ (H-2b)] mice were purchased from Jackson Laboratories (Bar Harbor, ME). BALB/c mice were used as LR-PLT and whole blood donors at 8 – 12 weeks of age, while BALB.B BM donors and C57BL/6 recipients were utilized at 6 – 8 weeks of age. BALB.B, BALB/c, and C57BL/6 donors for seroanalyses and/or in vivo clearance assays were utilized at 8 – 12 weeks of age. Female TCR75×Thy1.1 (H-2b) mice were a generous gift from Dr. Pat Bucey 21. All mice were housed in Emory University Department of Animal Resources facilities and all studies and procedures were carried out in accordance with Emory University’s Institutional Animal Care and Use Committee guidelines.
Antibodies for Flow Cytometry
Antibodies were purchased from BD Pharmingen (PE anti-mouse CD41, PE anti-TER119/erythroid, PE Rat IgG2b, APC goat anti-mouse Igs, FITC anti-mouse CD19, FITC anti-mouse CD4, FITC anti-mouse CD8α, PE anti-mouse CD3ε, APC anti-mouse CD3ε, FITC Rat IgG2a, k, and FITC anti-mouse CD229.1/Ly9.1) and eBioscience (PE Rat IgG1 and APC anti-mouse CD229/Ly9).
Leukocyte Reduced Platelet Rich Plasma Preparation
LR-PLT products were harvested as previously described 11, and transfusion dose and schedule were based upon existing protocols in mice 22–24. Briefly, donor PLT rich plasma (PRP) was isolated by centrifugation and was passed over a Neonatal Purecell PL High Efficiency Leukocyte Reduction Filter (Pall Corporation, Port Washington, NY). LR-PLT transfusions contained 1x108 total PLTs and were transfused through the tail-vein. All PLT handling was performed at room temperature. PLTs, residual RBCs, and residual leukocytes were enumerated as previously described 11. A “swirl” test was performed on all LR-PLT concentrates to test the quality of platelets in solution 25.
Isolation of Whole Blood
Whole blood was collected in 1:8 ACD and washed three times with 1x DPBS at 394×g for 15 minutes. The washed whole blood was re-suspended in 1x DPBS at a 1:5 dilution and 500 µl transfusions were given by tail vein.
Adoptive Transfer of Donor Specific CD4+ T cells
TCR75 whole splenocytes were liberated by mechanical disruption and incubated in RBC lysis buffer (2 mL/spleen) for 5 minutes at room temperature. The cells were washed once in 1x DPBS, washed three times with warm 1x RPMI, and then incubated for 20 minutes with 5 µM CFSE (Invitrogen, Eugene, OR) in a 37°C water-bath. Cells were then washed two times with cold 1x RPMI supplemented with 10% fetal bovine serum (FBS), and washed twice with 1x DPBS prior to re-suspension in 1x DPBS. 1×106 CFSE labeled TCR75 whole splenocytes were adoptively transferred via tail vein injection and allowed to circulate for 24 hours prior to experimentation.
CTLA4-Ig treatment
CTLA4-Ig was a generous gift from the laboratory of Dr. Christian P. Larsen (Emory University, GA). In the BMT and TCR75 studies, C57BL/6 recipients were administered 500 µg of CTLA4-Ig i.p. two hours prior to the first LR-PLT transfusion. To control for this treatment process in the BMT experiments, parallel groups were treated with 500 µg of human IgG1 isotype control antibody (BioXCell, West Lebanon, NH) i.p.
Bone Marrow Transplantation
Twenty-four hours prior to BMT, all recipients were conditioned with sub-lethal gamma irradiation treatments (700 rads). Bone marrow was harvested from femora and tibiae in 1x RMPI with 10% FBS. Cells were washed three times in 1x DPBS. During the third wash, bone marrow cells were enumerated using a hemocytometer, and then re-suspended at 10×106 cells/mL in 1x DPBS. 500 µl (5×106 bone marrow cells) transfusions were injected into the tail veins of all recipients. Engraftment was monitored six weeks post-BMT by staining peripheral blood leukocytes with APC anti-mouse CD229/Ly9 (1:100) for 30 minutes at 4°C. The samples were washed three times in FACS buffer, and then stained with FITC anti-mouse CD229.1/Ly9.1 (1:50) and PE anti-mouse CD3ε (1:100) in FACS buffer for 30 minutes at 4°C. The cells were washed three times and re-suspended in FACS buffer. Samples were run on a FACS Caliber and analyzed by FlowJo. 10,000 CD3+ events were collected. Engraftment was measured as percent donor CD229.1+ cells.
Seroanalysis: Indirect Immunofluorescence Staining
Sera was diluted 1:10 in FACS buffer and incubated with BALB.B or BALB/c splenocyte targets for 30 minutes at 4°C. The samples were then washed three times and stained with APC goat anti-mouse Ig (1:100), FITC anti-mouse CD19 (1:100) and PE anti-mouse CD3ε (1:100) in FACS buffer for 30 minutes at 4°C, in the dark. Samples were run on a FACS Caliber and analyzed on FlowJo. A CD19− CD3+ parent gate was utilized to avoid nonspecific background signal due to the B cell receptor and Fc receptor expressing cells. A positive response was defined as two standard deviations above the mean MFI of sera from naïve C57BL/6 mice.
