Abstract
Background and Aims Gene flow from crops to their wild relatives has the potential to alter population growth rates and demography of hybrid populations, especially when a new crop has been genetically modified (GM). This study introduces a comprehensive approach to assess this potential for altered population fitness, and uses a combination of demographic data in two habitat types and mathematical (matrix) models that include crop rotations and outcrossing between parental species.
Methods Full life-cycle demographic rates, including seed bank survival, of non-GM Brassica rapa × B. napus F1 hybrids and their parent species were estimated from experiments in both agricultural and semi-natural habitats. Altered fitness potential was modelled using periodic matrices including crop rotations and outcrossing between parent species.
Key Results The demographic vital rates (i.e. for major stage transitions) of the hybrid population were intermediate between or lower than both parental species. The population growth rate (λ) of hybrids indicated decreases in both habitat types, and in a semi-natural habitat hybrids became extinct at two sites. Elasticity analyses indicated that seed bank survival was the greatest contributor to λ. In agricultural habitats, hybrid populations were projected to decline, but with persistence times up to 20 years. The seed bank survival rate was the main driver determining persistence. It was found that λ of the hybrids was largely determined by parental seed bank survival and subsequent replenishment of the hybrid population through outcrossing of B. rapa with B. napus.
Conclusions Hybrid persistence was found to be highly dependent on the seed bank, suggesting that targeting hybrid seed survival could be an important management option in controlling hybrid persistence. For local risk mitigation, an increased focus on the wild parent is suggested. Management actions, such as control of B. rapa, could indirectly reduce hybrid populations by blocking hybrid replenishment.
Keywords: Brassica napus, Brassica rapa, demography, fitness, gene flow, genetically modified crops, hybridization, introgression, management, crop rotation
INTRODUCTION
Gene flow and hybridization between crops and their wild relatives is common and has been so since the beginning of agriculture (Ellstrand, 2003). A substantial number of molecular studies have revealed permanent incorporation – introgression – of crop genes into the genomes of wild and weedy relatives (Kwit et al., 2011; Ellstrand et al., 2013). In the case of planting of genetically modified (GM) crops this incorporation of (trans)genes into wild relatives is generally considered as undesirable (Chandler and Dunwell, 2008; Schierenbeck and Ellstrand, 2009) and has been found in several cases (Ellstrand et al., 2010). This includes the occurrence of transgenic hybrids among Brassica species (Warwick et al., 2008).
Gene flow between crops and wild relatives does not per se constitute a risk to the environment, but could alter the dynamics of plant populations, changing their population growth rates (‘fitness’) and persistence both inside and outside an agricultural environment. Such increased fitness might lead to environmentally damaging changes in agricultural management with regard to herbicide use and weeding practices (Hawes et al., 2003), or changes in overall structure and function of ecosystems (Chandler and Dunwell, 2008; Kwit et al., 2011). For transgenes, hybrid populations could also act as a temporal bridge between successive crops through outcrossing (Squire et al., 2011). Crop genes have been found to persist for several years in and around agricultural fields through introgression into wild relatives (Ellstrand et al., 2013) or through the persistence of volunteers and plants growing outside the agricultural fields, i.e. feral plants (Pivard et al., 2008; Schafer et al., 2011; Devos et al., 2012).
Current guidelines on the environmental risk assessment of growing GM plants in the European Union (EFSA, 2010) emphasize that there might be environmental consequences both inside and outside agricultural habitats that result from changes in population dynamic caused by gene flow among crops and wild relatives. Similar points have been made in North America (Snow et al., 2005). Therefore, assessments need to include a consideration of the dynamics of hybrids in both agricultural and semi-natural habitats.
Quantification of the effects of gene flow of GM crops involves both the potential to produce viable offspring (compatibility) and the consequences of the gene flow for population viability, i.e. fitness (Hails and Morley, 2005). The compatibility of crops with many wild relatives is well characterized (Ellstrand et al., 2013), including precise estimates of hybridization and flower synchrony rates for Brassica (Norris et al., 2004; Devos et al., 2009). In particular, hybridization between wild turnip (B. rapa) and oilseed rape (OSR) B. napus has been shown to occur naturally (Wilkinson et al., 2003; Jørgensen et al., 2009; Luijten et al., 2014). The second stage in assessing risks – the consequences for plant population fitness – requires experimental demographic data for hybrids relative to the parental species; see Hooftman et al. (2007), Campbell et al. (2009) and Snow et al. (2010) for examples, and specifically Snow et al. (1999), Allainguillaume et al. (2006), Jørgensen et al. (2009) and Vacher et al. (2011) for examples involving Brassica. However, most of these studies have not included the full life cycle or have combined data from different experiments, reducing the validity of any predictions to assess fitness in terms of the population growth rate (Hails and Morley, 2005).
Accurate assessment of changes in fitness of hybrids must incorporate several fundamental ecological considerations (Hails and Morley, 2005). First, fitness is an integrated measure of performance over all life stages requiring demographic data for all major stage transitions, i.e. vital rates. Such data are best combined by employing population models (Bullock, 1999). Several studies into crop–wild hybrids have considered above-ground stages (e.g. Hooftman et al., 2007; Vacher et al., 2011; Yang et al., 2011; Cao et al., 2014), but none has included simultaneous measures of seed bank survival.
Second, fitness is habitat-dependent and so studies within single environments have only a limited value for risk assessment (Ridley and Ellstrand, 2009; Snow et al., 2010). In particular, crop–wild relative hybrids are usually studied in agricultural conditions, on tilled soils and sometimes with fertilizer and weed control (Hails and Morley, 2005). This may be useful for determining changes in weed infestations in crops, but not for assessing impacts on semi-natural habitats. In this paper we report experiments from both habitat types. Some previous studies have reported lower F1 hybrid fitness in semi-natural habitats (Allainguillaume et al., 2006; Jørgensen et al., 2009; Haider et al., 2009), while other systems have reported F1 hybrid vigour (e.g. Hooftman et al., 2007) in which crop genes provide an adaptive advantage to hybrids under agricultural or similar nutrient-rich conditions (Hartman et al., 2013). We investigated whether the latter is also the case in Brassica.
In agricultural systems, a third consideration is that the persistence of hybrids needs to be assessed in the context of farming practices. OSR – Brassica napus – is grown in rotations with other crops across the world (Sweet et al., 2004; Bishnoi et al., 2007; Beckie et al., 2011; Seymour et al., 2012). For the UK, OSR may be used as a break crop one year in three or four (Sweet et al., 2004). In the intervening years Brassica volunteers or hybrids are generally controlled by specific herbicides and cultivation practices. However, persistence over the intervening period in the seed bank will determine hybrid and volunteer emergence in the following OSR crop.
Previous studies of crop–wild relative hybrids have addressed some of these three considerations: for example, inclusion of most life history stages in an estimate of fitness (Hooftman et al., 2005, 2007); and consideration of natural habitats in which a wild relative is established (Allainguillaume et al., 2006, 2009). To our knowledge no study has considered all three considerations simultaneously and none has considered persistence in the context of agricultural rotations. Despite several studies performed on weed seed bank dynamics and management (e.g. Gruber et al., 2005; Pekrun et al., 2006; Bohan et al., 2011), the relative contribution of seed bank dynamics compared with adult life stage dynamics on hybrid population persistence in agricultural systems is not well described. Furthermore, no study has analysed the influence of parental species dynamics on hybrid demography.
