Abstract
Coronary computed tomographic angiography (CCTA) employing CT scanners of 64-detector rows or greater represents a novel noninvasive method for detection of coronary artery disease (CAD), providing excellent diagnostic information when compared to invasive angiography. In addition to its high diagnostic performance, prior studies have shown that CCTA can provide important prognostic information, although these prior studies have been generally limited to small cohorts at single centers. The Coronary CT Angiography EvaluatioN For clinical Outcomes: An InterRnational Multicenter Registry, or CONFIRM, is a large, prospective, multinational, dynamic observational cohort study of patients undergoing CCTA. This registry currently represents more than 32,000 consecutive adults suspected of having CAD who underwent ≥ 64–detector row CCTA at 12 centers in 6 countries between 2005 and 2009. Based on its large sample size and adequate statistical power, the data derived from CONFIRM registry has and will continue to provide key answers to many important topics regarding CCTA. Based on its multisite international national design, the results derived from CONFIRM should be considered as more generalizable than prior smaller single-center studies. This article summarizes the current status of several studies from CONFIRM registry.
Keywords: Prognosis, Coronary artery disease, Coronary CT angiography
INTRODUCTION
Over the last decade, there have been significant technological advances involving multi-detector computed tomography (CT) technology, including the introduction of 64-detector scanners, as well as high-definition, dual-source, fast-pitch helical and wide area detector CT; all of which have resulted in improved spatial resolution, temporal resolution and volume coverage. These significant advances in CT technology have allowed coronary CT angiography (CCTA) to emerge as an attractive non-invasive alternative to invasive angiography, by providing detailed information about the coronary anatomy. Several prior studies have demonstrated high diagnostic performance of CCTA in comparison to invasive coronary angiography. In a recent meta-analysis of 27 studies using 16 to 64-slice scanner, CCTA was reported to have a diagnostic sensitivity, specificity, negative predictive value (NPV), and positive predictive values (PPV) of 94%, 83%, 48%, and 99%, respectively (1). In addition, the ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography)—the first prospective multicenter study evaluating 230 stable patients without known coronary artery disease undergoing CCTA before invasive coronary angiography(2)—demonstrated a diagnostic sensitivity, specificity, NPV, and PPV of 95%, 83%, 64%, and 99%, respectively, compared to a quantitative coronary angiographic reference standard. Importantly, these results included scoring of all vessel segments, without exclusion of patients based on baseline heart rate, coronary artery calcium score (CAC), or body mass index. Subsequent to the ACCURACY study, two additional prospective multicenter studies also demonstrated high sensitivity, negative predictive value, and high diagnostic accuracy of 64-slice CCTA compared to invasive coronary angiogram—in particular for patients without known CAD—with study results similar to the ACCURACY trial (3, 4).
One additional potential benefit of CCTA is its ability to characterize and quantify coronary artery plaque and arterial wall features in addition to providing accurate evaluation of degree of coronary stenosis. Numerous prior studies have compared the ability of CCTA to characterize and quantify coronary plaque versus intravascular ultrasound (IVUS) (5). In a recent meta-analysis of 33 studies that comprised 946 patients, CCTA was noted to demonstrate excellent correlation to IVUS for other atherosclerotic measures including cross sectional area, plaque area, area stenosis, and plaque volume (6).
In addition to its high diagnostic performance against invasive angiography and IVUS, CCTA findings of CAD have been evaluated for its ability to predict future adverse CAD events. The first large single center prognostic study evaluated the association of all-cause mortality with CCTA defined extent and severity of CAD in 1,127 patients with suspected disease (7). In this study, the per-patient, per-vessel and per-segment stenosis severity by CCTA was scored as minimal (<30%), mild (30% to 49%), moderate (50% to 69%), or severe (≥70%). Atherosclerotic plaque was assessed by 3 distinct methods: 1) presence or absence of moderate or severe plaque; 2) coronary artery plaque burden as categorized by a modified Duke CAD index; and 3) simple clinical scores grading plaque extent and distribution. During a 15 month follow-up period, CCTA predictors of death included proximal left anterior descending artery (LAD) stenosis and number of vessels with ≥50% and ≥70% stenosis (all p <0.0001). Furthermore, survival worsened with higher-risk Duke scores, ranging from 96% survival for 1 stenosis ≥70% or 2 stenoses 50% (p <0.013) to 85% survival for ≥50% left main (LM) artery stenosis (p <0.0001). In addition, clinical scores measuring plaque burden and distribution predicted 5 to 6% higher absolute death rate (6.6% vs. 1.6% and 8.4% vs. 2.5%; p <0.05 for both). Importantly, a negative CCTA was noted to provide an extremely low risk for death. A recent meta-analysis of 18 studies with 16-64slice CCTA showed that the pooled annualized event rate for obstructive (any vessel with >50% luminal stenosis) versus normal CCTA was 8.8% versus 0.17% per year for major adverse cardiovascular events (MACE) (p <0.05) and 3.2 % versus 0.15% for death or myocardial infarction (MI) (p <0.05). In the same study the pooled negative likelihood ratio for MACE after normal CCTA findings was 0.008 (95% confidence interval [CI]: 0.0004 to 0.17, p <0.001), and the positive likelihood ratio was 1.70 (95%CI: 0.31 to 0.52, p <0.001) (8).
