Abstract
Pituitary adenylate cyclase activating peptide (PACAP) is found in human trigeminocervical complex and can trigger migraine. PACAP levels were measured using a sensitive radioimmunoassay. Stimulation of the superior sagittal sinus (SSS) in cat elevated PACAP levels in cranial blood. Patients with moderate or severe migraine headache had elevated PACAP in the external jugular vein during headache (n = 15), that was reduced 1 h after treatment with sumatriptan 6 mg (n = 11), and further reduced interictally (n = 9). The data suggest PACAP, or its receptors, are a promising target for migraine therapeutics.
Introduction
Migraine is the most common cause of neurological disability worldwide.1 Migraine is a brain disorder2 requiring management with acute and preventive therapies.3 The best outcome with acute therapy for oral treatments is about one third of patients are pain free at 2 h.4 New approaches are in development and more are needed.5
Pituitary adenylate cyclase activating peptide (PACAP) is found in human trigeminocervical complex.6 Infusion of PACAP-38 into migraineurs triggers migraine.7 Having previously shown that activation of the trigeminovascular system led to the release of CGRP,8 we hypothesized that such activation would also result in the extracranial release of PACAP, and that this activation would be seen in migraine. The work was first reported, in part, in abstract form at the Seventh International Headache Congress, Toronto, Canada, 16–20 September 1995.9
Methods
Experimental animals
All experiments described in this study were approved by the Animal Care and Ethics Committee of the University of New South Wales and conformed to its guidelines.
Preparatory surgery
Five adult cats of either sex were studied (mean ± standard deviation, 3.3 ± 0.8 kg). Cats were anesthetised with an intraperitoneal injection of α-chloralose (70 mg/kg), with supplementary doses (20 mg/kg i.v.) given throughout the experiment. Polyethylene catheters were inserted into the femoral artery and vein to allow continuous measurement of blood pressure and heart rate and to administer drugs and fluids. A third catheter was inserted into one external jugular vein to permit sampling of blood. Cats were intubated and ventilated with 30% oxygen in air. End-expiratory CO2 was maintained at 3.5–4%. Cats were immobilized by intermittent intravenous administration of gallamine triethiodide (20 mg). Testing for sympathetic responses to noxious stimulation was conducted at regular intervals to assess the depth of anesthesia.
The superior sagittal sinus (SSS) was exposed,10 and stimulated with supramaximal square-wave stimulus-isolated shocks (100 V, 500 μsec duration, 10 Hz). Blood (5 mL) was taken before, and after, 7–8 min of stimulation. The volume of blood was replaced by an equivalent amount of plasma expander.
Patients
Data were collected from 15 patients who had presented to the Prince of Wales Hospital, Sydney, with a history of migraine with or without aura.11 Aura was not present at sampling in any patient and no patient was on a migraine preventive.
Inclusion criteria were to have between one and six attacks of migraine per month of at least moderate severity, and be aged between 18 and 60 years. They should have had a history of migraine for at least 1 year and if female, be using adequate contraceptive measures. Exclusion criteria were concomitant ergot use, medication overuse, pregnancy, lactation, a history of cardiovascular disease or other significant systemic or psychiatric disease. The study was conducted prior to the use of online registries.
The patients gave informed consent after an explanation of the procedure. They were rested supine and blood was sampled from the external jugular vein ipsilateral to the headache. They were then treated with sumatriptan 6 mg s.c. injection and sampling repeated at 60 minutes. All patients studied had severe unilateral headache present at the time of sampling.
The study was approved the Institutional Ethics Committee of the Prince of Wales and Prince Henry Hospitals.
Peptide assays
Blood was collected into tubes prepared with aprotonin (Trasylol, 5000 IU) and heparin (500 IU), and kept on ice until centrifugation at 2000 rpm for 15 min. Plasma was aliquoted, coded, and stored at −30°C until being freeze-dried for transport for assay.
Calcitonin gene-related peptide
Immunoreactive CGRP was quantitated using a rabbit antiserum (R-8429) raised against synthetic rat CGRP conjugated to bovine serum albumin (BSA) and used at a final dilution of 1:37,500. Iodinated CGRP was purified by HPLC allowing measurements of CGRP-like material with a minimum detection of 10 pmol/L. The CGRP antiserum showed no cross-reaction with related peptides.12 CGRP-like immunoreactivity is hereafter described as CGRP accepting the limitations of radioimmunoassay (RIA).