In this paper we address these three considerations in a study of F1 hybrids between OSR, B. napus, and its wild relative B. rapa. B. rapa is highly compatible with B. napus with a high potential of crop gene introgression (Devos et al., 2009; Liu et al., 2013) and co-occurs with it either as a weed or in road- and river-side populations in close proximity to crops (Wilkinson et al., 2003; Luijten et al., 2014). Using a combination of demographic studies and population modelling, we address the following four hypotheses. (1) Differences in vital rates between hybrids and parental species lead to changes in population fitness. (2) Inherited crop plant traits in hybrids cause low fitness compared with the wild relative in semi-natural habitats, but higher performance compared with wild relatives in agricultural conditions. (3) Crop rotations can modify the persistence time of hybrid populations in agricultural systems. (4) Interactions with the parental populations contribute strongly to the demographic dynamics of hybrid populations.
MATERIALS AND METHODS
Plant material, hybridization and hybrid identification
Brassica rapa seed was collected from semi-natural populations at three typical riparian sites in central England in July 2002, namely Bath, Radley and Wytham (Supplementary Data Fig. S1). The vegetation at each site comprised unmanaged tall herb and grass communities, with locally abundant B. rapa.
F1 hybrid seed was produced by growing 30 plants originating from the Radley population in the glasshouse. Following emasculation, B. rapa flowers were hand-pollinated by Brassica napus ‘Apex’ a conventional winter OSR cultivar, and then isolated against secondary pollination using sealed bags. No GM material was used in this study. After harvest, the seeds from the maternal plants were pooled. Hybridization using self-compatible species generally produces a mixture of hybrid and selfed seed, so the proportion of hybrid seed was determined using flow cytometry on 55 germinants (Supplementary Data Methods S1). The proportion of true hybrids among the plants surviving to pod production in the hybrid-sown plots (see below) was also determined using flow cytometry on leaf tissue collected in situ.
Experimental design
At the three semi-natural sites in which we collected the seeds, five blocks were laid out, each containing four plots. The design enabled comparison of the performance of the three types (B. rapa, B. napus and hybrids) under undisturbed and disturbed conditions (see below). A plot comprised two parallel lines 2·5 m in length and 0·5 m apart. One line was disturbed by removing vegetation and turf 0·2 m in width and 0·05 m in depth. We sowed 50 seeds individually along the row at 0·05-m intervals. The other line was left undisturbed and 100 seeds were sown individually at 0·025-m intervals. One plot per block was sown with B. rapa, one with B. napus (OSR) ‘Apex’, one with hybrid seeds and the fourth was left unsown to estimate germination from any existing B. rapa seed bank. Both the disturbance treatments within plots and the sowing treatments among plots were randomly assigned. In total, 6750 seeds were sown at each site. We did not identify statistical differences between the disturbance treatments for any demographic measure (Supplementary Data Methods S2), so in the remainder of the study these data are pooled for each plant type. To assess seed survival in the seed bank, ten nylon mesh bags per species per site (i.e. 3 × 30 in total) containing 50 seeds each were buried at a depth of 10 cm.
A similar randomized block experiment was set up in an agricultural environment at the National Institute of Agricultural Botany in Cambridge (NIAB; Supplementary Data Fig. S1). Three blocks of 10 × 4 m were laid out in an arable field, which was cultivated and sown in September 2002. Each block comprised five 2·5-m lines sown with 50 seeds at 0·05-m intervals. Five types of seed were randomly assigned to the five lines: B. napus (OSR ‘Apex’), hybrids and B. rapa from each of the three field sites from which seeds were collected. A sixth line was left unsown to monitor background germination. A fourth block, being part of an accompanying experiment (unpublished), contained three repeats of the six sowing lines. Data from these three latter repeats were pooled for each plant type and comprised a fourth block in the data analysis. The field was caged against birds and irrigated, fertilized and treated with pesticides as would be an OSR crop.
Plant vital rates
All germinants in all plots were monitored 1 week after sowing and subsequently every 3 weeks until death or seed production. In addition to (1) germination and (2) survival rates among life stages, we measured (3) fecundity. Fecundity was estimated by counting pods on all plants in the semi-natural habitats and of five random plants per line in the arable field, and by counting seeds per pod for five random pods per plant. Furthermore we measured: (4) seed viability, estimated by germination of seed samples at 20 °C on moist cotton wool following 48 h of chilling at −20 °C, with ongoing dormancy and so remaining viability of non-germinants determined by tetrazolium staining; and (5) seed bank survival, estimated using half of the buried seed bags in the following spring (overwinter survival) and the remainder in the following autumn (annual survival). The number of intact seeds was counted and viability was tested using tetrazolium staining. Staining was taken as a more reliable method than in vitro germination tests in which seeds may remain dormant or seedlings die before the hypocotyl is visible.
The estimation of germination rates for B. rapa, and of all vital rates for true hybrids, is not straightforward due to a resident B. rapa seed bank, and ‘hybrid seed’ being a mixture of true hybrids and B. rapa. This was resolved by using control line estimates of the resident seed bank, estimates of the proportion of sown seed that was truly hybrid and measures of B. rapa vital rates from the relevant experimental lines in a series of equations to calculate true hybrid vital rates using least squares estimation (Supplementary Data Methods S2). Where there were no significant differences in vital rates among parental species and the hybrid, they were considered to be equivalent for the following model calculations.
Population growth rates and elasticity analyses
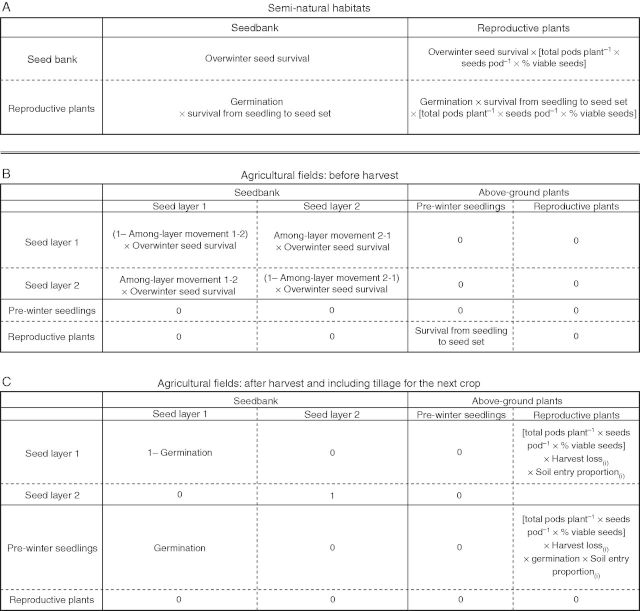
We constructed density-independent, temporally explicit projection matrices of point-populations, including the transitions among life stages: seed production, adult plants and un-germinated viable seeds in the seed bank (Fig. 1). As these plants are annual, no between-year survival of adult plants was included. Transition matrices were established for each parental species and hybrids separately for each plot, combining the set of measured vital rates for that plot.
Fig. 1.
Matrices used in this study. (A) The stage-structured matrix used for semi-natural populations. (B, C) Periodic matrices for the agricultural populations for each year in the rotation. Rotation-dependent parameters (i) are the proportion of seeds entering the soil dependent on the type of tillage and harvest loss, being 5 % in years the OSR crop is planted.