In vivo Survival of BALB.B Splenocyte Targets
C57BL/6 and BALB.B splenocytes were harvested in 1x RPMI with 10% FBS. Splenocytes were incubated in RBC lysis buffer (2 mL/spleen) for 5 minutes at room temperature. Cells were then washed three times in 1x DPBS. During the third wash, cells were enumerated using a hemocytometer. C57BL/6 and BALB.B splenocyte targets were labeled with CFSEhi (3 uM) or CFSElo (2.5 nM), respectively at 37°C for 10 minutes. Samples were mixed at equal volumes to generate 10×106 cells/mL per target population. 500 µl transfusions were given through tail veins (total dose of 5×106 cells per target). Approximately 18 hours post infusion, splenocytes were isolated and target survival was assessed by flow cytometry; BALB.B targets were normalized to C57BL/6 targets in each animal to control for mouse-to-mouse differences in injection volume and harvesting efficiency.
Statistics
Statistical analysis was performed using one-way ANOVA with Dunnett’s post-test and column statistics. Significance was determined by a P value less than 0.05.
Results
Anti-donor antibody responses following LR-PLT transfusions are abrogated by CTLA4-Ig
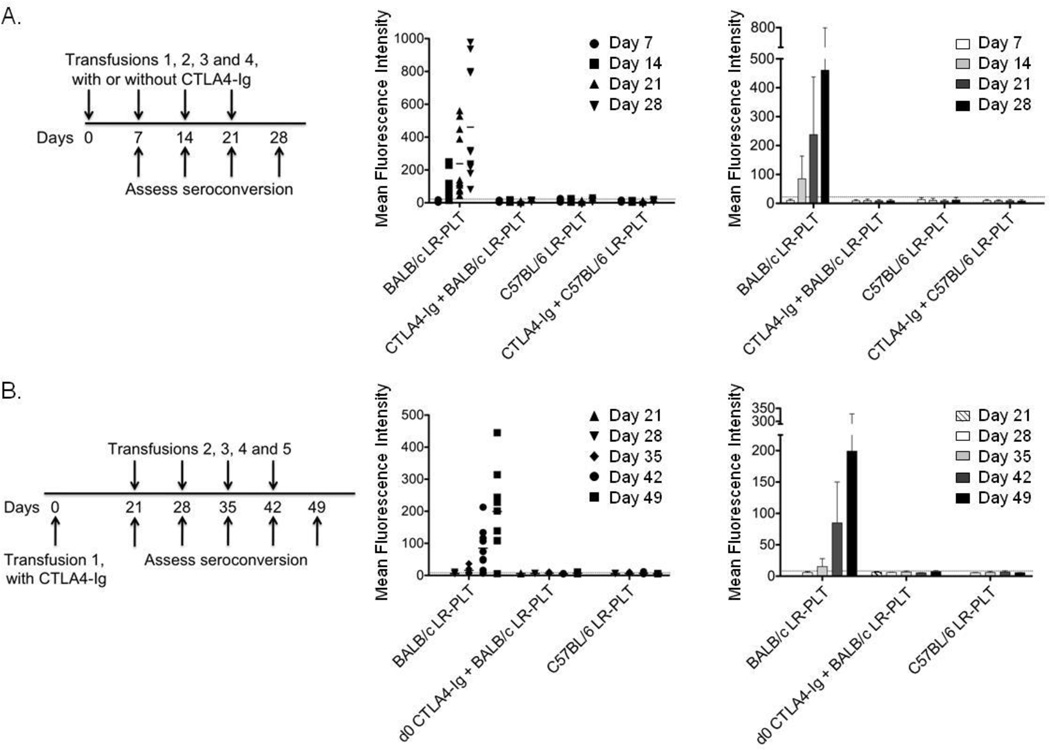
C57BL/6 (H-2b) recipients received four weekly LR-PLT transfusions from BALB/c (H-2d) or control syngeneic C57BL/6 donors. An injection of CTLA4-Ig was given two hours prior to each transfusion.
Whereas untreated mice made robust antibody responses to BALB/c LR-PLT that increased with ongoing transfusion, CTLA4-Ig treatment prevented the induction of detectable alloantibodies at all time points (Figure 1A). A single dose of CTLA4-Ig at the time of the first LR-PLT transfusion was sufficient to prevent alloimmunization by four additional BALB/c LR-PLT transfusions as long as 49 days after CTLA4-Ig administration (Figure 1B). Together, these data demonstrate that CTLA4-Ig effectively inhibits the humoral response to transfused allogeneic LR-PLTs.
Figure 1. CTLA4-Ig prevents humoral alloimmunization to LR-PLT.
(A) C57BL/6 recipients were administered a 500 µg dose of CTLA4-Ig i.p. two hours prior to each weekly transfusion of BALB/c LR-PLT concentrates. Sera was collected one week after each transfusion and tested for anti-donor antibodies using BALB/c splenocyte targets by indirect immunofluorescence staining. The middle panel shows individual animals whereas the right panel demonstrates combined data. (B) On day 0, C57BL/6 recipients were administered a single 500 µg dose of CTLA4-Ig i.p. two hours prior to a BALB/c LR-PLT transfusion. Recipients received four subsequent transfusions a week apart, on days 21, 28, 35, and 42. Sera was collected one week after each transfusion and tested for anti-BALB antibodies by indirect immunofluorescence staining using BALB/c splenocyte targets. Responders in (A) and (B) were defined as having an MFI two standard deviations above the mean MFI of the background sera from naïve C57BL/6 mice (dotted line). Error bars represent the mean ± SD. The combined data from three independent experiments are shown.
