Abstract
Sirtuin 1 is a nicotinamide adenine dinucleotide-dependent protein deacetylase which regulates longevity and improves metabolism. Activation of Sirtuin 1 confers beneficial effects in models of neurodegenerative diseases. We and others have provided convincing evidence that overexpression of Sirtuin 1 plays a neuroprotective role in mouse models of Huntington's disease. In this study, we report that SRT2104, a small molecule Sirtuin 1 activator, penetrated the blood–brain barrier, attenuated brain atrophy, improved motor function, and extended survival in a mouse model of Huntington's disease. These findings imply a novel therapeutic strategy for Huntington's disease by targeting Sirtuin 1.
Introduction
Huntington's disease (HD) is a hereditary neurodegenerative disorder, which is caused by a CAG trinucleotide repeat expansion in huntingtin gene. Currently, there are no pharmacological interventions available to delay the onset or reverse the progression of this devastating disease.1 Previously, we and others have provided convincing evidence that genetically increasing Sirtuin 1 (SIRT1) shows neuroprotection in several mouse models of HD, whereas a deficiency of SIRT1 exacerbates the HD phenotype.2,3 Indeed, many connections exist between the deacetylation function of SIRT1 and its role in neuroprotection.4 As a result, pharmacological interventions targeting SIRT1 might provide a new therapeutic strategy for HD.
During the past decade, SIRT1 has been the focus of intense investigation because of its ability to extend lifespan in lower organisms and beneficial effects in various models of human disorders such as diabetes, neurodegeneration, and cancer.4 Therefore, there has been great attention to characterize Sirtuin-activating compounds (STACs) that can modulate the ability of SIRT1 to deacetylate substrate proteins.5 Recently, two studies have definitively demonstrated that SIRT1 can be pharmacologically activated by small molecule STACs in vitro under certain conditions.6,7 Moreover, STACs extended lifespan in mice fed the regular diet and protected neurons against degeneration in Alzheimer's disease models.8,9
In this study, we validated the brain penetration of SRT2104, a newly characterized STAC,10 and found that SRT2104 improved motor performance on the balance beam, attenuated brain atrophy, and increased survival in the N171-82Q HD mice. SRT2104 has been proven to be safe and possess biological effects in both healthy and elderly populations of human phase I clinical trials.11,12 Our results suggest a novel therapeutic strategy for HD.
Materials and Methods
Mice and drug administration
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. Male N171-82Q HD mice were mated to female hybrid mice (B6C3F1/J; Jackson Laboratory, Bar harbor, ME). Male mice were used in our studies as reported previously.13 SRT2104 (>98% purity) was synthesized by Sirtris, a GlaxoSmithKline company. 0.5% SRT2104 containing diet (Harlan 2018SX, Frederick, MD) or control diet were provided to the mice from 6 weeks of age until the end of study.
Drug concentration measurement
Eight brains were dissected from wild-type (WT) mice fed SRT2104 for 6 month. SRT2104 was quantified in brain homogenate via LC/MS/MS at Alliance Pharma (Malvern, PA). SRT2104 was extracted with acetonitrile followed by centrifugation; the supernatant was diluted into an equal volume of water and directly injected onto a reverse phase LC system. The eluent flowed into a triple quadruple mass spectrometer running atmospheric pressure chemical ionization set to detect a parent-ion m/z of 517.3 and product-ion m/z of 203.3. Peak area ratios of standards were used to calibrate the LC/MS/MS and to subsequently quantify SRT2104 concentrations from the brain samples obtained during the study.
Behavioral test, body weight, and survival
All mice were randomly divided into different groups. Each group contained 10–12 mice at the beginning of experiments for survival and behavioral tests. Motor function was assessed on an 80-cm-long and 11-mm-wide cylindrical beam as described.14 All the mice tested were at 12, 18, and 24 weeks of age.
The body weight of the mice was recorded every week. Survival was monitored daily by experienced investigators.