Although numerous other studies have examined the potential for prognostication using CCTA results, most have been limited to small sample sizes in single-center studies. The Coronary CT Angiography EvaluatioN For clinical Outcomes: An InterRnational Multicenter Registry, or CONFIRM, study was subsequently established as a large, prospective, multinational dynamic observational cohort study of patients undergoing 64-slice CCTA. At present, the CONFIRM registry is comprised of 2 phases. Phase I CONFIRM is a derivation cohort that details demographic, clinical and CT findings for 27,125 consecutive patients (≥18 years of age), who were enrolled at 12 sites in 6 countries (United States, Canada, Germany, Switzerland, Italy, South Korea) between 2005 and 2009. These patients were followed for 2.3 ± 1.1 years for a primary endpoint of all-cause death. Phase II CONFIRM is a distinct non-overlapping validation cohort, detailing identical elements to Phase I and with event follow-up for 4,682 patients at 6 sites in 4 countries (United States, Canada, Austria, South Korea)(9). The CONFIRM sites were chosen on the basis of adequate volume, proficiency at performing and interpreting CCTA, diverse patient populations and a mix of private and academic facilities in order to ensure a broad-based sample of patients. The CONFIRM cohorts included symptomatic subjects with suspected but without known CAD, symptomatic subjects with known CAD, and asymptomatic patients. The patients were followed up after CCTA to identify adverse CAD events including all-cause death, myocardial infarction, unstable angina, target vessel revascularization, and CAD-related hospitalization. There are multiple ongoing studies using this dynamic registry, which may lead to significant changes in clinical practice. This articles summarizes some of the current key findings from the CONFIRM registry.
1. Clinical risk scoring prior to imaging
1a. Pretest likelihood of angiographically significant CAD by the invasive angiography-based guideline probabilities overestimates the actual prevalence of CAD
Guidelines for management of patients with suspected CAD rely on the pretest probabilities of angiographically significant CAD, which recognizes age, sex and angina typicality as chief predictors. These guidelines were originally created by vital reports from Coronary Artery Surgery Study (CASS) Registry (10), Diamond and Forrester (11), and the Pyror et al. method (12, 13). Importantly, the prevalence of angiographically significant CAD in these studies was derived from patients who were referred for invasive coronary angiography, based on symptoms or other clinical indications. Whether these estimates are accurate in a contemporary setting amongst individuals referred for non-invasive angiography had not been well studied to date.
Cheng et al. identified 14,048 consecutive patients from the CONFIRM registry with suspected CAD who underwent CCTA, and examined the reliability of the guidelines probabilities in patients who were referred to CCTA (14). The observed prevalence of obstructive CAD by CCTA was compared to the expected prevalence of CAD calculated based on the table of probabilities in 2002 American College of Cardiology / American Heart Association clinical practice guideline for management of patient with stable angina.
The observed prevalence of ≥50% and ≥70% coronary stenosis were substantially lower than those predicted by guideline probabilities in all patients, including those with atypical angina (15% versus 47% for ≥50% coronary stenosis, 7% versus 37% for ≥70% coronary stenosis) and typical angina (29% versus 86% for ≥50% coronary stenosis, 19% versus 71% for ≥70% coronary stenosis)(Figure1). The marked overestimation of disease prevalence by the standard probability methods was found at all participating centers and across all sex and age subgroups. Additionally, the prevalence of CAD was noted to be more severely overestimated in women, in whom the estimate prevalence of ≥50% stenosis and prevalence shown by CTA was 41% vs. 13%, respectively (p <0.001). Furthermore, the estimate for ≥70% stenosis was 26%, while the observed prevalence was only 6% (p <0.001). Nevertheless, while overestimating the stenosis severity, symptoms were significantly associated with the presence of obstructive coronary stenosis. Typical angina was associated with the highest prevalence of ≥50% coronary stenosis (40% in men, 19% in women) and ≥70% coronary stenosis (27% in men, 11% in women) compared with other symptom categories (p <0.001 for all).
Figure 1.