Platelet-specific CD4+ T cell division is prevented by costimulatory blockade
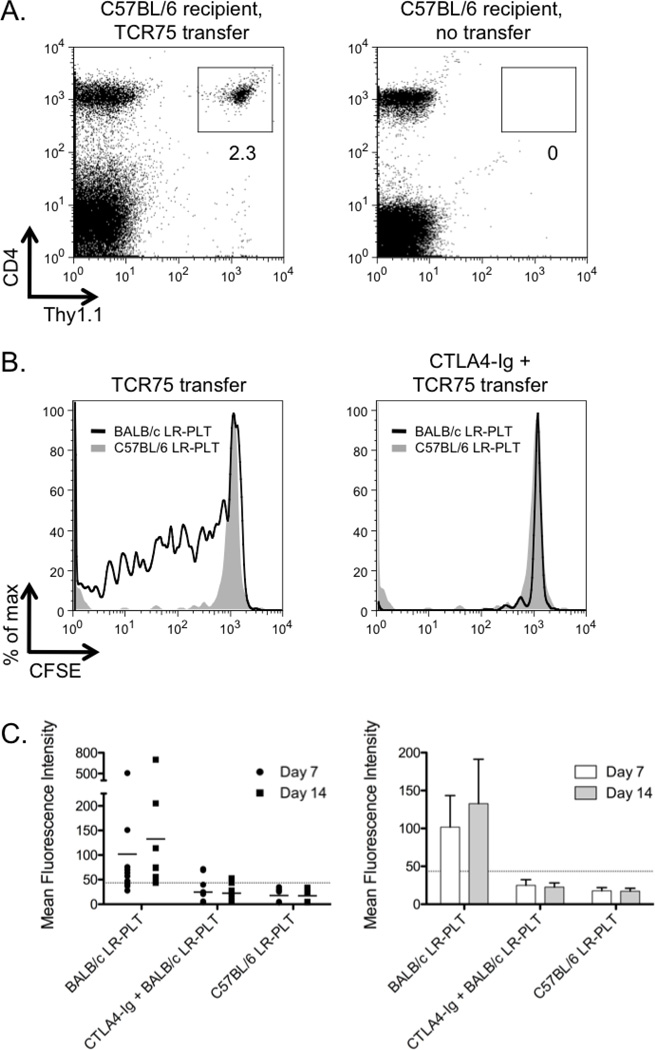
The predicted mechanism by which CTLA4-Ig prevents immune responses is by affecting CD4+ T cell activation and differentiation. To test this hypothesis in the context of LR-PLT transfusion, we made use of a tractable TCR transgenic murine system. The TCR75 transgenic mouse expresses a TCR on CD4+ T cells that reacts with a peptide derived from the BALB/c H-2Kd MHC class I molecule presented by the C57BL/6 MHC class II, I-Ab (Kd54–68/I-Ab) 21. Thus, TCR75 recognizes a peptide from donor (BALB/c) MHC class I alloantigen (Kd) processed and presented by recipient APCs. TCR75 mice were bred onto a Thy1.1 congeneic background to allow visualization after adoptive transfer into wild-type C57BL/6 recipients (Figure 2A). CFSE labeled TCR75 cells were adoptively transferred into naïve C57BL/6 recipients 24 hours prior to transfusion with LR-PLTs. Mice were transfused with BALB/c LR-PLTs, and splenocytes were harvested 5 days post transfusion.
Figure 2. CTLA4-Ig treatment inhibits donor specific CD4+ T cell responses to platelet transfusions.
(A) Representative dot plots illustrate the ability to readily discriminate adoptively transferred TCR75×Thy1.1 splenocytes from C57BL/6×Thy1.2 recipients using the Thy T cell congenic marker. (B) CFSE labeled TCR75 splenocytes were adoptively transferred into C57BL/6 recipients. Twenty-four hours later, recipients received a LR-PLT transfusion from either BALB/c (dark line) or syngeneic C57BL/6 (shaded histogram) donors. Some mice were treated with 500 µg of CTLA4-Ig i.p two hours prior to transfusion. Five days post-transfusion, splenocytes were harvested and stained with anti-CD4 and anti-Thy1.1; TCR75 cells were visualized by gating on CD4+Thy1.1+ events and division was measured by dilution. The illustrated histograms are representative of the trend observed in three independent experiments. (C) TCR75 splenocytes were adoptively transferred into C57BL/6 recipients. Twenty-four hours later, recipients received a LR-PLT transfusion from either BALB/c or syngeneic C57BL/6 mice. Some animals received 500 µg of CTLA4-Ig i.p two hours prior to transfusion. Sera were collected at seven and fourteen days post transfusion and tested for anti-donor antibodies by indirect immunofluorescence staining using BALB/c splenocyte targets. Error bars represent the mean ± SD. The combined data from three independent experiments are shown.
TCR75 cells proliferate robustly in response to BALB/c LR-PLT transfusion, as indicated by CFSE dilution (Figure 2B). The proliferation was not a non-specific result of LR-PLT transfusion that activates T cells in the absence of cognate peptide antigen, as no proliferation was detected in control animals receiving syngeneic LR-PLTs (Figure 2B). Administration of CTLA4-Ig effectively prevented proliferation of TCR75 cells in response to transfusion of BALB/c LR-PLTs (Figure 2B). The adoptive transfer of TCR75 did not alter the general nature of the underlying immune response, as anti-H-2d alloantibodies were detected in TCR75 recipients that received BALB/c LR-PLT (Figure 2C). In addition to preventing CD4+ T cell proliferation, CTLA4-Ig prevented the induction of detectable alloantibodies, even with the increased precursor frequency due to TCR75 adoptive transfer (Figure 2C).