For semi-natural habitats, we used the matrix of Fig. 1A, allowing growth of hybrids and B. rapa in all years. Note that B. napus had no recorded fecundity in these semi-natural environments and therefore λ = 0, with only 0·3 % of seeds planted surviving until flowering and none surviving until seed-set. The predicted median for long-term population stochastic growth rate (λ) and the 95 % confidence intervals for λ were calculated from 100 000 runs, with re-sampling from plot-based sets of vital rates. For seed bank survival the average seed bank mortality among all samples per plant type was used in all calculations. Elasticity estimations were performed post hoc on the mean values among runs of the individual matrix elements (Caswell, 2000). Elasticity is a measure of the importance of each matrix element calculated as the relative change in λ following a change to each element.
In an agricultural habitat, B. napus (OSR) will be sown once every 3 or 4 years, in rotation with cereals or other broadleaved crops such as sugar beet. To simulate the effects of crop rotation, we employed periodic matrices (Caswell, 2000; Mertens et al., 2002). The crop rotation scenarios we simulated were those used in an earlier field trial into the implications of management of growing herbicide-tolerant OSR, and represent typical UK crop rotations (BRIGHT; Sweet et al., 2004). The rotations were: (1) a 3-year rotation with OSR followed by two autumn-sown cereals (e.g. wheat), i.e. the ‘BRIGHT 1’ scenario of OSR–wheat–wheat; and (2) a 4-year rotation similar to (1), but with a spring sown broadleaved crop (e.g. sugar beet) separating the two years of autumn-sown cereals, i.e. the ‘BRIGHT 3’ scenario of OSR–wheat–sugar beet–wheat. The main differences between the two scenarios are the length of the rotation and the timing of tillage. Late tillage in the spring-sown crop results in lower incorporation of Brassica seeds into the soil (8·5 % vs. 2 %; Gruber et al., 2005) and so a higher loss of seeds from the population. Rotation details are provided in Supplementary Data Methods S3. A hypothetical ‘continuous OSR rotation’ scenario was included as control, in which OSR was grown every year, allowing us to explore the effects of adding representative crop rotations on hybrid population growth (λ) compared with no rotations. We investigated which life-cycle elements affected λ the most (elasticity). Furthermore, we explored the effect of altering rotation length on λ and elasticities by adding hypothetical 2- and 5- to 8-year length rotations.
Separating pre- and post-harvest processes, we used a periodic matrix model with outcrossing, which is adapted from the model in Hooftman et al. (2007). Below we give a summary of the model, and give a detailed description of model and its parameters in Supplementary Data Methods S3. The model is written in Matlab v. 7.14.0.739 (Mathworks, Natick, MA, USA); the code can be obtained from the corresponding author. The periodic model consists of four stages all expressed in density m−2: seeds in two seed bank layers (shallow and deep), emerged seedlings and reproductive plants (Fig. 1). In addition to the usual demographic parameters, there are additional parameters required to account for characteristics of the agricultural habitat (Claessen et al., 2005): the proportion of seeds escaping harvest; the proportion of those seeds entering the soil; seed movement between the shallow and deep soil layers; and seed survival after early and spring ploughing. For parameter values see Supplementary Data Methods S3.
All three species are modelled as growing at the same time in the same field and being able to cross with each other. Following an initial pulse of B. rapa, representing establishment of a weed population, hybrids are formed by reciprocal outcrossing between B. rapa and OSR from t = 1 onwards. The number of hybrid seeds formed depends on the outcrossing rate, the reproductive densities of the parental species, the fecundity of the maternal species and the fecundity of hybrids itself (Hooftman et al., 2007). Outcrossing was set at 3 %, following Jørgensen et al. (2009) for a 1 : 1 mixture with 44·5 plants m−2. We tested for the effect of parental lines on hybrid population dynamics through new hybrid formation. The formation of hybrid seeds by the two parents was assumed to be too small to substantially influence the dynamics of the parental species
OSR is sown at the start of the rotation and is represented as emerged seedlings following immediate germination. We initiated the model with a sown OSR crop of 60 plants m−2, approximating a commercial density (Jørgensen et al., 2009). Seed banks were assumed to be empty initially. A single-pulse immigration event from B. rapa (e.g. as impurities in the seed) was assumed in this first year, with an initial density one order of magnitude lower (6 m−2). The model is run for a fixed period (t_max) of 100 years (BRIGHT 3 and control) and 102 years for the BRIGHT 1 scenario, allowing rotation cycles to be completed. For each run and for each species λ is calculated as:
| (1) |
As for the semi-natural habitat the predicted median of the long-term stochastic population growth rate (λ) and the 95 % confidence intervals for λ are based on 100 000 runs, re-sampling from complete sets of vital rates per plot, and hence including any correlations among vital rates. The seed bank survival data were assumed to be the same as in the semi-natural habitat. The sequences of simulated matrices for the hybrid, B. rapa and B. napus were stored, allowing the same sequences to be used in the elasticity calculations below.
Elasticity (ε) was calculated iteratively. As hybrids and parental species are simulated to grow together, continual hybridization replenishes the seed bank of the hybrid. Therefore, changes in parental vital rates could influence the λ of hybrid populations. We included the indirect effect of alterations in matrix transitions of the parental populations on the λ of the hybrid population in our elasticity calculations. We refer to these cross plant type elasticities as ‘interactive elasticity’. To calculate such interactive elasticities we extended standard elasticity calculations (Caswell, 2000). The first component, the sensitivity (S, eqn 2a) we re-defined as ‘the average proportional change in λ of the hybrid population, caused by changing a single transition in any plant type’. To do this we elevated in turn individual matrix entries (αij) in the (a) and (b) matrices (eqn 2a) for the hybrid, B. rapa and B. napus by 10 % following Hooftman et al. (2008). We calculated the proportional change in λ of hybrids caused by elevating each elements independently and calculated the median over 100 000 runs (sensitivity = S), which was subsequently normalized and λ-corrected to elasticity (ε) using standard methodology (Caswell, 2000). We averaged this elasticity over the (a) and (b) matrices (eqn 2a).
The new addition we introduce to the above standard methodology is that we now incorporate in the elasticity calculation both hybrid population dynamics itself as well as the contribution from parental dynamics to the λ of the hybrids via the formation of new hybrid seeds. We did this by weighting each element with the overall summed sensitivity of the full matrices (eqn 2b). Note that eqns (2a) and (2b) include normalization to ensure elasticities are comparable. For comparative analysis, we split the matrix elements into three categories adapted from Silvertown et al. (1996): (1) the seed bank – i.e. seed bank survival and among seed bank transitions; (2) fecundity; and (3) growth – i.e. transitions among growing plants (Supplementary Data Methods S3).
| (2a) |
with 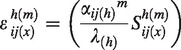 including
including 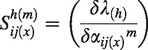
| (2b) |
with including where = elasticity of matrix element αij of species x on λh; = interactive elasticity of transition type y of species x on λh; m = periodic matrix: (a) or (b); x = species: hybrid (h), B. rapa (r) and B. napus (n); = sensitivity of matrix element αij in periodic matrix m of species x on λh; αij = stochastic matrix element with row i and column j; λh = stochastic population growth rate of the hybrid population (eqn 1); y = transition type: seed bank (y1), growth (y2) and fecundity (y3).