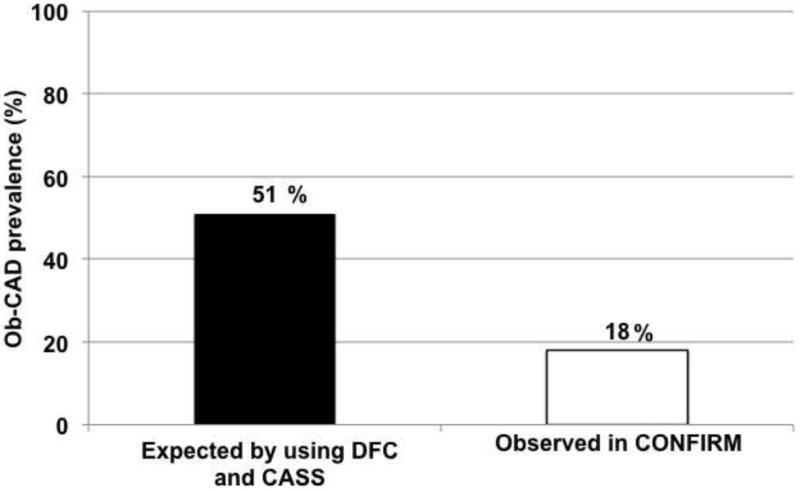
Overall Obstructive CAD prevalence in patients with Non Angina, Atypical Angina, and Typical Angina. Observed prevalence (yellow bars) was lower than expected prevalence (red bars) for angiographically confirmed ≥50% stenotic coronary artery disease. (18% versus 51%; p <0.001). Ob-CAD = obstructive coronary artery disease, NonAng = Nonanginal chest pain, AtypAng = Atypical chest pain, TypAng = Typical Angina, DFC = Diamond and Forrester classification, CASS = Coronary artery surgery study
The explanation for these findings regarding the difference between predicted and observed prevalences of obstructive coronary stenosis are likely multifactorial, but may be due to the formation of guidelines based on patients in historical studies in individuals who were referred for invasive rather than non-invasive angiography, and therefore may overestimate the prevalence of CAD compared with the population referred for CCTA. In this regard, the results of this study may serve as a contemporary update for pretest probability estimates of CAD in populations referred for non-invasive imaging such as CONFIRM, and may be useful for identifying a significant proportion of low- or intermediate-likelihood patients in whom additional testing may not be necessary.
2. Prognostic value of CCTA in patients without known CAD
2a. Symptomatic patients at intermediate pretest risk with calcium 0
Prior studies have demonstrated that a non-negligible proportion of symptomatic patients presenting with chest pain and CAC scores of 0 do, in fact, possess obstructive CAD; with this findings especially noted among patients with a high pre-test risk of obstructive CAD (15-18). However, the prevalence of obstructive CAD among patients with CAC scores of 0, who have a lower pre-test risk of CAD, had not been previously examined. Additionally, the prognostic importance of obstructive CAD among patients with a CAC score of 0 and the incremental prognostic value of CAC scoring over CCTA had previously remained unclear. Villines and colleagues focused on the prognostic value of CCTA in symptomatic patients with CAC scores of 0(19). They identified 10,037 symptomatic patients without known CAD, who underwent concomitant CCTA and CAC scoring from CONFIRM registry, and assessed the prevalence of obstructive CAD with CAC score of 0 (Figure 2). Importantly, among patients with CAC score of 0, 84% had no CAD, but 13% had non-obstructive CAD and 3.5% had obstructive CAD defined as ≥ 50% stenosis. In addition, they noted that a CAC score > 0 had a high sensitivity and positive predictive value for identifying obstructive CAD, 89% and 96% respectively, while the overall specificity and negative predictive value were not high, 59% and 29% respectively. In a multivariate analysis for prediction of obstructive CAD among symptomatic patients without CAC, family history of CAD and smoking were found to be the strongest predictors.
Figure 2.
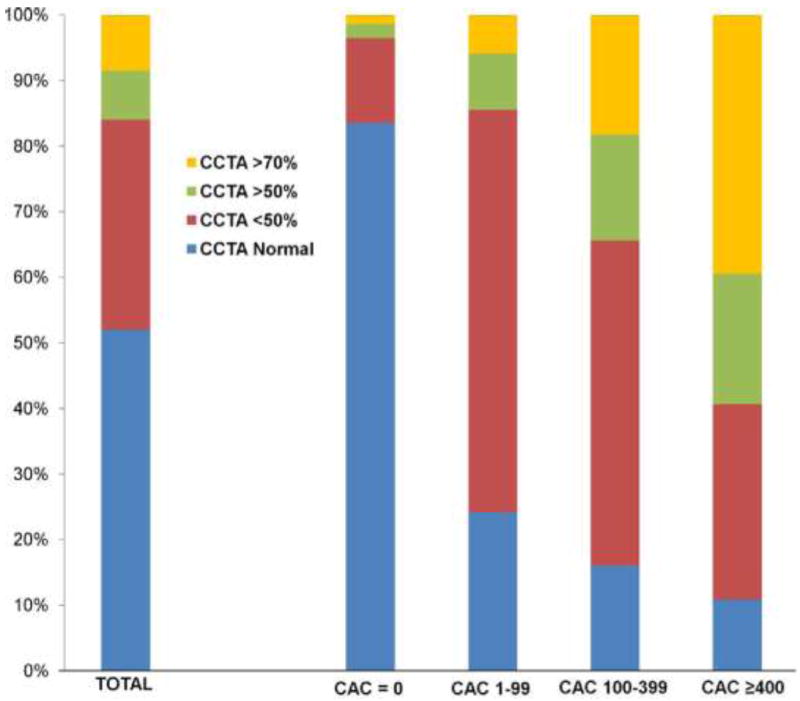
Coronary Artery stenosis on CCTA. Increasing frequency of obstructive disease with increasing coronary artery calcification (CAC) (Agaston) scores is demonstrated. *p <0.001 between groups. CCTA = coronary computed tomography angiography.