In vivo structural MRI acquisition and quantification of brain volume
In vivo structural MRI studies were performed on a vertical bore 9.4-T magnetic resonance imager (Bruker Biospin, Billerica, MA) with a triple-axis gradient and an animal imaging probe. The detailed image capture and analysis procedures were described in our previous study.14
Statistics
Results are expressed as mean ± SE. Behavioral and body weight data were analyzed by two-way repeated analysis of variance (ANOVA). Survival curve was analyzed by Kaplan–Meier analysis. Brain volume comparisons between groups were conducted by one-way ANOVA with Holm–Sidak Post-hoc test. Differences were considered significant at P < 0.05.
Results
SRT2104 penetrated the blood–brain barrier
In order to validate brain penetration of SRT2104 in mice, mice were fed 0.5% SRT2104 containing diet for 6 months, brain tissue was harvested and the concentrations of SRT2104 in the brain were measured. The concentrations of SRT2104 in the mouse brain were 2.23 ± 0.61 μmol/L (mean ± SE, n = 8). Our data confirmed the feasibility of systematic administration of SRT2104 in a preclinical trial of HD.
SRT2104 improved motor function and increased survival in N171-82Q HD mice
WT and N171-82Q HD mice were fed 0.5% SRT2104 diet or control diet from 6 weeks of age until the end of study. Motor function was evaluated on balance beam tests. HD mice displayed motor deficiency starting from 12 weeks of age, indicated by extended traverse time on the balance beam (Fig.1A); in contrast, HD mice fed SRT2104 diets exhibited improved motor performance compared to HD mice fed control diets, indicated by the shortened traverse time on the balance beam at 12, 18, and 24 weeks (Fig.1A). SRT2104 had no effect on traverse time in WT mice. These results indicated that SRT2104 ameliorated motor deficits in N171-82Q HD mice.
Figure 1.
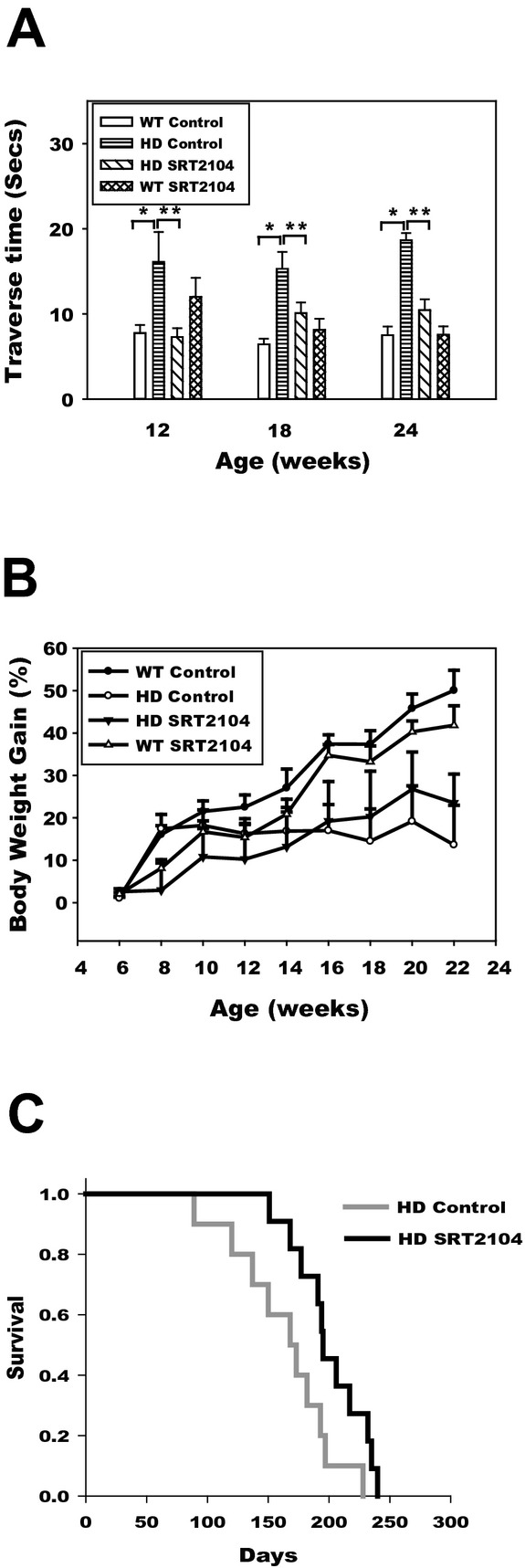