RESULTS
Vital rates of hybrids and parental species
The hybrids went extinct in two of the three semi-natural sites, whereas B. rapa plants survived to seed set at all sites. Flow cytometry indicated that 65 % of the seeds resulting from the hybridization method were hybrid. The proportion of hybrids among surviving plants of the hybrid lines decreased at all semi-natural sites compared with the initial seed population (Fisher’s exact test: P < 0·01), indicating that hybrids had a lower survival rate than B. rapa (Supplementary Data Fig. S2). All following analyses that tested for differences between hybrids and B. rapa are for the semi-natural site in which hybrids survived (Wytham). In all semi-natural habitats, most B. napus (OSR) plants did not persist beyond the vegetative phase, with only seven plants flowering and none producing pods out of the 2250 B. napus seeds sown (Table 1); hence no full life-cycle characteristics can be given and λ is 0. In contrast, in the agricultural habitat the proportion of hybrids among the surviving plants in the hybrid lines did not decrease compared with that of the initial seed population, with 75 % of plants sampled being hybrid (binomial comparison; P = 0·63).
Table 1.
Estimated vital rates in (a) a semi-natural (Wytham) and (b) an agricultural habitat; identical vital rates are given where differences are not significant
| (a) Semi-natural habitat | ||||||||
|---|---|---|---|---|---|---|---|---|
| Lambda (λ) | Germination (***) | Survival from seedling to adult | Survival until flowering (*) | Survival until seed set (**) | Fecundity (P = 0·06) | Overwinter seed survival (***) | Annual seed survival (***) | |
| B. rapa | 1·44 | 0·062 | 0·790 | 0·519 | 0·679 | 53·64 | 0·623 | 0·623 |
| Hybrid (B. rapa × B. napus) | 0·64 | 0·277 | 0·790 | 0·519 | 0·056 | 3·27 | 0·623 | 0·623 |
| B. napus | 0 | 0·08 | 0·790 | 0·259 | 0 | 0 | 0·012 | 0·012 |
| (b) Agricultural habitat | ||||||||
|---|---|---|---|---|---|---|---|---|
| Lambda (λ)† | Germination | Survival from seedling to adult | Survival until flowering | Survival until seed set | Pods per plant (*) | Seeds per pod (***) | Viability (***) | |
| B. rapa | 8·8‡ | 0·355 | 0·812 | 0·939 | 0·948 | 324 | 13·7 | 0·984 |
| Hybrid (B. rapa × B. napus) | 8·9‡ | 0·355 | 0·812 | 0·939 | 0·948 | 19·4 | 1·81 | 0·596 |
| B. napus | 14·9‡ | 0·355 | 0·812 | 0·939 | 0·948 | 324 | 23·4 | 0·984 |
† Seed survival assumed to be the same as in the semi-natural habitat.
‡ In a hypothetical 1-year rotation with continuous OSR growth; see Table 3.
*P < 0·05; **P < 0·01; ***P < 0·001.
Semi-natural habitats
We identified significant demographic differences between the parents and hybrids at Wytham (Table 1). Hybrids had three times the germination rate (F1,4 = 45·7; P = 0·003) but one-twelfth the late season survival compared with their collective parent types (F1,4 = 39·5; P = 0·003). Fecundity was marginally significantly different between hybrids and B. rapa, with B. rapa individuals producing 15 times more seeds than hybrid individuals (F1,4 = 6·77; P = 0·060). No differences were found for plant survival from early to late season.
We found substantially lower seed survival in the soil for B. napus than for B. rapa and hybrids for both over-winter (F2,3 = 12·75; P = 0·034) and annual survival (F2,5 = 40·2; P = 0·001) over all sites (Table 1). B. napus seed survival was close to zero (1·2 %). However, B. rapa and hybrids did not differ, and over-winter mortality did not differ from annual mortality. This suggests that the majority of seed mortality took place over the winter.
At Wytham the mean λ for B. rapa indicated an increasing population (Table 2), although confidence intervals encompassed 1 (population stability). For the hybrid, λ values indicated a population decline over the full confidence range (λ < 1). Elasticity analysis (Table 2) showed that the overwinter seed survival rate was the most important transition for hybrid populations, whereas both fecundity and survival had high elasticities for B. rapa.
Table 2.
Population growth rate (λ), confidence intervals and elasticities for the individual vital rates
| B. rapa† | Hybrid | |
|---|---|---|
| Population growth rate (λ) | 1·44 | 0·64 |
| 95 % Confidence interval | 0·62–5·53 | 0·62–0·79 |
| Elasticities | ||
| Overwinter seed bank survival | 0·40 | 0·99 |
| Survival rates and fecundity‡ | 0·60 | 0·01 |
† In the habitat (Wytham) in which both hybrids and B. rapa survived.
‡ Survival rates and fecundity co-occur in loops only and therefore all have the same elasticity.
Agricultural habitat
In the agricultural habitat, both parental species and hybrids survived to set seed. Hybrids had a 16 times fewer pods per plant than B. napus and B. rapa (F1,7 = 8·05; P = 0·025) with no difference between the two parental species (Table 1). Hybrids also had a 6·5–12 times fewer seeds per pod, with B. napus having the highest number (F2,6 = 84·7; P < 0·001). Hybrid seeds had only about half of the viability of the parental species (F1,7 = 306; P < 0·001), with B. napus and B. rapa seeds having equal viability (Table 1). This resulted in substantial differences in overall fecundity: 21 viable seeds per maternal plant for hybrids compared with 4368 (B. rapa) and 7460 (B. napus). We found no differences among the parental species and hybrids in germination or survival.
These vital rates resulted in a projected decline of both the B. rapa and hybrid population in the agricultural habitat (Table 3). However, the relatively high mean stochastic λ values (0·77–0·89) suggest a potential for these populations to persist for decades: a 90 % reduction in population size would take 9–20 years, depending on the rotation. The confidence intervals around these growth rates also indicate that there is some probability of population increase, albeit low.
Table 3.
Population growth rates (λ), time to 90 % reduction of the population and elasticities from three rotation scenarios
| 3-year rotation† |
4-year rotation‡ |
Continuous OSR rotation§ |
||||
|---|---|---|---|---|---|---|
| B. rapa | Hybrid | B. rapa | Hybrid | B. rapa | Hybrid | |
| Population growth rate (λ) | 0·89 | 0·87 | 0·74 | 0·75 | 8·8 | 8·9 |
| 95 % Confidence interval | 0·63–1·28 | 0·62–1·28 | 0·59–1·00 | 0·59–1·00 | 2·1–37·5 | 2·0–34·4 |
| 90 % Reduction time (years) (confidence interval) | 20 (7–∞) | 20 (6–∞) | 9 (6–∞) | 9 (6–∞) | – | – |
| (Interactive) elasticities | ||||||
| Hybrid seed bank survival | – | 0·23 | – | 0·17 | – | 0·02 |
| Hybrid growth | – | ≈ 0 | – | ≈ 0 | – | ≈ 0 |
| Hybrid fecundity | – | ≈ 0 | – | ≈ 0 | – | 0·01 |
| Affected by B. rapa seed bank survival | 0·43 | 0·71 | 0·61 | 0·80 | 0·05 | ≈ 0 |
| Affected by B. rapa growth | 0·03 | 0·03 | 0·03 | 0·02 | 0·04 | 0·14 |
| Affected by B. rapa fecundity | 0·54 | 0·02 | 0·37 | 0·01 | 0·91 | 0·83 |
| Affected by B. napus | – | ≈ 0 | – | ≈ 0 | – | ≈ 0 |
† Rotation of B. napus (OSR) and two autumn-sown cereals.