Pituitary adenylate cyclase activating peptide
Immunoreactive PACAP-38 was measured using a commercial RIA kit from Peninsula (Merseyside, St. Helens, UK). Briefly, 100 μL of sample or a standard solution of PACAP-27 (1–128 pg/mL) in RIA buffer (0.05 mol/L NaCl, 0.1% BSA, 0.1% Triton X-100, 0.01% NaN3) were incubated with 100 μL PACAP-27 antiserum at a final dilution of 1:120,000 at +4°C for 24 h. A volume of 100 μL of iodinated PACAP-27 (∼10,000 cpm) in RIA buffer was added and the mixture incubated for 24 h at +4°C. Antibody-bound tracer was separated from free tracer by the addition of 100 μL goat anti-rabbit IgG serum and 100 μL normal rabbit serum. After incubation at room temperature for 90 min, 500 μL of cold RIA buffer was added and the samples centrifuged at 1700g for 30 min. The tubes were gently aspirated and the radioactivity of the precipitates measured. The ID50 was about 4 pmol/L and the detection limit 1 pmol/L. The degree of cross-reaction of the PACAP-38 antiserum with PACAP-27 was 0.01%.13 PACAP-38-like immunoreactivity is hereafter described as PACAP accepting the limitations of RIA, which include that shorter fragments with the appropriate site retained would also be included.
Statistical analysis
PACAP estimates and the data analysis were done blind to the treatment allocation. Group data are presented as mean ± standard error (SE), unless otherwise indicated. Groups were compared using the Wilcoxon signed-rank test. Significance was assessed at the P < 0.05 level.
Results
Cat study
There was no significant change in physiological parameters during stimulation (Table1). There was a significant increase in CGRP levels following SSS stimulation from 148 ± 7 to 230 ± 22 pmol/L (Z = 2.02, P < 0.05).
Table 1.
Cardiovascular data for cat experiments
| Control | SSS stimulation | |
|---|---|---|
| Heart rate (/min) | 201 ± 12 | 203 ± 9 |
| Blood pressure (mmHg) | 105 ± 6 | 103 ± 5 |
SSS, superior sagittal sinus.
PACAP levels rose substantially from 112 ± 25 to 287 ± 38 pmol/L (Z = 2.02, P < 0.05). The results are depicted in Figure1.
Figure 1.
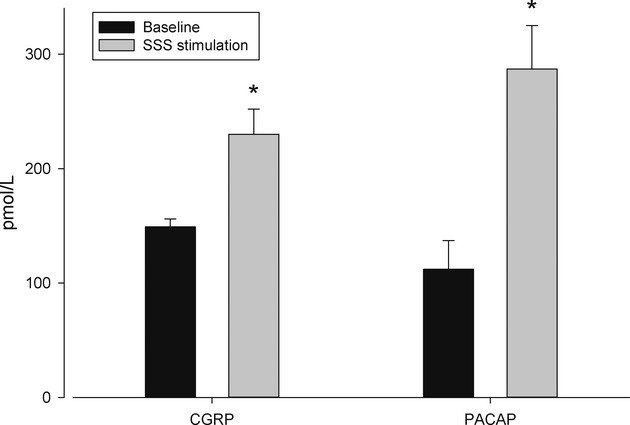
Peptide levels (mean ± SEM, error bars) in the blood of the external jugular vein of the cat before, (black column) and during (shaded column) stimulation of the superior sagittal sinus. There are significant increases (denoted by the asterisk) in the levels of CGRP and PACAP. All data are in pmol/L. CGRP, calcitonin gene-related peptide; PACAP, pituitary adenylate cyclase activating polypeptide.
Human study
The patients studied had migraine with (n = 2) or migraine without aura (n = 13). Fifteen patients (13 females) aged 37 ± 8 years (mean ± SD) were sampled at a migraine attack length of 13 h (3–24; median and range) when pain was moderate or severe. PACAP levels were 36 ± 3 pmol/L (n = 15) during the attack when pain was moderate or severe.
Of the 15 subjects, 11 were treated with sumatriptan 6 mg by subcutaneous injection. At 1 h post injection, 9 of 11 had mild or no pain.
Four patients preferred not to have a second blood sample. Further sampling at 1 h after postsumatriptan demonstrated the PACAP level had reduced consistent with headache amelioration to 26 ± 3 pmol/L (Z = 2.3, P < 0.02, n = 11; Fig.2).