CTLA4-Ig treatment prevents LR-PLT transfusion induced BMT rejection
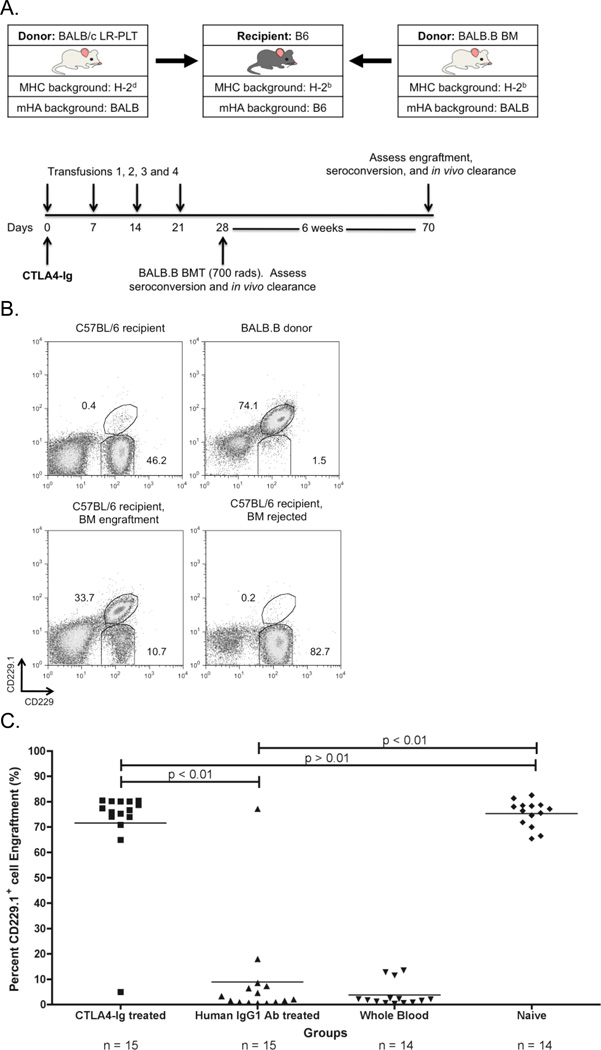
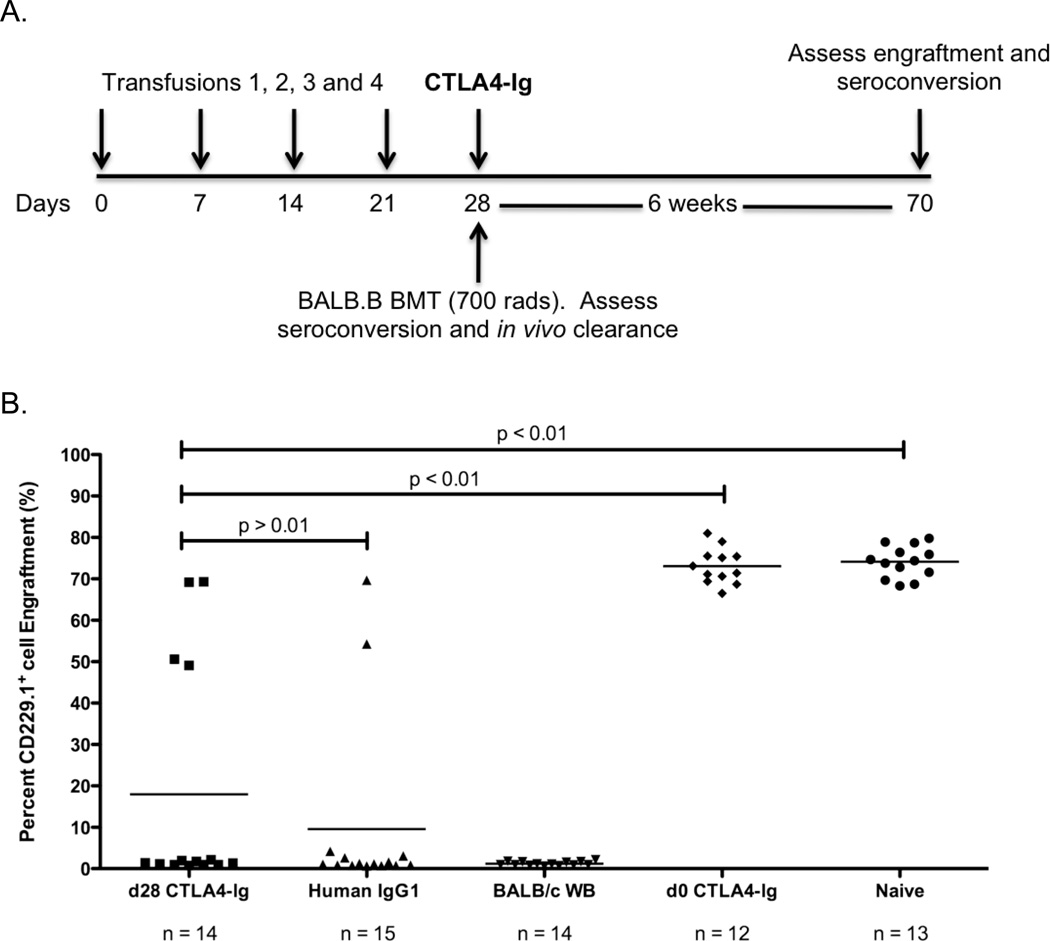
CD4+ T cells activation correlates with alloantibody responses to transfused platelets 22, 24, 26, 27. In addition, depletion of CD4+ T cells abrogates alloantibody induction by LR-PLT transfusion 28. Finally, CD4+ T cells are required for the induction of T cells involved in BMT rejection across mHA barriers (Patel, SR., et al, manuscript in submission). Thus, we hypothesized that CTLA4-Ig would also prevent LR-PLT induced BMT rejection (see Figure 3A for experimental design). C57BL/6 (H-2b) recipients were transfused weekly (four times) with BALB/c (H-2d; MHC and mHA-mismatched) LR-PLT concentrates. One week after the last transfusion, recipients received a BALB.B (H-2b; MHC-matched and mHA-mismatched) BMT under reduced intensity conditions. Two hours prior to the first transfusion, some animals received a single dose of CTLA4-Ig; control mice received human IgG1 isotype control antibody.