Interestingly, the results of this study demonstrated that there was no significant differences in all cause mortality irrespective of obstructive CAD among patients with CAC score 0 (Figure 3). However, among those with CAC score of zero, patients with obstructive CAD experienced higher rates (3.9%) of major adverse events—inclusive of all-mortality, nonfatal MI, or coronary revascularization—compared to patients (0.8%) with no CAD or non-obstructive CAD (p <0.001). The investigators explained these findings by pointing out that the majority of patients with CAC scores of 0 and obstructive CAD possessed single-vessel disease and that coronary revascularization in this setting has never been demonstrated to improve survival. Finally, they also noted that based on Receiver-Operator Characteristics (ROC) curves, CAC score in symptomatic patients did not add incremental prognostic information for predicting major adverse events when compared with CAD extent on CCTA (CCTA area under the curve = 0.825; CAC+CCTA area under the curve = 0.826; p=0.84) (Figure 4).
Figure 3.
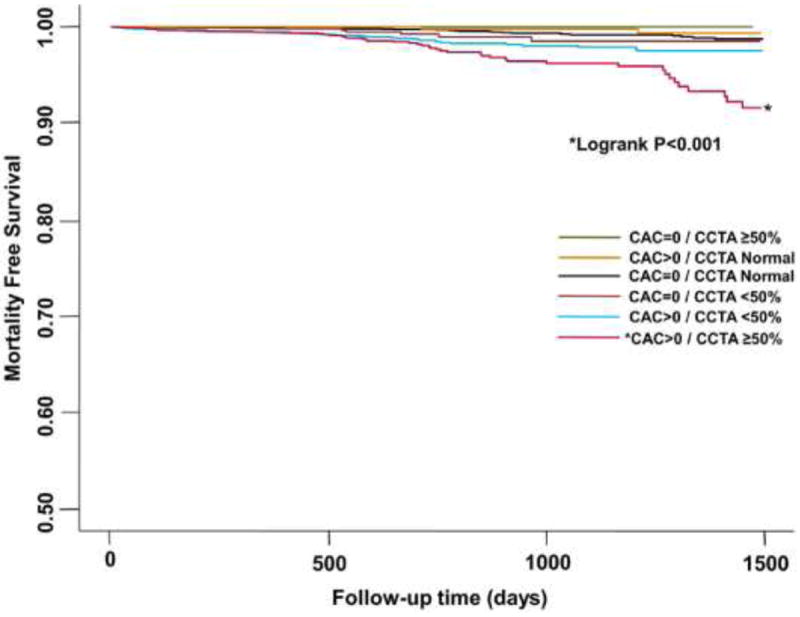
Survival According to CAC score and Stenosis Severity. All-cause mortality-free survival among patients according to CAC score and the severity of coronary artery disease on coronary CTA.
Figure 4.
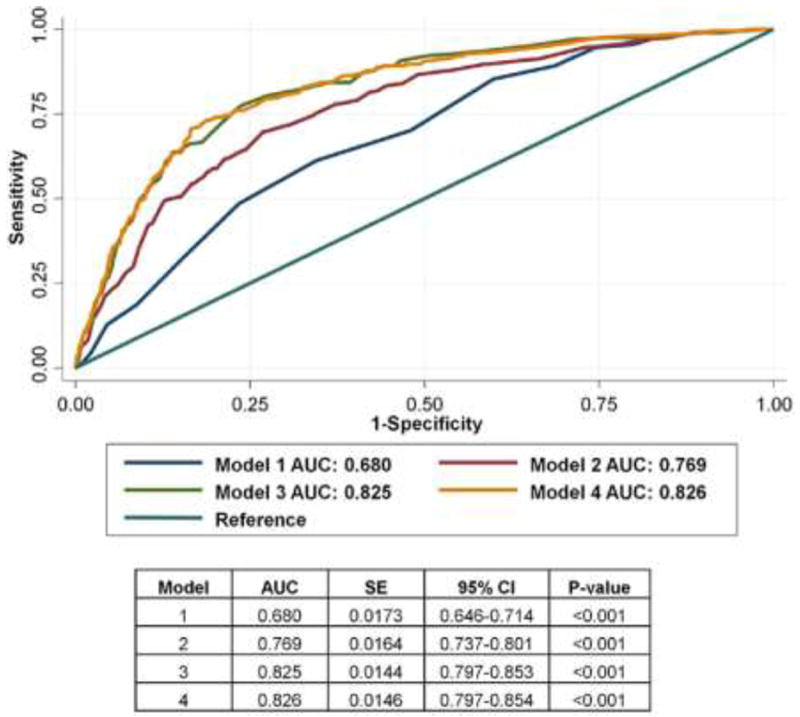
Receiver-Operator Characteristic Curves: Major Adverse Events. Receiver-operator characteristic curves of 4 models for predicting composite major adverse events in 8,907 patients over a median of 2.1 years. CAC score categories were defined as: 0, ≤100, 101 to 400, and >400. Model 2 (Morise score + CAC category) compared to Model 1 (Morise score only): p <0.001. Model 3 (Morise score + number vessels ≥50% stenosis) compared to Model 2 (Morise score + CAC category): p <0.001. Model 4 (Morise score + CAC category + number vessels ≥50% stenosis) compared to Model 2 (Morise score + CAC category): p <0.001. Model 3 compared to Model 4: p = 0.84. AUC = area under the curve; CAC = coronary artery calcification; CI = confidence interval.