SRT2104 ameliorated motor deficits and increased survival in N171-82Q HD mice. Mice were fed 0.5% SRT2104 containing diet from 6 weeks of age until the end of life. (A) Mice were trained on 11-mm cylindrical beam and then tested at 12, 18, and 24 weeks of age. The traverse time on the beam was recorded. The longer traverse time indicates impaired motor function. The values are the mean ± SE, two-way (group and age) repeated ANOVA tests were used. *P < 0.01 compared with the values of wild-type (WT) diet group; **P < 0.05 compared with the values of HD diet group. n = 10–12 mice. (B) Body weight was recorded weekly. The values are the mean ± SE, n = 10–12 mice. (C) Survival was monitored daily by experienced operators. The mice were considered to be at the end of life when they were unable to right themselves after being placed on their backs and initiate movement after being gently prodded for 30 sec. n = 10–12 mice. Log rank analysis was used to compare survival data between two groups.
N171-82Q HD mice also lose body weight after the onset of disease; SRT2104 did not prevent body weight loss in HD mice and did not alter body weight in WT mice (Fig.1B). N171-82Q HD mice have short lifespan, the median lifespan of HD mice was 168 days, while the HD mice fed SRT2104 diet was 195 days (P = 0.03). Thus, SRT2104 increased the median lifespan of N171-82Q mice ∼16% compared with HD mice in control diet (Fig.1C).
SRT2104 attenuated brain atrophy in N171-82Q mice
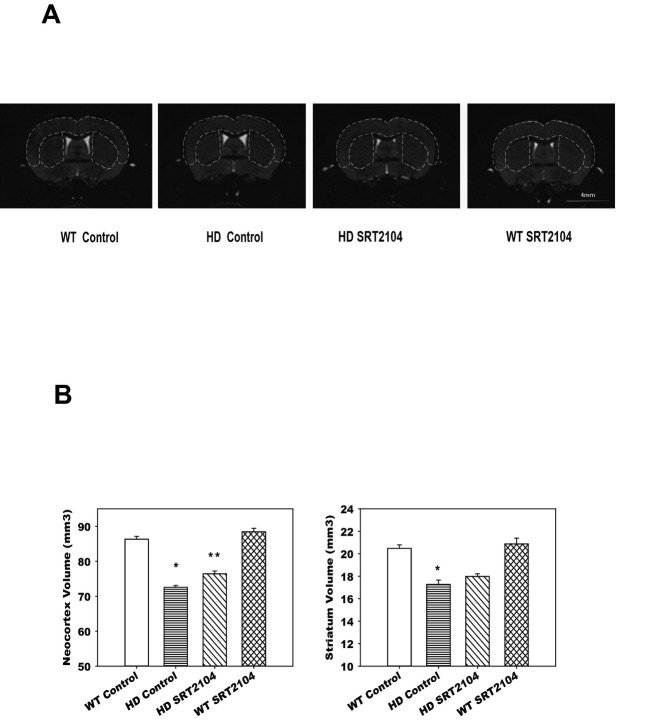
The selective regional brain volume loss is one of the key pathological features in N171-82Q HD mice and is a valuable measure of efficacy for treatment in preclinical trials.15 Brain volumes were measured at 22 weeks of age by structural MRI. As we previously reported, neocortex and striatum displayed the most dramatic volume loss in this model. HD mice fed SRT2104 diet had significant preservation of neocortex volume (P < 0.05) (Fig.2). Striatum volume in HD mice fed SRT2104 had a trend of better preservation, but did not reach statistical significant difference compared to HD mice fed control diet (P = 0.068) (Fig.2). These data support that SRT2104 rescues regional brain atrophy in N171-82Q HD mice. SRT2104 had no effect on brain volumes in WT mice.