Figure 3. CTLA4-Ig LR-PLT transfusion induced BMT rejection.
(A) Experimental model testing the ability of CTLA4-Ig to prevent platelet induced rejection of an MHC-matched BMT. BALB/c platelet donors were MHC- and mHA-mismatched, whereas BALB.B bone marrow donors were MHC-matched but mHA-mismatched with respect to the C57BL/6 recipients. Recipients received a single 500 µg dose of CTLA4-Ig or human IgG1 i.p. two hours prior to the first transfusion. Recipients received four LR-PLT transfusions, a week apart. After the fourth transfusion, recipients received a BALB.B BMT under reduced intensity conditions. in vivo survival of BALB.B targets was performed after BMT (see Figure 4). (B) The CD229+ congenic markers. Representative dot plots illustrate the ability to detect C57BL/6 recipient CD229+ (top left panel) and BALB.B donor CD229.1+ (right panel) cells by flow cytometry. Engraftment is demonstrated as the presence of a double positive CD229.1+ CD229+ population (bottom left panel) and rejection as the absence of this double positive population (bottom right panel). Chimerism is indicated by the presence of both the double positive CD229.1+ CD229+ and the single positive CD229+ populations. (C) BALB.B BMT engraftment results. Engraftment is represented as percent CD229.1+ cells in the peripheral blood; the horizontal lines denote the mean of each group. Rejection was measured as having a percent CD229.1+ cells engraftment two standard deviations above the mean of the positive control group known to reject, recipients treated with isotype control antibody human IgG1. Statistics were generated using column statistics and a one-way ANOVA with Dunnett’s post-test. The combined data from three independent experiments are shown.
The combined results of three experiments demonstrate that while 14/15 (93%) of recipients transfused with BALB/c LR-PLTs rejected BMT in the presence of control human IgG1 injection, only 1/15 (7%) of CTLA4-Ig treated recipients rejected BMT (Figure 3C). The extent of engraftment promoted by CTLA4-Ig treatment prior to LR-PLT transfusion was equivalent to engraftment in naïve mice (Figure 3C). Together, these data demonstrate that a single dose of CTLA4-Ig at the time of the initial antigen exposure prevents LR-PLT transfusion induced rejection of MHC-matched BMT.
Engrafting Recipients Treated with CTLA4-Ig Lack Detectable BALB Specific Immunity
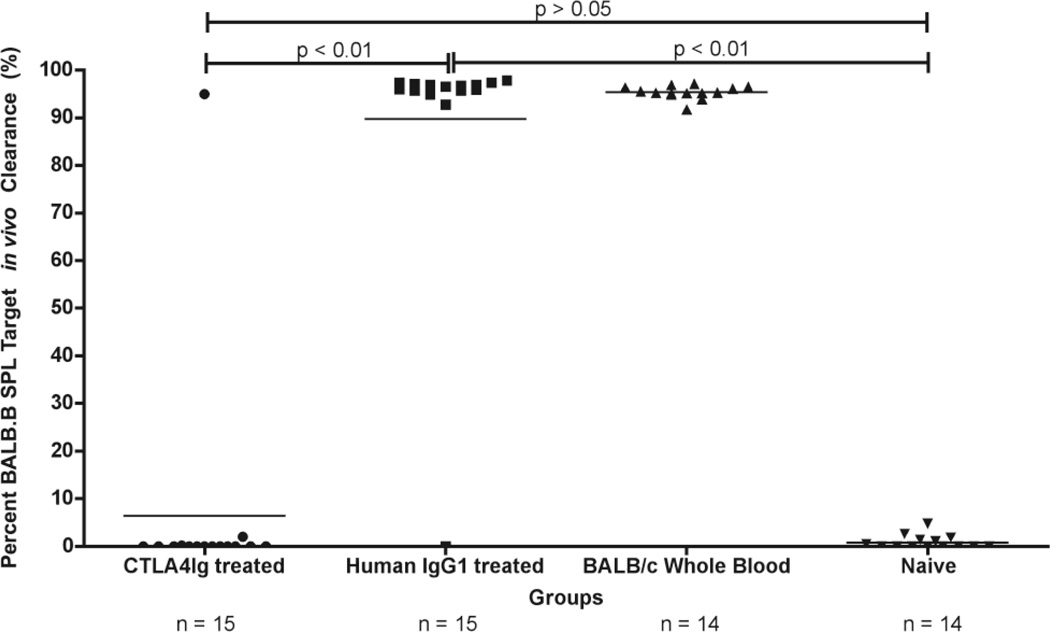
LR-PLT transfusion induced rejection of BMT across mHA barriers (in the BALB→B6 strain combination) depends upon T cell immunity, but not antibodies (Patel, SR., et al, in submission). Although engraftment of the BALB.B BMT in the presence of CTLA4-Ig treatment suggests immunosuppression, it is also possible that the failure to reject the BMT in CTLA4-Ig treated recipients was a result of the CTLA4-Ig altering the microenvironment to promote bone marrow engraftment. Thus, to directly test immunity, in vivo clearance assays were performed by monitoring the fate of BALB.B splenocyte targets injected along with an equal number of control C57BL/6 splenocytes.