‡ Rotation of B. napus (OSR), two autumn-sown cereals with a spring-sown broadleaved crop in between.
§ Hypothetical continuous OSR cultivation without other crops.
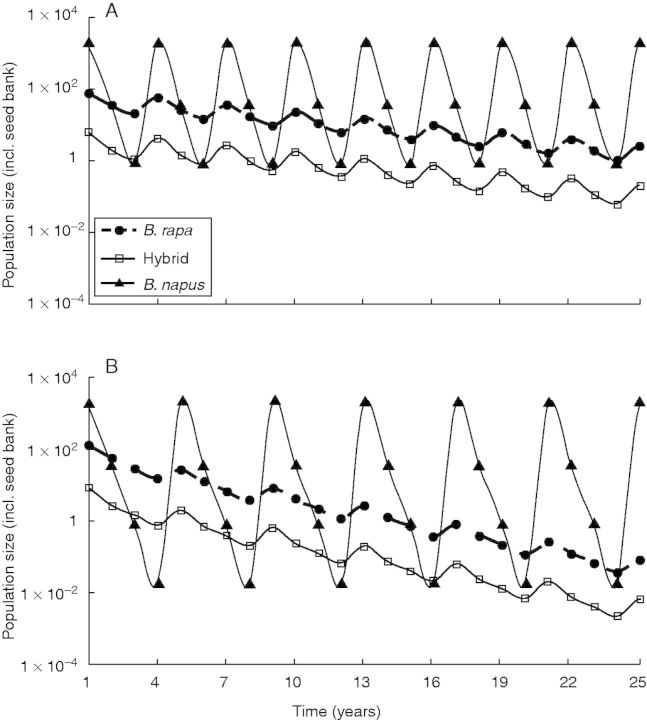
The parental species and hybrid all showed density cycles caused by reproduction – or sowing for OSR – occurring only in years in which OSR was the crop (‘OSR years’; Fig. 2). In intervening years, populations of B. rapa and hybrids were reduced through seed mortality in the soil and death of plants following germination from the upper soil layer. As a consequence, λ values were lower under the 4-year rotation scenario because of the longer interval between years in which seed set was possible (see extended analyses below). Note that B. napus is sown as a crop in these simulations, and hence has a λ of 1.
Fig. 2.
Population sizes in crop rotation scenarios for B. rapa, B. napus and the hybrid, with hybrids formed by outcrossing between the parents from t = 1 onwards. (A) A 3-year rotation of oilseed rape (OSR) and two autumn sown cereals (BRIGHT 1). (B) A 4-year rotation of OSR, an autumn sown cereal, a broadleaved crop and an autumn sown cereal (BRIGHT 3). The difference between both scenarios is the length of the period between planting an OSR crop. Populations start from emerged seedlings only, mimicking an immigration event and first time OSR cultivation.
Hybrid growth rates followed the growth rates of B. rapa closely, with similar confidence intervals (Table 3). The mechanism here is that the low fecundity of hybrids is partially compensated for by new F1 hybrid seeds in OSR years. This is confirmed by the interactive elasticities (Table 3); the hybrid population growth rate was mostly driven by the dynamics of the wild parent. The foremost ‘interactive elasticity’ of the hybrid λ was in the seed bank transitions of B. rapa. This suggests that the hybrid population is largely dependent on the ability of the wild parent to survive in the seed bank, complete its life cycle in OSR years and cross with OSR to form new F1 hybrid offspring. This contrasts with the dynamics of B. rapa itself for which elasticity is balanced among seed bank and fecundity vital rates.
By contrast, in the hypothetical control scenario of continuous OSR production the λ values of both B. rapa and hybrids were mostly sensitive to above-ground dynamics, especially fecundity of B. rapa. This result indicates that the importance of the seed bank – outlined above – is caused by agricultural rotations in which germinants are controlled in non-OSR years.
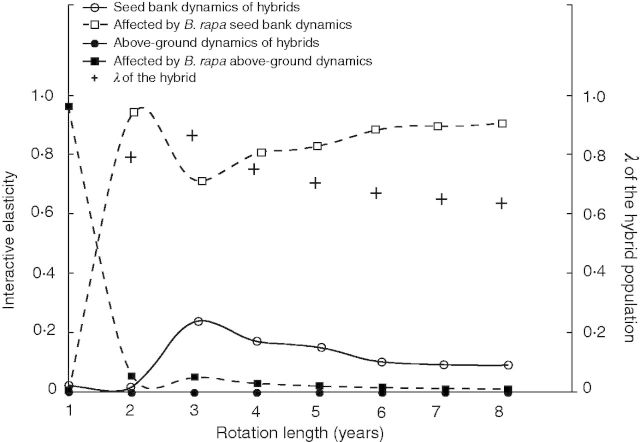
We examined the effect of rotation length further by removing or adding non-OSR crops into the rotations (Fig. 3). As the length of the rotation increases high elasticity of above-ground stages in the continuous OSR scenario is replaced by high elasticity of B. rapa below-ground seed bank transitions (≈0·9), with a retained lower elasticity of the seed bank transitions of the hybrids itself (≈0·1). The results are identical for the two BRIGHT scenarios. This is because no sequential combinations of the spring-sown broadleaved crop and OSR are feasible, which would have differentiated between both scenarios based on the differing proportion of seeds incorporated in the soil between autumn- and spring-sown crops (Supplementary Data Methods S3).
Fig. 3.
Simulated changes in ‘interactive’ elasticity of hybrid and B. rapa above- or below-ground vital rates with hypothetical shortened or lengthened rotations. The proportional importance in influencing the growth rate of the hybrid population is shown for the matrix elements that denote either above- or below-ground dynamics of the hybrid population itself or of its wild parent through replenishment. The influence of B. napus on the hybrid population growth rate is not shown as its elasticity ≈ 0 for all elements.
DISCUSSION
We found substantial fitness differences between the F1 hybrid of Brassica rapa × Brassica napus (OSR) and both parental species. Furthermore, the relative contributions of different life stages to fitness differences depend upon habitat type, i.e. semi-natural or agricultural, and type of rotation. Combining manipulative experiments and modelling, as we have done here, strongly facilitates understanding of the population dynamics of these systems. Better understanding is an important step in managing hybrid populations and designing mitigation strategies against environmental damage from the presence of hybrids. Such strategy considerations could also include testing for the stability of introgression through recombination and for the maintenance of introgressed genes in the recipient populations (Devos et al., 2009; Ellstrand et al., 2013; Garnier et al., 2014).