Figure 2.
SRT2104 attenuated brain atrophy in N171-82Q mice. Mice were fed 0.5% SRT2104 containing diet from 6 weeks of age. Then, mice were anesthetized with isoflurane (1%), respiration was monitored and the temperature was maintained during the entire scan. Images were acquired by a three-dimensional T2-weighted fast spin echo sequence with the following parameters: echo time (TE)/repetition time (TR) = 40/700 msec, resolution = 0.1 × 0.1 × 0.1 mm, echo train length = 4, number of average = 2 and flip angle = 40°. The imaging resolution and contrast were sufficient for automatic volumetric characterization of the mouse brains and substructures. The intensity-normalized images were submitted by the Diffeomap software to a linux cluster, which runs Large Deformation Diffeomorphic Metric Mapping (LDDMM). The transformations encode morphological differences between subject and template images and can be analyzed with deformation-based morphometry (DBM) to detect regional changes in brain volume. Twenty-nine different brain regions were automatically segmented and the volume of each brain region was calculated. (A) Representative MRI images in mice from indicated groups. (B) The volumes of neocortex and striatum were measured by structural MRI at 22 weeks of age. Values are mean ± SE from four to six mice. *P < 0.05 compared with the WT control group; **P < 0.05 compared with the HD control group (ANOVA with Holm–Sidak Post-hoc test).
Discussion
SIRT1 deacetylates a variety of substrates and thus it is critical in diverse biological events including stress resistance, metabolism, and aging. Genetic upregulation of SIRT1 has protective effects on several neurodegenerative diseases, including HD.2,3 In this study, we confirmed brain penetration of a SIRT1 activator SRT2104, and provided the first evidence that a small molecule SIRT1 activator ameliorated motor deficits, rescued brain atrophy, and extended survival in HD mouse model. The beneficial effects of SRT2104 in HD mice provided a proof of concept for developing STACs for the treatment of HD as well as other neurodegenerative disorders with similar pathogenic mechanisms.
Studies in worms indicated that increased Sir2 (homolog of SIRT1) protected neurons against polyglutamine-mediated toxicity.16We and others demonstrated neuroprotection mediated by SIRT1 in mammalian models of HD.2,3 Thus, the specific SIRT1 activator(s) provide unique tools to dissect the pharmacological effects by targeting SIRT1 in HD. SRT2104 was derived from the optimization of previously lead structure – the imidazo [1,2-b] thiazole scaffold. The combination of biochemical activity and pharmacokinetic profile featured this compound currently developed in clinical trials. It was reported that SRT2104 is a specific SIRT1 activator.10 In our study, we confirmed that SRT2104 penetrates the blood–brain barrier and our data suggest protective effects of SRT2104 in a HD mouse model. These findings provide promising results in further development of STACs for treatment of HD, although the variability of pharmacokinetics among individuals’ hints that further optimization might be needed.11 Nevertheless, this is the first proof of concept that activation of SIRT1 by small molecule compound is beneficial in HD.
Although the promising results of SRT2104 in an HD mouse model encourage us to further pursue treatment strategy in this direction, we need to mention that the drugs targeting SIRT1 for clinical use are still in their infancy. A recent study indicates that Selisistat, a SIRT1/Sir2 inhibitor, alleviates pathology in Drosophila and mammalian cell and mouse models of HD mice.17 It is intriguing that NF-κB p65 subunit, a reported SIRT2 substrate,18 was deacetylated by Selisistat at the concentrations used in the study. It is interesting to know whether Selisistat, at the concentrations used in this study, also inhibits SIRT2, because SIRT2 inhibition was reported to provide protective effects in HD.19
The results urge us to further explore the role of SIRT1 in different disease stages and develop more specific SIRT1 modulators. Although the signaling pathway regulated by SIRT1 in HD mice needs to be further addressed, our results support developing STACs as therapeutic agents for HD.