2b. CAD severity for prediction of all cause death in patients without known CAD: Relation to Age and Gender
The high diagnostic performance of CCTA for CAD detection and exclusion has been previously examined (2-4). Min et al. examined the all-cause mortality in relation to CAD severity in 24,775 patients undergoing ≥ 64-detector row CCTA without known CAD using the CONFIRM registry (20). In risk-adjusted analysis, both per-patient obstructive (>50% stenosis) (hazard ratio [HR]: 2.60; 95% confidence interval [CI]: 1.94 to 3.49; p <0.0001) and non-obstructive (HR: 1.60; 95%CI: 1.18 to 2.16; p=0.002) CAD conferred increased risk of mortality compared with patients without evidence of CAD. This data highlights the clinical importance of non-obstructive coronary artery disease—which comprises a significant number of patients for whom functional testing might be expected to be negative—and its strong relationship with all-cause mortality.
The investigators also noted a dose-response relationship to the number of coronary vessels exhibiting obstructive CAD and all-cause mortality, with increasing risk observed for non-obstructive (HR: 1.62; 95% CI: 1.20 to 2.19; p = 0.002), obstructive 1-vessel (HR: 2.00; 95% CI: 1.43 to 2.82; p <0.0001), 2-vessel (HR: 2.92; 95% CI: 2.00 to 4.25; p <0.0001), or 3-vessel or left main (HR: 3.70; 95% CI: 2.58 to 5.29; p <0.0001) CAD (Figure 5). Importantly, the absence of CAD was associated with a very low annualized death rate of 0.28%.
Figure 5.
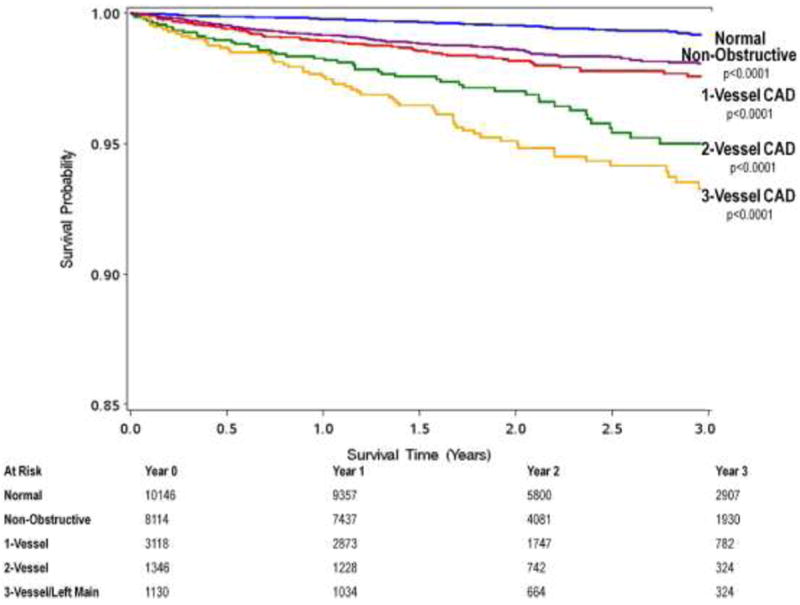
Unadjusted All-cause 3-Year Kaplan-Meier Survival by the Presence, Extent and Severity of CAD by CCTA. There is a dose response relationship of mortality to increasing numbers of vessels with obstructive coronary artery disease. CAD = coronary artery disease, CCTA = coronary computed tomography angiography.
Furthermore, due to the size of this study, the investigators were able to explore gender- and age-related differences in CAD-related outcomes. Adjusted hazard ratios for the prediction of all-cause mortality for non-obstructive CAD and 3-vessel/left main disease for females were higher than for males (HR: 4.21; 95% CI: 2.47 to 7.18; p <0.0001 vs. HR: 3.27; 95% CI: 1.96 to 5.45; p <0.0001). Moreover, when stratified by age into <65 years versus ≥65 years, younger subjects experienced higher hazards ratios for death for 2-vessel (HR: 4.00; 95%CI: 2.16 to 7.40; p <0.001, HR: 2.46; 95%CI: 1.51 to 4.02; p=0.0003) or 3-vessel (HR: 6.19; 95%CI: 3.43 to 11.2; p <0.0001, HR: 3.10; 95%CI: 1.95 to 4.92; p <0.0001) obstructive CAD when compared to older patients. Furthermore, the investigators noted that although the pretest likelihood of obstructive CAD as estimated by the Diamond Forrester tabular (11, 21) methods was highly predictive of obstructive CAD by CCTA in this study, it was not predictive for all-cause mortality. These suggest that a revision in the way we assess stable symptomatic patients with suspected CAD may be needed, and the CONFIRM investigators are currently attempting to improve these methods.
Currently, the CONFIRM investigators are also investigating the prognostic value of CCTA findings for major adverse cardiac events including but not limited to all-cause death, such as non-fatal myocardial infarction and late target vessel revascularization, in patients without known CAD (22, 23).