Hypotheses
Three of our four hypotheses were substantiated. Our first hypothesis was that we would detect differences in vital rates, and ultimately in the fitness – i.e. the population growth rate – of hybrids compared with the parental species. This was indeed the case, with hybrids having lower fitness or being intermediate in fitness between the two parental species in the semi-natural habitat in which hybrids survived, as is generally expected for F1 hybrids (Burke and Arnold, 2001). The hybrid growth rate indicated population decrease (λ < 1) compared with B. rapa which had a positive population growth rate, and OSR which had a growth rate of zero. In the agricultural habitat both hybrids and B. rapa had only potential population growth rates of λ > 1 when rotations were not considered. When including rotations mean λ dropped below 1. These findings of a low F1 hybrid fitness are in line with other results for Brassica hybrids, e.g. as reported by Allainguillaume et al. (2006), Jørgensen et al. (2009) and Haider et al. (2009). We acknowledge that there could be substantial vital rate differences among OSR cultivars (Lutman et al., 2003, 2005); we consider ‘Apex’ a representative example open-pollinated cultivar as it has been cultivated for many years in the UK and used in many studies such as Sweet et al. (2004) and Lutman et al. (2005).
Our second hypothesis was not substantiated. We suggested that inherited crop plant traits would lead to a growth rate for hybrids below that of B. rapa in semi-natural habitats, but higher than B. rapa in agricultural conditions because of pre-adaptation of crop genes to the crop environment. However, hybrids performed worse than B. rapa because of low fecundity in both semi-natural and agricultural conditions. Furthermore, hybrid fitness differed substantially across sites, being zero at two of the three semi-natural sites. Our elasticity analysis showed that the demographic dynamics of hybrids were very different from those of B. rapa: hybrid dynamics were largely dependent on the seed bank in both semi-natural and agricultural habitats.
Our third hypothesis was substantiated. We suggested that rotations could modify the projected persistence of hybrids in agricultural systems as has been found for weed species (Bohan et al., 2011; Gulden et al., 2011). In rotations including non-OSR years, i.e. years without the possibility for Brassica seed set, growth rates indicated population decreases (λ < 1) and the dominant matrix elasticities switched from above-ground dynamics to seed bank survival. However, hybrid populations could still persist for >20 years under the assumptions of our model, parameterized using our field data. Comparing the 3- and 4-year rotations, the declines of B. rapa and hybrids were more precipitous in the latter. Further examination showed that because the seed bank of both the wild Brassica and hybrids is only replenished when OSR is grown, under a longer rotation more seeds have died by the time a new OSR crop is sown. The rate of hybrid decline was little affected by its fecundity, but was determined by hybrid seed bank survival and, most importantly, by the seed bank dynamics of B. rapa. This last point confirms our last hypothesis. Interactions with the parental populations, in terms of continual hybridization events, strongly affect the population dynamics and growth rate of hybrid populations.
The role of the seed bank
Overall, in agricultural habitats the dynamics of the seed bank proved to be the dominant driver of the hybrid life cycle. This importantly includes the seed bank dynamics of the wild parents in a situation in which F1 hybrids can be formed continually and hybrid fecundity is low. OSR is one of many crops grown in rotation. The resulting periodicity of rotations means the seed bank and its accompanying management is of prime importance for many weeds (Bohan et al., 2011; Gulden et al., 2011), including weedy hybrids as shown here. Our elasticity analysis revealed that in Brassica the seed-bank-related transitions are the most influential and contribute most to the differences in population persistence, a conclusion which we share with Claessen et al. (2005). In both habitat types the hybrid population was estimated to be in decline but hybrids could persist for decades in agricultural habitats through survival in the seed bank combined with periodic continual hybridization events.
In considering fitness of hybrids in the Brassicaceae and other families, fecundity is the most frequently studied trait using either GM or conventional plants as model systems (e.g. Snow et al., 1999; Allainguillaume et al., 2006; Jørgensen et al., 2009), with other traits much more rarely studied. However, it seems it is those poorly studied traits, such as seed bank survival, that are the most important to populations in both semi-natural and agricultural settings. This result strengthens the call for better insights into interactions between the seed bank, the environment and management (Gruber et al., 2005; Bohan et al., 2011; Gulden et al., 2011).
The explicit novel finding from this study is that, particularly in an agricultural setting, the seed bank survival of the wild relative (B. rapa) is the most influential for the hybrid growth rate. The factors influencing these demographic transitions (number of seeds escaping harvest, movement between layers, etc.) are influenced by management and would benefit from future experimental study. However, we expect that in cases where Brassica hybrid fecundity is restored in backcross generations (see, for example, Jørgensen et al., 2009; Snow et al., 2010) the dependence on the parental species would decrease, whereas higher outcrossing rates would further increase dependence on the parental seed bank dynamics.
Introgression beyond F1 hybrids
We made the simplifying assumption that the fitness of hybrids is well described by the low fitness of the F1 generation, but recognize that our conclusions are dependent upon this assumption. The subsequent process of backcrossing was not studied here but has been studied experimentally for B. rapa × B. napus hybrids by Jorgensen and co-workers (summarized by Jørgensen et al., 2009) and recently by Liu et al. (2013). Those studies show that Brassica hybrids have highly variable fitness (Devos et al., 2009; Jørgensen et al., 2009). Furthermore, several studies suggest that F1 hybrids might have reduced fitness compared with later backcrosses (Burke and Arnold, 2001; Snow et al., 2010; Yang et al., 2011) and fitness could bounce back in later generations towards that of the wild parent (Liu et al., 2013; Cao et al., 2014). Therefore, our fitness estimates of Brassica hybrids are likely at the lower end of what might happen in reality. Despite these issues, low fecundity of hybrids would result in very few such backcrosses and the hybrid population being dominated by newly created F1 hybrids during OSR crop years. Moreover, other factors that we did not study can arrest development of further generations; for example, male fitness in later generations could be impaired, flower morphologies become incompatible or flowering seasons become asynchronous (Norris et al., 2004; Warwick et al., 2004; Jørgensen et al., 2009). Furthermore, chromosomal selection might be biased (de Jong and Hesse, 2012), resulting in unequal segregation of traits that affect fitness, selecting against later generation hybrids.
As long as an additional transgene in hybrids is not subject to its intended stress benefit outside of the crop, such as diseases or insects, its introgression rate could be based on neutral segregation ratios across generations. However, in those cases where these stresses remain, such as herbicide spraying, tolerant hybrids could have an additional benefit and so increase their presence in the population (Devos et al., 2012; Gressel, 2014). Transgene presence may also interact with the hybridization process; a transgene will be subject to positive or negative genetic hitchhiking based on its position on the chromosome as well as affecting the likelihood of selection of that chromosomal segment (Hartman et al., 2013). Furthermore, the transgene could incur fitness costs or benefits to plant growth (Snow et al., 1999; Hails and Morley, 2005; de Jong and Rong, 2013). Therefore, for an environmental risk assessment of GM crops, the potential for survival of hybrids based on population dynamics is a first step, which has to be complemented by the further impact of transgenic traits on hybrid survival. Garnier et al. (2014) provide a modelling framework that could be used.
CONCLUSIONS
In this study we assessed which aspects of the life cycle of hybrids and their parents were of principal importance for the persistence of hybrids. We found that changes in seed bank survival rate may have the greatest impact on long-term persistence of hybrid populations. We suggest that more research should focus on traits that alter seed persistence dynamics or seed dormancy. Similarly, changes in management can interact with changes in fitness: the timing and depth of tillage are known to influence the level of dormancy and survival of seeds and thus the longevity of the wild relative or hybrid seed bank (Gruber et al., 2005; Pekrun et al., 2005, 2006). Therefore, there may be the potential for changes in the timing and depth of tillage operations to control hybrid populations. These suggestions are based on the assumption that hybrid populations are undesirable in and among crop fields and their presence needs to be managed (Snow et al., 2005; Chandler and Dunwell, 2008; Schierenbeck and Ellstrand, 2009).