Figure 2.
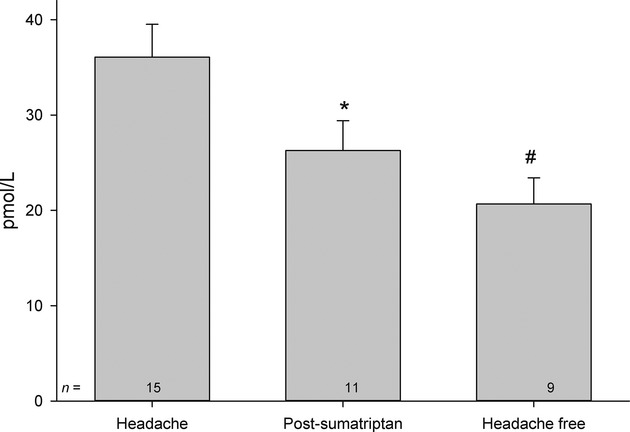
Elevation of pituitary adenylate cyclase activating peptide (PACAP) during headache and its reduction after treatment with sumatriptan 6 mg s.c. (postsumatriptan, *P < 0.05 compared to headache). Interattack levels of PACAP (headache free) are less than attack levels (#P < 0.05). PACAP, pituitary adenylate cyclase activating polypeptide.
Sampling of nine of the initial cohort of 15 patients between attacks demonstrated a PACAP level of 21 ± 3 pmol/L, which was less than the ictal level (Z = 2.7, P < 0.01; Fig.2).
Discussion
Here we report elevations in the level of PACAP and CGRP in the cranial circulation of the cat with nociceptive dural stimulation. Similarly, we report reduction in PACAP levels with amelioration of migraine headache when treated with sumatriptan, and lower levels of PACAP between attacks when compared with attacks. The substantial elevations in PACAP levels in cat and humans during trigeminovascular activation reflect a robust change that is likely to be biologically meaningful and the reduction after treatment with sumatriptan further links the change to acute migraine. The effects seen repeat for PACAP those reported for CGRP14 that correctly predicted the efficacy of CGRP receptor antagonists, such as olcegepant.15 The data suggest targeting PACAP mechanisms could produce antimigraine effects.
PACAP is a member of the vasoactive intestinal polypeptide (VIP)/secretin/glucagon neuropeptide super-family.16 PACAP acts at a specific receptor PAC1, and at two other sites, VPAC1 and VPAC2, the latter two also being activated by VIP.17 Infusion of PACAP-38 into migraineurs triggers migraine,7 and indeed produces vasodilatation and headache in control nonmigraine subjects.18 In contrast, VIP, while a potent vasodilator, does not produce headache in controls or migraineurs19 suggesting a role for the PAC1 receptor in triggering migraine. Most recently it was shown that PACAP levels are elevated in acute migraine,20 just as we have seen.
PACAP mechanisms interact with head pain at a number of loci. PACAP can be found in human trigeminocervical complex,6 in the trigeminal and sphenopalatine ganglia.21 Modulation of PAC1 and VPAC receptors can inhibit trigeminovascular second order transmission.22 PACAP plays a role in nitroglycerin-triggered trigeminovascular activation in mice.23 PACAP effects may be seen at suprabulbar sites, such as the paraventricular nucleus of the hypothalamus,24 and the hypothalamic region is implicated in migraine pathophysiology.25
In conclusion, we have shown elevated levels of PACAP in cat with nociceptive dural stimulation, and in migraineurs during spontaneous migraine. Moreover, successful acute migraine treatment with sumatriptan significantly reduces PACAP levels in responders. The data suggest the PAC1 receptor or the PACAP peptide represent potential novel approaches for migraine therapeutics.
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia, by grants from the Australian Brain Foundation, and the Swedish Research Council (grant no. 5958). A. S. Zagami was in receipt of a NHMRC postgraduate scholarship.