The results from three combined experiments demonstrated that only 1/15 (7%) of the BALB/c LR-PLT transfused recipients treated with CTLA4-Ig cleared the BALB.B splenocyte targets in vivo (Figure 4). In contrast, 14/14 (100%) of the rejecting BALB/c LR-PLT transfused recipients treated with human IgG1 cleared the BALB.B splenocyte targets in vivo (Figure 4). Together, these data demonstrate that a single dose of CTLA4-Ig is sufficient to prevent donor mHA specific alloimmunity in response to four BALB/c LR-PLT transfusions and the subsequent rejection of an MHC-matched BALB.B BMT expressing the target mHA(s).
Figure 4. Effects of CTLA4-Ig on alloimmunization in BMT recipients transfused with LR-PLT products.
BALB specific immunity was assessed by in vivo survival of BALB.B splenocyte targets in BMT recipients. The horizontal line denotes the mean of each group. Statistics were generated using column statistics and a one-way ANOVA with Dunnett’s post-test. The data shown in both panels is the combined data from three separate experiments.
CTLA4-Ig treatment at the time of MHC-matched BMT (after LR-PLT transfusion) does not prevent BMT rejection
Although we have demonstrated that CTLA4-Ig administered at the time of the first of four LR-PLT transfusions is sufficient to prevent the rejection of a subsequent BMT, these data do not determine at which point the CTLA4-Ig is exerting its effects. The half-life of CTLA4-Ig in a mouse has been estimated at roughly 6 days 29; thus, there may be sufficient amounts of the reagent present at the time of BMT to directly prevent rejection regardless of effects upon immune responses to LR-PLT transfusion. To test these different scenarios, CTLA4-Ig treatment was delayed until after LR-PLT transfusion. C57BL/6 (H-2b) recipients were transfused four times weekly with BALB/c (H-2d: MHC- and mHA-mismatched) LR-PLT concentrates (Figure 5A). One week after the last transfusion, recipients received a BALB.B (H-2b; MHC-matched and mHA-mismatched) BMT under reduced intensity conditions with either a single dose of CTLA4-Ig or human IgG1 isotype control antibody. Six weeks later, BMT engraftment was assessed using CD229 congenic markers. An additional control group was included that received CTLA4-Ig at the time of the first LR-PLT transfusion.
Figure 5. Delayed CTLA4-Ig treatment is ineffective at preventing LR-PLT induced BMT rejection.
(A) Experimental model testing the ability of CTLA4-Ig to prevent platelet induced rejection of an MHC-matched BMT when administered at the time of BMT. Recipients received four LR-PLT transfusions, a week apart. After the fourth transfusion, recipients received a BALB.B BMT under reduced intensity conditions with or without a single 500 µg dose of CTLA4-Ig or human IgG1 i.p. two hours prior to transplantation. (B) Engraftment is represented as percent CD229.1+ cells in the peripheral blood; the horizontal lines denote the mean of each group. Rejection was measured as having a percent CD229.1+ cells engraftment two standard deviations above the mean of the positive control group known to reject, recipients treated with isotype control antibody human IgG1. Statistics were generated using column statistics and a one-way ANOVA with Dunnett’s post-test. The combined data from three independent experiments are shown.
The combined results of three experiments demonstrated that only 4/14 (29%) of the BALB/c LR-PLT transfused recipients treated with a single CTLA4-Ig dose at the time of BMT engrafted the BALB.B BMT (Figure 5B). Of mice that received human IgG1 isotype control antibody, 2/15 (13%) engrafted the BALB.B BMT while 13/13 (100%) of the untreated naïve recipients engrafted the BALB.B BMT (Figure 5B). This was not due to the CTLA4-Ig not being active, as 12/12 (100%) of the BALB/c LR-PLT transfused recipients treated with a single CTLA4-Ig dose at the time of the first LR-PLT transfusion engrafted the BALB.B BMT (Figure 5B). These data demonstrate that CTLA4-Ig loses its effectiveness when administered after LR-PLT transfusion but prior to BMT.
Discussion
Currently, the approved interventions to decrease humoral alloimmunization are limited to filter leukoreduction of platelet products, and when possible, matching donor/recipient antigens, although the latter is not typically done for platelets prior to immunization. UV treatment of platelet units has also been demonstrated to decrease humoral alloimmunization in humans 4. However, neither stringent filter leukoreduction nor UV irradiation completely prevents humoral alloimmunization. It is unclear to what extent any of the above approaches affect cellular immunization to mHAs in platelet products as it relates to inducing BMT rejection.
Currently, no approved pharmacological interventions are employed to prevent alloimmunization to platelets. Broad-spectrum immunosuppressants in general clinical use may in theory decrease alloimmunization to platelets. However, these agents are associated with many serious side effects, including opportunistic infections and toxicities that can result in morbidity and/or mortality 30–32. In contrast, monoclonal antibodies or fusion proteins that target critical immunological pathways required for optimum lymphocyte activation (e.g. CTLA4-Ig), are less toxic and have milder side-effect profiles, while still affecting adaptive immunity 16, 33. Although immunosuppression is always a clinical concern, CTLA4-Ig (Abatacept) is FDA approved for use in patients with rheumatoid arthritis, and the extent of immunosuppression is typically well tolerated.
Herein, we demonstrate that CTLA4-Ig inhibits both humoral and cellular alloimmunization, preventing antibodies to MHC alloantigens and preventing rejection of bone marrow across mHA barriers under reduced intensity conditioning. Thus, in an animal model, CTLA4-Ig mitigates the two known immunological sequelae of platelet transfusion. To the best of our knowledge, there are no data on the effects of CTLA4-Ig on alloimmunization to platelet transfusions in humans. However, it has been reported that CTLA4-Ig (along with cyclosporine) induced anergy of T cells from human patients with chronic immune thrombocytopenic purpura (ITP) stimulated with platelet antigens in vitro 34, 35.