For hybrids with low fecundity, as long as a new inherited (GM) trait does not increase fecundity, local risk mitigation could additionally focus on the wild relative. To manage hybrid presence, selective herbicide treatments could be effective even in those cases in which herbicide-tolerant F1 hybrids occur, as herbicides would still kill the wild relative. Such management would reduce hybrid populations indirectly by blocking seed bank replenishment. We stress that the role crop rotations play in hybrid population persistence deserves greater attention as they strongly influence the dynamics of both hybrids and wild relatives.
B. napus, both as a crop (OSR) and as a feral plant, is a particularly pertinent case study, due to the regular presence of wild relatives (B. rapa) in both semi-natural habitats and as crop weeds (Wilkinson et al., 2003; Luijten et al., 2014). We emphasize, however, that the principles and approach presented here have a broader applicability elsewhere, and the techniques applied to study dynamics in semi-natural habitats can reveal new insights when applied to crop systems.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Fig. S1: Map of the experimental locations and seed collections of B. rapa. Fig. S2: Proportion of hybrids confirmed by flow cytometry from plants grown from the original seed sample under controlled conditions compared with plants sampled at seed set in the experimental sites. Methods S1: Hybrid identification. Methods S2: Vital rates and full among-line statistics Methods S3: Periodic matrix model.
ACKNOWLEDGEMENTS
We thank two anonymous reviewers for their comments that greatly improved an earlier version of the manuscript. This study was funded by the BBSRC (GM114157) and NERC-CEH (NEC04432, NEC04630 and NEC04940).
LITERATURE CITED
- Allainguillaume J, Alexander M, Bullock JM, et al. 2006. Fitness of hybrids between rapeseed (Brassica napus) and wild Brassica rapa in natural habitats. Molecular Ecology 15: 1175–1184. [DOI] [PubMed] [Google Scholar]
- Allainguillaume J, Harwood T, Ford CS, et al. 2009. Rapeseed cytoplasm gives advantage in wild relatives and complicates genetically modified crop biocontainment. New Phytologist 183: 1201–1211. [DOI] [PubMed] [Google Scholar]
- Beckie HJ, Harker KN, Légère A, Morrison MJ, Séguin-Swartz G, Falk KC. 2011. GM canola: the Canadian experience. Farm Policy Journal 8: 43–49. [Google Scholar]
- Bishnoi U, Zurres K, Cebert E, Mentreddy RS. 2007. Agronomic and economic performance of winter canola in south-eastern US. Journal of Agricultural Sciences 3: 263–268. [Google Scholar]
- Bohan DA, Powers SJ, Champion G, et al. 2011. Modelling rotations: can crop sequences explain arable weed seed bank dynamics. Weed Research 51: 422–432. [Google Scholar]
- Bullock JM. 1999. Using population matrix models to target GMO risk assessment. Aspects of Applied Biology 53: 205–212. [Google Scholar]
- Burke JM, Arnold ML. 2001. Genetics and fitness of hybrids. Annual Review in Genetics 35: 31–52. [DOI] [PubMed] [Google Scholar]
- Campbell LG, Snow AA, Sweeney PM, Ketner JM. 2009. Rapid evolution in crop-weed hybrids under artificial selection for divergent life histories. Evolutionary Applications 2: 172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Stewart CN, Zheng M, et al. 2014. Stable Bacillus thuringiensis transgene introgression from Brassica napus to wild mustard B. juncea. Plant Science 227: 45–50. [DOI] [PubMed] [Google Scholar]
- Caswell H. 2000. Matrix population models . Sunderland, MA: Sinauer Publishers. [Google Scholar]
- Chandler S, Dunwell JM. 2008. Gene flow, risk assessment and the environmental release of transgenic plants. Critical Reviews in Plant Sciences 27: 25–49. [Google Scholar]
- Claessen D, Gilligan CA, Lutman PJW, van den Bosch F. 2005. Which traits promote persistence of feral GM crops? Part 1: implications of environmental stochasticity. Oikos 110: 20–29. [Google Scholar]
- de Jong TJ, Hesse E. 2012. Selection against hybrids in mixed populations of Brassica rapa and Brassica napus: model and synthesis. New Phytologist 194: 1134–1142. [DOI] [PubMed] [Google Scholar]
- de Jong TJ, Rong J. 2013. Crop to wild gene flow: does more sophisticated research provide better risk assessment? Environmental Science & Policy 27: 135–140. [Google Scholar]
- Devos Y, De Schrijver A, Reheul D. 2009. Quantifying the introgressive hybridisation propensity between transgenic oilseed rape and its wild/weedy relatives. Environmental Monitoring and Assessment 149: 303–322. [DOI] [PubMed] [Google Scholar]
- Devos Y, Hails RS, Messéan A, Perry JN, Squire GR. 2012. Feral genetically modified herbicide tolerant oilseed rape from seed import spills: are concerns scientifically justified? Transgenic Research 2: 1–21. [DOI] [PubMed] [Google Scholar]
- EFSA. 2010. Guidance on the environmental risk assessment of genetically modified plants. The EFSA Journal 1879: 1–111. [Google Scholar]
- Ellstrand NC. 2003. Dangerous liaisons? When cultivated plants mate with their wild relatives. Baltimore, MD: John Hopkins University press. [Google Scholar]
- Ellstrand NC, Heredia SM, Leak-Garcia JA, et al. 2010. Crops gone wild: evolution of weeds and invasives from domesticated ancestors. Evolutionary Applications 3: 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellstrand NC, Meirmans P, Rong J, et al. 2013. Introgression of crop alleles into wild or weedy populations. Annual Reviews in Ecology, Evolution and Systematics 44: 325–345. [Google Scholar]
- Garnier A, Darmency H, Tricault Y, Chèvre AM, Lecomte J. 2014. A stochastic cellular model with uncertainty analysis to assess the risk of transgene invasion after crop-wild hybridization: oilseed rape and wild radish as a case study. Ecological Modelling 276: 85–94. [Google Scholar]
- Gressel J. 2014. Dealing with transgene flow of crop protection traits from crops to their relatives. Pest Management Science 7: doi 10.1002/ps.3850. [DOI] [PubMed] [Google Scholar]
- Gruber S, Pekrun C, Claupein W. 2005. Life cycle and potential gene flow of volunteer oilseed rape in different tillage systems . Weed Research 45: 83–93. [Google Scholar]
- Gulden RH, Lewis DW, Froese JC, et al. 2011. The effect of rotation and in-crop weed management on the germinable weed seed bank after 10 years. Weed Science 59: 553–561. [Google Scholar]
- Haider N, Allainguillaume J, Wilkinson M. 2009. Spontaneous capture of oilseed rape (Brassica napus) chloroplasts by wild B. rapa: implications for the use of chloroplast transformation for biocontainment. Current Genetics 55: 139–150. [DOI] [PubMed] [Google Scholar]
- Hails RS, Morley K. 2005. Genes invading new generations: a risk assessment perspective. Trends in Ecology and Evolution 20: 245–252. [DOI] [PubMed] [Google Scholar]