Conflict of Interest
A. Z. has nothing to disclose. L. E. is on the Advisory Board of Labrys, and has received grant support from Merck. P. J. G. is on Advisory Boards for Allergan, Colucid, MAP pharmaceuticals, Merck, Sharpe and Dohme, eNeura, Autonomic Technologies Inc., Boston Scientific, Eli-Lilly, Medtronic, Linde gases, Arteaus, AlderBio, and BristolMyerSquibb. P. J. G. has consulted for Pfizer, Nevrocorp, Lundbeck, Zogenix, Impax, Zosano and DrReddy, and has been compensated for expert legal testimony. He has grant support from Allergan, Amgen, and Merck. P. J. G. has received honoraria for editorial work from Journal Watch Neurology and for developing educational materials and teaching for the American Headache Society.
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Akerman S, Holland P, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12:570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Sprenger T. Current practice and future directions in the management of migraine: acute and preventive. Lancet Neurol. 2010;9:285–298. doi: 10.1016/S1474-4422(10)70005-3. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin, 5-HT1B/1D agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358:1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- 5.Goadsby PJ. Therapeutic prospects for migraine: can paradise be regained? Ann Neurol. 2013;74:423–434. doi: 10.1002/ana.23996. [DOI] [PubMed] [Google Scholar]
- 6.Uddman R, Tajti J, Hou M, et al. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia. 2002;22:112–116. doi: 10.1046/j.1468-2982.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- 7.Schytz HW, Birk S, Wienecke T, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132:16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- 8.Zagami AS, Goadsby PJ, Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides. 1990;16:69–75. doi: 10.1016/0143-4179(90)90114-e. [DOI] [PubMed] [Google Scholar]
- 9.Zagami AS, Edvinsson L, Hoskin KL, Goadsby PJ. Stimulation of the superior sagittal sinus causes extracranial release of PACAP. Cephalalgia. 1995;15(suppl 14):109. [Google Scholar]
- 10.Goadsby PJ, Zagami AS. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain. 1991;114:1001–1011. doi: 10.1093/brain/114.2.1001. [DOI] [PubMed] [Google Scholar]
- 11.Headache Classification Committee of The International Headache Society. The international classification of headache disorders (second edition) Cephalalgia. 2004;24(suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 12.Grunditz T, Ekman R, Hakanson R, et al. Calcitonin gene-related peptide in thyroid nerve fibres and C cells. Effects on thyroid hormone secretion and response to hypercalcaemia. Endocrinology. 1986;119:2313–2324. doi: 10.1210/endo-119-5-2313. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Malmberg AB, Yaksh TL, et al. Capsaicin-evoked release of pituitary adenylate cyclase activating peptide (PACAP) and calcitonin gene-related peptide (CGRP) from rat spinal cord in vivo. Regul Pept. 1997;69:83–87. doi: 10.1016/s0167-0115(97)02133-2. Mar 26. [DOI] [PubMed] [Google Scholar]
- 14.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:761–766. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 15.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 16.Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Regul Pept. 1992;37:287–303. [PubMed] [Google Scholar]
- 17.Harmar AJ, Fahrenkrug J, Gozes I, et al. IUPHAR reviews 1: pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Br J Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin FM, Asghar MS, Guo S, et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia. 2012;32:140–149. doi: 10.1177/0333102411431333. Jan. [DOI] [PubMed] [Google Scholar]
- 19.Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilatation but does not induce migraine. Cephalalgia. 2008;28:226–236. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 20.Tuka B, Helyes Z, Markovics A, et al. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia. 2013;33:1085–1095. doi: 10.1177/0333102413483931. [DOI] [PubMed] [Google Scholar]
- 21.Csati A, Tajti J, Kuris A, et al. Distribution of vasoactive intestinal polypeptide, pituitary adenylate cyclase activating peptide, nitric oxide synthase and their receptors in human and rat sphenopalatine ganglion. Neuroscience. 2012;202:158–168. doi: 10.1016/j.neuroscience.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 22.Akerman S, Goadsby PJ. VPAC1 and PAC1 receptor antagonists inhibit activation of the parasympathetic outflow to the cranial vasculature to prevent atuonomic responses and neuronal firing in the trigeminocervical complex. Cephalalgia. 2009;29(suppl 1):130. [Google Scholar]
- 23.Markovics A, Kormos V, Gaszner B, et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis. 2012;45:633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Robert C, Bourgeais L, Arreto CD, et al. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci. 2013;33:8827–8840. doi: 10.1523/JNEUROSCI.0439-13.2013. May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin triggered migraine attacks. Brain. 2014;137:232–242. doi: 10.1093/brain/awt320. [DOI] [PubMed] [Google Scholar]