2c. Studies of Prognostic value of CCTA findings in selected population
Current American Diabetes Association guidelines endorse the cardiovascular disease prevention for patients with diabetes mellitus (DM) based on an observational and population-based studies demonstrating excess cardiovascular risk and death attributable to diabetes (24). However, prior data examining the incremental risk of DM for the future adverse CAD events have generally lacked information regarding CAD prevalence and severity based on CCTA findings (25-28). Rana et al identified 23,643 consecutive individuals undergoing ≥ 64-detector row CCTA without known CAD from CONFIRM registry (Diabetes Care 2012 in press). In this study, 3,370 individuals with DM who were then propensity-matched in 1:2 fashion to 6,740 unique non-DM individuals. The presence of CAD on CCTA was graded as none, non-obstructive (1-49% stenosis), or obstructive (≥50% stenosis). DM individuals possessed higher frequencies of obstructive CAD (37% vs. 27%, p <0.0001) and lower frequencies of normal arteries (28% vs. 36%, p <0.0001) compared to non-DM individuals. In addition, CAD extent was higher for DM versus non-DM individuals for obstructive 1-vessel disease [VD] (19% vs. 14%, p<0.0001), 2-VD (9% vs. 7%, p<0.0001) and 3-VD (9% vs. 5%, p <0.0001) with higher per-segment stenosis in the proximal and mid segments of every coronary artery (p <0.001 for all). Furthermore, the risk of mortality for DM individuals was higher than those non-DM individuals for those with no CAD, (hazard ratio [HR]: 3.64; 95%CI: 1.67 to 7.92; p=0.001), non-obstructive CAD (HR: 5.23; 95%CI: 2.55 to 10.7; p <0.0001), 1-VD (HR: 6.39; 95%CI: 2.98 to 13.7; p <0.0001), 2-VD (HR 12.33: 95%CI: 5.62 to 27.1; p <0.0001) and 3-VD (HR: 13.25; 95%CI: 6.15 to 28.6; p <0.0001) (Figure6). This data demonstrated that the risk of mortality was more than 5-fold higher for DM individuals with non-obstructive CAD and almost 10-fold higher for those with obstructive CAD compared to non-DM individuals. Furthermore, when CAD extent and severity was stratified on a per-vessel analysis as no CAD, nonobstructive CAD, obstructive1-VD, obstructive 2-VD or obstructive 3-VD, the risk of mortality associated with the presence of DM, resulted in a step up in the CAD risk category, as compared to those with non-DM individuals. The CONFIRM investigators are also currently evaluating the mortality risk for DM and non-DM individuals enrolled into CONFIRM with a longer follow-up period.
Figure 6.
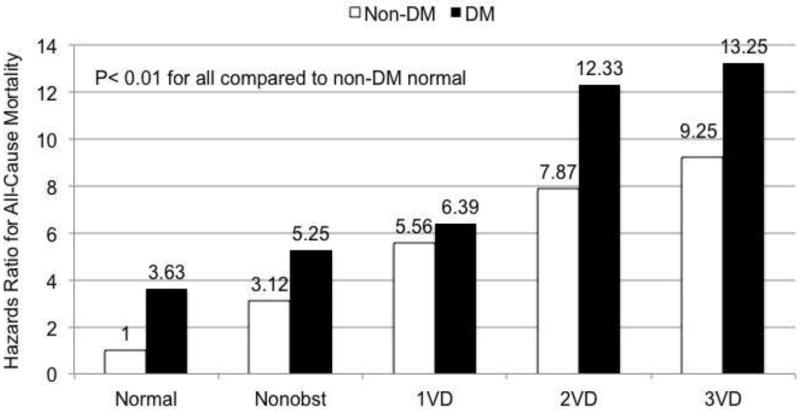
Multivariable risk-adjusted hazards ratio for coronary artery disease and severity on a per-vessel basis for matched individuals with and without diabetes. Non-DM = non-diabetes mellitus; DM = diabetes mellitus; Non obst = Non-obstructive CAD <50% maximal per-patient stenosis; 1VD = 1-vessel obstructive CAD >50% stenosis; 2VD = 2-vessel obstructive CAD >50% stenosis; 3VD = 3-vessel obstructive CAD >50% stenosis.
There are currently numerous ongoing studies in development that are examining the differential prognostic value of CCTA findings based on gender (29), body mass index (Labounty et al), ethnicity (30), chronic kidney disease (Chow et al), family history of CAD (31), smoking (Cheng et al and Labounty et al), and hypertension (32).
2d. Prognostic value of CAD severity for prediction of mortality risk: Relation to left ventricular ejection fraction
Previously, the examination of the prognostic value of CAD severity and left ventricular ejection fraction (LVEF) by CCTA was limited to single center studies(33). Chow et al. examined the prognostic value of CAD severity, LVEF, and clinical variables for predicting all-cause mortality in 14,064 patients without known CAD who underwent ≥ 64-slice CCTA(34). The National Cholesterol Education Program-Adult Treatment Panel III risk was calculated as a summary measure of clinical characteristics denoting risk. A multivariate Cox model for predicting all-cause mortality revealed that abnormal LVEF (hazard ratio [HR]: 2.74; 95%CI: 2.12 to 3.51)—defined as <50%--and CAD severity (HR: 1.58; 95% CI: 1.42 to 1.76) were independent predictors after adjusting for clinical characteristics. Furthermore, Chow also assessed the improvement of reclassification based on CAD severity and LVEF using net reclassification improvement (NRI) method(35). Patient reclassification was significantly improved when LVEF was added to clinical variables alone (NRI: 22.5%; p <0.001) and when CAD severity was added to the model of clinical variable and LVEF (NRI: 17.8%; p <0.001). At present, CONFIRM investigators are examining whether not only abnormal versus normal LVEF is useful for enhancing risk stratification, but also whether the degree of left ventricular dysfunction by CCTA improves risk stratification for all-cause mortality(36).