Acknowledgments
This research was conducted under a research agreement between Sirtris, a GSK company and Johns Hopkins University. Funding was provided by Sirtris, a GSK company; CHDI Foundation; National Institutes of Health NS082338 (to W. D.).
Author Contribution
M. J. is the first author and the corresponding author, contributed to design of the study, conduction of experiments, interpretation of the data, and writing of the manuscript. W. D. is the corresponding author, contributed to overview of the experimental design, interpretation of the data, and writing of the manuscript. J. Z. contributed to behavioral experiments, bodyweight recording, change of diets for the mice, and editing the manuscript. Q. P. contributed to mouse breeding, genotyping, and colony maintenance. Z. H., J.-Y. Z., and S. M. conducted MRI scan and data analysis. J.-L. E., G.-P. V., R. F., and V. S. contributed to the preparation of SRT2104, measurement of brain SRT2104 concentration, and discussion.
Conflict of Interest
J. L. E., G. P. V., R. V. & V. S. are/were employees of GSK and hold/have held stock in GSK. J. H. U. has no conflict of interest.
References
- 1.Ross CA, Aylward EH, Wild EJ, et al. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10:204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- 2.Jeong H, Cohen DE, Cui L, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang M, Wang J, Fu J, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nat Med. 2011;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubbard BP, Gomes AP, Dai H, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakshminarasimhan M, Curth U, Moniot S, et al. Molecular architecture of the human protein deacetylase Sirt1 and its regulation by AROS and resveratrol. Biosci Rep. 2013;33:395–404. doi: 10.1042/BSR20120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell SJ, Martin-Montalvo A, Mercken EM, et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graff J, Kahn M, Samiei A, et al. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J Neurosci. 2013;33:8951–8960. doi: 10.1523/JNEUROSCI.5657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng PY, Bemis JE, Disch JS, et al. The identification of the SIRT1 activator SRT2104 as a clinical candidate. Lett Drug Des Discov. 2013;10:793–797. [Google Scholar]
- 11.Hoffmann E, Wald J, Lavu S, et al. Pharmacokinetics and tolerability of SRT2104, a first-in-class small molecule activator of SIRT1, after single and repeated oral administration in man. Br J Clin Pharmacol. 2012;75:186–196. doi: 10.1111/j.1365-2125.2012.04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libri V, Brown AP, Gambarota G, et al. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One. 2013;7:e51395. doi: 10.1371/journal.pone.0051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan W, Guo Z, Jiang H, et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang M, Peng Q, Liu X, et al. Small-molecule TrkB receptor agonists improve motor function and extend survival in a mouse model of Huntington's disease. Hum Mol Genet. 2013;22:2462–2470. doi: 10.1093/hmg/ddt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y, Peng Q, Hou Z, et al. Structural MRI detects progressive regional brain atrophy and neuroprotective effects in N171-82Q Huntington's disease mouse model. Neuroimage. 2011;56:1027–1034. doi: 10.1016/j.neuroimage.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker JA, Arango M, Abderrahmane S, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 17.Smith MR, Syed A, Lukacsovich T, et al. A potent and selective Sirtuin 1 inhibitor alleviates pathology in multiple animal and cell models of Huntington's disease. Hum Mol Genet. 2014;23:2995–3007. doi: 10.1093/hmg/ddu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothgiesser KM, Erener S, Waibel S, et al. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci. 2010;123:4251–4258. doi: 10.1242/jcs.073783. [DOI] [PubMed] [Google Scholar]
- 19.Luthi-Carter R, Taylor DM, Pallos J, et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci USA. 2010;107:7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]