The extent to which CTLA4-Ig inhibits alloimmunization to human platelet transfusion will have to be determined by clinical trials. Although some platelet transfusions are given for acute blood loss, the majority of transfused patients receive platelets to treat thrombocytopenia from as a result of chronic disease; thus, logistical use of CTLA4-Ig in such patients is feasible. The data in the current study suggest that such efforts should focus on patients who are not yet immune, and if possible, have not yet been exposed to platelet transfusion. The lack of efficacy of CTLA4-Ig in our studies when given after initiation of platelet transfusion therapy is consistent with the known properties of CTLA4-Ig and known requirements of T cells regarding costimulation signals. Memory T cells have lower thresholds of activation than do naïve T cells, and necessitate much less costimulation (if any) to reactivate into fully mature effector cells 13, 36. Memory alloimmunity against donor antigens can arise from previous transfusion, transplantation, or pregnancy. Thus, our data suggest that CTLA4-Ig may have less efficacy in patients with such histories. In addition, cross-reactivity of pathogen specific T cells with alloantigens has been reported (i.e. heterologous immunity) 37, and this may contribute to the lack of efficacy of CTLA4-Ig in some settings.
There are a number of known costimulatory pathways for which blocking reagents could be examined, including CTLA4-Ig (CD28/B7 pathway), anti-CD40 (CD40/CD40L pathway), anti-LFA-1 (LFA-1/ICAM pathway), LFA3-Ig (LFA-3/CD2 pathway), and others 33, 38, 39. Animal studies using the above costimulatory blockade regimens have demonstrated efficacy in prolonging renal, cardiac, pancreatic, and skin graft survival 38–42. However, of these costimulatory blockade reagents, only two are currently FDA approved; CTLA4-Ig for psoriasis and renal transplantation and LFA3-Ig for psoriasis 16, 20, 43.
In addition to avoiding the induction of a refractory state to platelet transfusions, prevention of HLA antibody formation will also benefit candidates for solid organ transplantation. Similarly, while the present report focuses on cellular sequela of LR-PLT transfusion in the setting of BMT, it may also be possible to extend the use of CTLA4-Ig in preventing platelet transfusion induced alloimmunization for solid organ transplantation. Patients with hepatic and renal failures can receive aggressive platelet transfusion support prior to transplant. This exposure to donor antigen may contribute to rejection of the transplant if donor mHA alloimmunity occurs in response to the platelet transfusions; however, such effects have not been formally tested and are thus theoretical at this point. Because the current findings are in a murine system, testing this hypothesis in a human setting would be required prior to drawing clinical conclusions. Nonetheless, these findings demonstrate a novel clinical indication for CTLA4-Ig in preventing humoral alloimmunization and rejection of an HLA-matched BMT across mHA barriers.
Acknowledgements
This work was funded in part by grants from NHLBI at the National Institutes of Health; grants R01HL092977 and R01HL105613.
Footnotes
The authors have no conflicts of interest to declare
References
- 1.Vassallo RR. Recognition and management of antibodies to human platelet antigens in platelet transfusion-refractory patients. Immunohematology. 2009;25:119–124. [PubMed] [Google Scholar]
- 2.Triulzi DJ. Transfusion-related acute lung injury: current concepts for the clinician. Anesth Analg. 2009;108:770–776. doi: 10.1213/ane.0b013e31819029b2. [DOI] [PubMed] [Google Scholar]
- 3.Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142:348–360. doi: 10.1111/j.1365-2141.2008.07189.x. [DOI] [PubMed] [Google Scholar]
- 4.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337:1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 5.Horan JT, Liesveld JL, Fenton P, Blumberg N, Walters MC. Hematopoietic stem cell transplantation for multiply transfused patients with sickle cell disease and thalassemia after low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte globulin. Bone Marrow Transplant. 2005;35:171–177. doi: 10.1038/sj.bmt.1704745. [DOI] [PubMed] [Google Scholar]
- 6.Resnick IB, Shapira MY, Slavin S. Nonmyeloablative stem cell transplantation and cell therapy for malignant and non-malignant diseases. Transpl Immunol. 2005;14:207–219. doi: 10.1016/j.trim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan R, Takahashi Y, McCoy JP, Espinoza-Delgado I, Dorrance C, Igarashi T, Lundqvist A, Barrett AJ, Young NS, Geller N, Childs RW. Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol. 2006;133:305–314. doi: 10.1111/j.1365-2141.2006.06019.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41:109–117. doi: 10.1038/sj.bmt.1705943. [DOI] [PubMed] [Google Scholar]
- 9.Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, Bredeson CN, Eapen M, Horowitz MM. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viollier R, Socie G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J, Cornish J, Ljungman P, Oneto R, Bekassy AN, Fuehrer M, Maury S, Schrezenmeier H, van Lint MT, Wojcik D, Locasciulli A, Passweg JR. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41:45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 11.Patel SR, Cadwell CM, Medford A, Zimring JC. Transfusion of minor histocompatibility antigen-mismatched platelets induces rejection of bone marrow transplants in mice. J Clin Invest. 2009;119:2787–2794. doi: 10.1172/JCI39590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229:271–293. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 13.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229:294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 15.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 16.Weaver TA, Charafeddine AH, Kirk AD. Costimulation blockade: towards clinical application. Front Biosci. 2008;13:2120–2139. doi: 10.2741/2829. [DOI] [PubMed] [Google Scholar]
- 17.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 19.Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyo J, Neumayer HH, Lang P, Larsen CP, Mancilla-Urrea E, Pestana JM, Block A, Duan T, Glicklich A, Gujrathi S, Vincenti F. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91:976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 20.Liscinsky M. FDA approves Nulojix for kidney transplant patients [monograph on the internet] 2011 Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm259184.htm.