3. Prognostic value of CCTA in patients with known CAD
3a. Prognostic value of CCTA in CABG patients
The utility of CCTA for risk stratification in coronary artery bypass graft (CABG) patients has not been fully examined. Small et al. identified 657 CABG patients who had undergone CCTA and assessed the prognostic value of unprotected coronary territory (UCT) or a summary of native vessel disease and graft patency: the coronary artery protection score (CAPS)(37). The investigators demonstrated that LVEF, creatinine, age, severity of native vessels disease were all univariate independent predictors for all-cause mortality (p <0.001). In multivariate analysis, adjusted for the EuroSCORE, both UCT (p = 0.004) and CAPS (p <0.001) were found to be predictors for all-cause mortality.
3b. Ongoing studies of Prognostic value of CCTA findings in patients with known CAD
Currently, CONFIRM investigators are also examining the prognostic value of CCTA findings assessing the presence of obstructive CAD, plaque burden and plaque distribution for predicting all-cause mortality or non-fatal myocardial infarction in patients with prior myocardial infarction or prior percutaneous coronary intervention, with results to be soon available.
CONCLUSION
To date, the CONFIRM observational cohort study has elicited numerous prognostic findings related to CAD detection by CCTA. Based on its multisite international approach—and ensuant large sample size and high statistical power—the results of the CONFIRM study may considered widely generalizable, and will be useful to address many further prognostic questions related to CAD in the future.
Acknowledgments
Dr. Min received modest speakers’ bureau and medical advisory board compensation and significant research support from GE Healthcare. Dr. Achenbach received grant support from Siemens and Bayer Schering Pharma and has served as a consultant for Servier. Dr. Al-Mallah received support from the American Heart Association, BCBS Foundation of Michigan, and Astellas. Dr. Cademartiri received grant support from GE Healthcare and has served on the Speakers’ Bureau of Bracco and as a consultant for Servier; Dr. Maffei received grant support from GE Healthcare. Dr. Chinnaiyan received grant support from Bayer Pharma and Blue Cross Blue Shield Blue Care MI. Dr. Chow received research and fellowship support from GE Healthcare, research support from Pfizer and AstraZeneca, and educational support from TeraRecon. Dr. Hausleiter received a research grant from Siemens Medical Systems. Dr. Kaufmann received institutional research support from GE Healthcare and grant support from Swiss National Science Foundation. Dr. Maffei received grant support from GE Healthcare Dr. Raff received grant support from Siemens, Blue Cross Blue Shield Blue Care MI, and Bayer Pharma.
Footnotes
Conflicts of interest: All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Mowatt G, Cook JA, Hillis GS, et al. 64-Slice Computed Tomography Angiography in the Diagnosis and Assessment of Coronary Artery Disease: Systematic Review and Meta-Analysis. Heart. 2008;94:1386–1393. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic Performance of 64-Multidetector Row Coronary Computed Tomographic Angiography for Evaluation of Coronary Artery Stenosis in Individuals Without Known Coronary Artery Disease: Results From the Prospective Multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) Trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic Performance of Coronary Angiography by 64-Row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 4.Meijboom WB, Meijs MFL, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 5.Pundziute G, Schuijf JD, Jukema JW, et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur Heart J. 2008;29:2373–2381. doi: 10.1093/eurheartj/ehn356. [DOI] [PubMed] [Google Scholar]
- 6.Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging. 2011;4:537–548. doi: 10.1016/j.jcmg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 8.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic Value of Cardiac Computed Tomography Angiography: A Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2011;57:1237–1247. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Min JK, Dunning A, Lin FY, et al. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr. 2011;5:84–92. doi: 10.1016/j.jcct.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Chaitman BR, Bourassa MG, Davis K, et al. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS) Circulation. 1981;64(2):360–367. doi: 10.1161/01.cir.64.2.360. [DOI] [PubMed] [Google Scholar]
- 11.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 12.Pryor DB, Harrell FE, Jr, Lee KL, Califf RM, Rosati RA. Estimating the likelihood of significant coronary artery disease. Am J Med. 1983;75:771–780. doi: 10.1016/0002-9343(83)90406-0. [DOI] [PubMed] [Google Scholar]