- Hartman Y, Uwimana B, Hooftman DAP, et al. 2013. Genomic and environmental selection patterns in two distinct lettuce crop-wild hybrid crosses. Evolutionary Applications 6: 569–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes C, Haughton AJ, Osborne JL, et al. 2003. Responses of plants and invertebrate trophic groups to contrasting herbicide regimes in the Farm Scale Evaluations of genetically modified herbicide-tolerant crops. Philosophical Transactions of the Royal Society, London B 358: 1899–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooftman DAP, Oostermeijer JGB, Jacobs MMJ, den Nijs JCM. 2005. Vital rates determine the performance advantage of crop-wild hybrids in Lettuce. Journal of Applied Ecology 42: 1086–1095. [Google Scholar]
- Hooftman DAP, de Jong MJ, Oostermeijer JGB, den Nijs JCM. 2007. Modelling the long-term consequences of crop-wild relative hybridisation: a case study using four generations of hybrids. Journal of Applied Ecology 44: 1035–1045. [Google Scholar]
- Hooftman DAP, Oostermeijer JGB, Marquard E, den Nijs JCM. 2008. Modelling the consequences of crop-wild relative gene flow: a sensitivity analysis of the effects of outcrossing rates and hybrid vigour breakdown in Lactuca. Journal of Applied Ecology 45: 1094–1103. [Google Scholar]
- Jørgensen RB, Hauser TP, D’Hertefeldt T, Andersen NS, Hooftman DAP. 2009. The variability of processes involved in transgene dispersal—case studies from Brassica and related genera. Environmental Science and Pollution Research 16: 389–395. [DOI] [PubMed] [Google Scholar]
- Kwit C, Moon HS, Warwick SI, Stewart CN. 2011. Transgene introgression in crop relatives: molecular evidence and mitigation strategies. Trends in Biotechnology 29: 284–293. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei W, Ma K, Li J, Liang Y, Darmency H. 2013. Consequences of gene flow between oilseed rape (Brassica napus) and its relatives. Plant Science 211: 42–51. [DOI] [PubMed] [Google Scholar]
- Luijten SH, Schidlo NS, Meirmans PG, de Jong TJ. 2014. Hybridisation and introgression between Brassica napus and B . rapa in the Netherlands. Plant Biology, in press. doi: 10.1111/plb.12197. [DOI] [PubMed] [Google Scholar]
- Lutman PJW, Freeman SE, Pekrun C. 2003. The long-term persistence of seeds of oilseed rape (Brassica napus) in arable fields. Journal of Agricultural Science 141: 231–240. [Google Scholar]
- Lutman PJW, Berry K, Payne RW, et al. 2005. Persistence of seeds from crops of conventional and herbicide tolerant oilseed rape (Brassica napus). Proceeding of the Royal Society B 272: 1909–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens SKF, van den Bosch F, Heesterbeek JAP. 2002. Weed populations and crop rotations: exploring dynamics of a structured periodic system. Ecological Applications 12: 1125–1141. [Google Scholar]
- Norris C, Sweet J, Parker J, Law J, den Nijs HCM, Bartsch D. 2004. Implications for hybridization and introgression between oilseed rape (Brassica napus) and wild turnip (B. rapa) from an agricultural perspective. In: den Nijs HCM, Bartsch D, Sweet J, eds. Introgression from genetically modified plants into wild relatives. Wallingford: CABI, 107–123. [Google Scholar]
- Pekrun C, Lane PW, Lutman PJW. 2005. Modelling seed bank dynamics of volunteer oilseed rape (Brassica napus). Agricultural Systems 84: 1–20. [Google Scholar]
- Pekrun C, Lutman PJW, Büsche A, Albertini A, Claupein W. 2006. Reducing potential gene escape in time by appropriate post-harvest tillage – Evidence from field experiments with oilseed rape at 10 sites in Europe. European Journal for Agronomy 25: 289–298. [Google Scholar]
- Pivard S, Adamczyk K, Lecomte J, et al. 2008. Where do the feral oilseed rape populations come from? A large-scale study of their possible origin in a farmland area. Journal of Applied Ecology 45: 476–485. [Google Scholar]
- Ridley CE, Ellstrand NC. 2009. Evolution of enhanced reproduction in the hybrid-derived invasive, California wild radish (Raphanus sativus). Biological Invasions 11: 2251–2264. [Google Scholar]
- Schafer MG, Ross AA, Londo JP, et al. 2011. The establishment of genetically engineered Canola populations in the US. PLoS One 6: e25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierenbeck KA, Ellstrand NC. 2009. Hybridisation and the evolution of invasiveness in plants and other organisms. Biological Invasions 11: 1093–1105. [Google Scholar]
- Seymour M, Kirkegaard JA, Peoples MB, White PF, French RJ. 2012. Break-crop benefits to wheat in Western Australia – insights from over three decades of research. Crop and Pasture Science 63: 1–16. [Google Scholar]
- Silvertown J, Franco M, Menges E. 1996. Interpretation of elasticity matrices as an aid to the management of plant populations for conservation. Conservation Biology 19: 591–597. [Google Scholar]
- Snow AA, Andersen B, Jørgensen RB. 1999. Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy B . rapa. Molecular Ecology 8: 605–615. [Google Scholar]
- Snow AA, Andow DA, Gepts P, et al. 2005. Genetically engineered organisms and the environment: current status and recommendations. Ecological Applications 15: 377–404. [Google Scholar]
- Snow AA, Culley TM, Campbell LG, Sweeney PM, Hegde SG, Ellstrand NC. 2010. Long-term persistence of crop alleles in weedy populations of wild radish (Raphanus raphanistrum). New Phytologist 186: 537–548. [DOI] [PubMed] [Google Scholar]
- Squire GR, Breckling B, Dietz-Pfeilstetter A, et al. 2011. Status of feral oilseed rape in Europe: its minor role as a GM impurity and its potential as a reservoir of transgene persistence. Environmental Science and Pollution Research 18: 111–115. [DOI] [PubMed] [Google Scholar]
- Sweet J, Simpson E, Law J, et al. 2004. Botanical and rotational implications of genetically modified herbicide tolerance in winter oilseed rape and sugar beet (BRIGHT Project) . London: Home-Grown Cereals Authority. [Google Scholar]
- Vacher C, Kossler TM, Hochberg ME, Weis AE. 2011. Impact of interspecific hybridisation between crops and weedy relatives on the evolution of flowering time in weedy phenotypes. PLoS One 6: e14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick SI, Beckie HJ, Simard MJ, et al. 2004. Environmental and agronomic consequences of herbicide-resistant (HR) canola in Canada. In: den Nijs HCM, Bartsch D, Sweet J, eds. Introgression from genetically modified plants into wild relatives. Wallingford: CABI, 323–337. [Google Scholar]
- Warwick SI, Legere A, Simard J, James T. 2008. Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population. Molecular Ecology 17: 1387–1395. [DOI] [PubMed] [Google Scholar]
- Wilkinson MJ, Elliott LJ, Allainguillaume J, et al. 2003. Hybridisation between Brassica napus and B. rapa on a national scale in the United Kingdom. Science 302: 456–459. [DOI] [PubMed] [Google Scholar]
- Yang X, Xia H, Wang W, et al. 2011. Transgenes for insect resistance reduce herbivory and enhance fecundity in advanced generations of crop-weed hybrids rice. Evolutionary Applications 4: 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]