- 21.Honjo K, Xu XY, Bucy RP. Heterogeneity of T cell clones specific for a single indirect alloantigenic epitope (I-Ab/H-2Kd54-68) that mediate transplant rejection. Transplantation. 2000;70:1516–1524. doi: 10.1097/00007890-200011270-00020. [DOI] [PubMed] [Google Scholar]
- 22.Bang A, Speck ER, Blanchette VS, Freedman J, Semple JW. Recipient humoral immunity against leukoreduced allogeneic platelets is suppressed by aminoguanidine, a selective inhibitor of inducible nitric oxide synthase. Blood. 1996;88:2959–2966. [PubMed] [Google Scholar]
- 23.Kao KJ. Effects of leukocyte depletion and UVB irradiation on alloantigenicity of major histocompatibility complex antigens in platelet concentrates: a comparative study. Blood. 1992;80:2931–2937. [PubMed] [Google Scholar]
- 24.Semple JW, Speck ER, Milev YP, Blanchette V, Freedman J. Indirect allorecognition of platelets by T helper cells during platelet transfusions correlates with anti-major histocompatibility complex antibody and cytotoxic T lymphocyte formation. Blood. 1995;86:805–812. [PubMed] [Google Scholar]
- 25.Stroncek DF, Rebulla P. Platelet transfusions. Lancet. 2007;370:427–438. doi: 10.1016/S0140-6736(07)61198-2. [DOI] [PubMed] [Google Scholar]
- 26.Bang KW, Speck ER, Blanchette VS, Freedman J, Semple JW. Unique processing pathways within recipient antigen-presenting cells determine IgG immunity against donor platelet MHC antigens. Blood. 2000;95:1735–1742. [PubMed] [Google Scholar]
- 27.Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, Freedman J. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–4254. [PubMed] [Google Scholar]
- 28.Gilson CR, Zimring JC. Alloimmunization to transfused platelets requires priming of CD4+ T cells in the splenic microenvironment in a murine model. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace PM, Rodgers JN, Leytze GM, Johnson JS, Linsley PS. Induction and reversal of long-lived specific unresponsiveness to a T-dependent antigen following CTLA4Ig treatment. J Immunol. 1995;154:5885–5895. [PubMed] [Google Scholar]
- 30.Dandel M, Hetzer R. Impact of immunosuppressive drugs on the development of cardiac allograft vasculopathy. Curr Vasc Pharmacol. 2010;8:706–719. doi: 10.2174/157016110792006923. [DOI] [PubMed] [Google Scholar]
- 31.Barraclough KA, Staatz CE, Isbel NM, McTaggart SJ. Review: Pharmacodynamic monitoring of immunosuppression in kidney transplantation. Nephrology (Carlton) 2010;15:522–532. doi: 10.1111/j.1440-1797.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- 32.Zaas DW. Update on medical complications involving the lungs. Curr Opin Organ Transplant. 2009;14:488–493. doi: 10.1097/MOT.0b013e32833065bd. [DOI] [PubMed] [Google Scholar]
- 33.Vincenti F, Kirk AD. What's next in the pipeline. Am J Transplant. 2008;8:1972–1981. doi: 10.1111/j.1600-6143.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 34.Peng J, Liu C, Liu D, Ren C, Li W, Wang Z, Xing N, Xu C, Chen X, Ji C, Zhang M, Hou M. Effects of B7-blocking agent and/or CsA on induction of platelet-specific T-cell anergy in chronic autoimmune thrombocytopenic purpura. Blood. 2003;101:2721–2726. doi: 10.1182/blood-2002-06-1666. [DOI] [PubMed] [Google Scholar]
- 35.Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood. 1991;78:2619–2625. [PubMed] [Google Scholar]
- 36.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 37.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 38.Gilson CR, Milas Z, Gangappa S, Hollenbaugh D, Pearson TC, Ford ML, Larsen CP. Anti-CD40 monoclonal antibody synergizes with CTLA4-Ig in promoting long-term graft survival in murine models of transplantation. J Immunol. 2009;183:1625–1635. doi: 10.4049/jimmunol.0900339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snanoudj R, Zuber J, Legendre C. Co-stimulation blockade as a new strategy in kidney transplantation: benefits and limits. Drugs. 2010;70:2121–2131. doi: 10.2165/11538140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Onodera K, Chandraker A, Schaub M, Stadlbauer TH, Korom S, Peach R, Linsley PS, Sayegh MH, Kupiec-Weglinski JW. CD28-B7 T cell costimulatory blockade by CTLA4Ig in sensitized rat recipients: induction of transplantation tolerance in association with depressed cell-mediated and humoral immune responses. J Immunol. 1997;159:1711–1717. [PubMed] [Google Scholar]
- 41.Azuma H, Chandraker A, Nadeau K, Hancock WW, Carpenter CB, Tilney NL, Sayegh MH. Blockade of T-cell costimulation prevents development of experimental chronic renal allograft rejection. Proc Natl Acad Sci U S A. 1996;93:12439–12444. doi: 10.1073/pnas.93.22.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weclawiak H, Kamar N, Ould-Mohamed A, Cardeau-Desangles I, Rostaing L. Biological agents in kidney transplantation: belatacept is entering the field. Expert Opin Biol Ther. 10:1501–1508. doi: 10.1517/14712598.2010.514901. [DOI] [PubMed] [Google Scholar]
- 43.Webber A, Hirose R, Vincenti F. Novel strategies in immunosuppression: issues in perspective. Transplantation. 2011;91:1057–1064. doi: 10.1097/TP.0b013e3182145306. [DOI] [PubMed] [Google Scholar]