- 13.Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM) Circulation. 2011;124:2423–2432. doi: 10.1161/CIRCULATIONAHA.111.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akram K, O’Donnell RE, King S, Superko HR, Agatston A, Voros S. Influence of symptomatic status on the prevalence of obstructive coronary artery disease in patients with zero calcium score. Atherosclerosis. 2009;203:533–537. doi: 10.1016/j.atherosclerosis.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Cademartiri F, Maffei E, Palumbo A, et al. Diagnostic accuracy of computed tomography coronary angiography in patients with a zero calcium score. Eur Radiol. 2010;20:81–87. doi: 10.1007/s00330-009-1529-9. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol. 2010;55:627–634. doi: 10.1016/j.jacc.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol. 2007;99:472–475. doi: 10.1016/j.amjcard.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 19.Villines TC, Hulten EA, Shaw LJ, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol. 2011;58:2533–2540. doi: 10.1016/j.jacc.2011.10.851. [DOI] [PubMed] [Google Scholar]
- 20.Min JK, Dunning A, Lin FY, et al. Age- and Sex-Related Differences in All-Cause Mortality Risk Based on Coronary Computed Tomography Angiography Findings: Results From the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 Patients Without Known Coronary Artery Disease. Journal of the American College of Cardiology. 2011;58:849–860. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 21.Diamond GA, Forrester JS, Hirsch M, et al. Application of conditional probability analysis to the clinical diagnosis of coronary artery disease. J Clin Invest. 1980;65:1210–1221. doi: 10.1172/JCI109776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achenbach S, Berman DS, Budoff MJ, et al. Abstract 14099: Prognostic Value of Coronary CT Angiography for the Prediction of Mortality and Non-Fatal Major Adverse Cardiac Events: Results from the Multinational CONFIRM Registry. Circulation. 2011;124(21 Supplement):A14099. [Google Scholar]
- 23.Wilson SR, Lin FY, Dunning AM, et al. Abstract 14999: Prognostic Value of Plaque Composition For the Prediction of Major Adverse Cardiovascular Events in Patients Without Known Coronary Artery Disease Undergoing 64-Detector Row Coronary CT Angiography: Results from 6,335 Patients in the Prospective Multinational CONFIRM Registry (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry. Circulation. 2011;124(21 Supplement):A14999. [Google Scholar]
- 24.Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 25.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 26.Whiteley L, Padmanabhan S, Hole D, Isles C. Should diabetes be considered a coronary heart disease risk equivalent?: results from 25 years of follow-up in the Renfrew and Paisley survey. Diabetes Care. 2005;28:1588–1593. doi: 10.2337/diacare.28.7.1588. [DOI] [PubMed] [Google Scholar]
- 27.Howard BV, Best LG, Galloway JM, et al. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care. 2006;29(2):391–397. doi: 10.2337/diacare.29.02.06.dc05-1299. [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM. Diabetes and coronary risk equivalency: what does it mean? Diabetes Care. 2006;29:457–460. doi: 10.2337/diacare.29.02.06.dc05-1904. [DOI] [PubMed] [Google Scholar]
- 29.Lin F, Chinnaiyan K, Dunning AM, et al. Gender differences in all-cause death by extent and severity of coronary arteyr disease by cardiac computed tomographic angiopgraphy: a matched analysis of the CONFIRM regitstry. J Am Coll Cardiol. 2011;57(14_Suppl_S):E773. [Google Scholar]
- 30.Hulten E, Villines Todd C, Dunning Allison L, et al. Coronary Artery Disease Burden by Coronary CT Angiography Predicts Mortality and Myocardial Infarction Across Multiple Ethnicities: Results from the CONFIRM Registry. Circulation. 2011;124:A15717. [Google Scholar]
- 31.Otaki Y, LaBounty T, Dunning A, et al. Young Patients with a Family History of Coronary Artery Disease Have Higher Prevalence, Increased Severity, and Worse Prognosis of Coronary Atherosclerosis: Results from 6308 Patients in the Prospective Multinational Confirm Registry (coronary Ct Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) J Am Coll Cardiol. 2012;59(13_Suppl_S):E1328. [Google Scholar]
- 32.LaBounty TM, Achenbach S, Al-Mallah M, et al. Hypertensive Individuals Have an Increased Prevalence of Coronary Artery Disease and Risk of Adverse Events: A Comparison of 15,091 Individuals from Confirm (coronary Computed Tomographic Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) J Am Coll Cardiol. 2012;59(13_Suppl_S):E1371. [Google Scholar]
- 33.Chow BJW, Wells GA, Chen L, et al. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol. 2010;55:1017–1028. doi: 10.1016/j.jacc.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Chow BJW, Small G, Yam Y, et al. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary computed tomography angiography evaluation for clinical outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging. 2011;4:463–472. doi: 10.1161/CIRCIMAGING.111.964155. [DOI] [PubMed] [Google Scholar]
- 35.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 36.Arsanjani R, LaBounty T, Gransar H, et al. Degree of left ventricular systolic dyscfunction by cardiac computed tomographic angiography improves risk stratification and discrimination of patients at risk for incident mortality: Results from 7907 patients in the prospective multicenter international CONFIRM study. J Am Coll Cardiol. 2012;59(13_Suppl_S):E1329. [Google Scholar]
- 37.Small GR, Yam Y, Chen L, et al. Prognostic Assessment of Coronary Artery Bypass Patients With 64-Slice Computed Tomography Angiography: Anatomical Information Is Incremental to Clinical Risk Prediction. Journal of the American College of Cardiology. 2011;58:2389–2395. doi: 10.1016/j.jacc.2011.08.047. [DOI] [PubMed] [Google Scholar]


