Abstract
6-[18F]-Fluoro-L-dopa (FDOPA) has been widely used as a biomarker for catecholamine synthesis, storage, and metabolism—its intense uptake in the striatum, and fainter uptake in other brain regions, is correlated with the symptoms and pathophysiology of Parkinson's disease (PD). 6-[18F]fluoro-m-tyrosine (FMT), which also targets L-amino acid decarboxylase, has potential advantages over FDOPA as a radiotracer because it does not form catechol-O-methyltransferase (COMT) metabolites. The purpose of the present study was to compare the regional distribution of these radiotracers in the brains of PD patients. 15 Parkinson's patients were studied with FMT and FDOPA positron emission tomography (PET) as well as high-resolution structural magnetic resonance imaging (MRI). MRI's were automatically parcellated into neuroanatomical regions of interest (ROIs) in Freesurfer (http://surfer.nmr.mgh.harvard.edu); region-specific uptake rate constants (Kocc) were generated from coregistered PET using a Patlak graphical approach. The essential findings were as follows: (1) regional Kocc were highly correlated between the radiotracers and in agreement with a previous FDOPA studies that used different ROI selection techniques; (2) FMT Kocc were higher in extrastriatal regions of relatively large uptake such as amygdala, pallidum, brainstem, hippocampus, entorhinal cortex, and thalamus, whereas cortical Kocc were similar between radiotracers; (3) while subcortical uptake of both radiotracers was related to disease duration and severity, cortical uptake was not. These results suggest that FMT may have advantages for examining pathologic changes within allocortical loop structures, which may contribute to cognitive and emotional symptoms of PD.
Keywords: Adult, Aged, Humans, Brain Mapping, Cerebral Cortex/metabolism/radionuclide imaging, Dopamine Agents/*diagnostic use/pharmacokinetics, Dihydroxyphenylalanine/*analogs & derivatives/drug effects/pharmacokinetics, Tyrosine/*analogs & derivatives/diagnostic use, Tomography, Parkinson Disease/*physiopathology/*radionuclide imaging, Research Support, U.S. Gov't, P.H.S.
Introduction
6-[18F]-Fluoro-L-dopa (FDOPA) is a radiopharmaceutical that follows the metabolic pathway of L-dopa (Brown et al., 1999b). In catecholaminergic neurons, FDOPA is decarboxylated by aromatic L-amino acid decarboxylase (AAAD) to fluorodopamine, which is taken up into synaptic vesicles by vesicular monoamine transporter type 2 (VMAT2) and cleared from the synaptic cleft by dopamine transporters (DAT; Endres et al., 1997). Specific uptake of FDOPA in the striatum as measured by positron emission tomography (PET) has been correlated with pathophysiology, duration, and functional status in Parkinson's disease (Nurmi et al., 2001). In addition to motor symptoms, PD patients suffer from cognitive and emotional symptoms that are not fully explained by nigrostriatal pathology. The ventral tegmental area (VTA) is relatively preserved in PD and contributes dopaminergic projections to many brain regions outside the striatum; several studies have related this extrastriatal uptake to non-motor symptoms (Bruck et al., 2005; Rinne et al., 2000).
Quantitation of FDOPA uptake is limited by the formation of catechol-O-methyltransferase (COMT) metabolites that decrease signal to background (DeJesus, 2003). For extrastriatal tissue, this is especially problematic, as specific uptake in many of these regions may be too low to be accurately measured by PET (Brown et al., 1999b). 6-[18F]fluorom-tyrosine (FMT), which also targets AAAD, has characteristics that potentially increase its sensitivity as a radiotracer; it has a 10-fold greater affinity for AAAD and is not a substrate for COMT. FMT and its metabolites also have poor affinity for DAT and VMAT2 (DeJesus, 2003).
Few studies have compared FMT to FDOPA as tools to study extrastriatal uptake; furthermore, many studies of extrastriatal FDOPA uptake have been done using normalized brain images. Since these studies were done, Freesurfer (http://surfer.nmr.mgh.harvard.edu) has been developed as a reliable tool for standard magnetic resonance imaging (MRI)-based cortical parcellation in native space. The goals of the present study were to compare the uptake profiles of FMT and FDOPA within Freesurfer-defined regions of interest (ROIs), to rank regional uptake values in comparison to previous studies, and to relate regional uptake to disease duration and severity of clinical symptoms.
Materials and Methods
15 subjects (mean age 60.3 years; SD, 6 years; 12 men) with Hoehn and Yahr stage 1-3 Idiopathic Parkinson Disease (PD) by UK brain bank criteria (Gibb and Lees, 1988), normal liver function, and absence of other major disease, were recruited from local movement disorders clinics. Of these subjects, 5 were taking monoamine oxidase inhibitors (MAOIs), 7 were taking dopamine agonists (pramipexole or ropinerole), and three were taking carbidopa/levodopa (750-1200 mg of levodopa daily).
Procedures included brain FDOPA and FMT PET imaging, magnetic resonance imaging (MRI), and Unified Parkinson Disease Rating Scale (UPDRS; Fahn et al., 1987) scoring by a movement disorders specialist (CG). Subjects were off anti-Parkinson medication for 18 hours prior to PET and UPDRS scoring. The local institutional review board approved the protocol, and informed consent was obtained from all participants.
FDOPA and FMT were synthesized by electrophilic fluorination of the appropriate stannylated precursors [for FMT, N-(trifluoroacetyl)-3-acetoxy-6-(trimethylstannyl)-lphenylalanine ethyl ester (ABX, Radeberg, Germany)] followed by flash chromatography over alumina, hydrolysis in HBr, separation by semi-prep HPLC and final workup to radiopharmaceutical purity (Namavari et al., 1993; Nickles et al., 1984).
Subjects were pretreated with carbidopa, 2.5 mg/kg orally prior to each PET scan, and 200 mg tolcapone (a COMT inhibitor) prior to the FDOPA scan. These pretreatment doses were given a mean of 59 (range 47-75, SD 9) minutes before the FDOPA scan and 65 (range 50-74, SD 7) minutes before the FMT scan. Since tolcapone has in rare cases been associated with liver damage (Keating and Lyseng-Williamson, 2005), serum AST, an index of liver function, was acquired at study enrollment and 2-4 weeks after the tolcapone dose.
PET images were obtained on a Siemens ECAT EXACT HR+ PET scanner with an axial intrinsic resolution of 4.7 mm. Following intravenous injection of 5.2 mCi ± 10% of radiopharmaceutical, 18 3D dynamic frames were acquired over 90 minutes. The mean within-subject difference in injected activity of FMT and FDOPA was 2%. The mean interval between FMT and FDOPA PET scans was 35 days.
MRI scans were obtained on a 3T SIGNA scanner using an 8-channel head coil, with higher-order shimming. A magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted volume (TR 6.6 ms, TE 2.8 ms, flip angle 8 degrees, inversion time 900 ms, field of view 260 mm, slice thickness 1.2 mm) was acquired for PET coregistration and automated parcellation into neuroanatomical ROIs. Digital communications in medicine (DICOM) single-slice images were first converted to Neuroimaging Informatics Technology Initiative (NIFTI) volumes in AFNI (http://afni.nimh.nih.gov) and then automatically segmented in Freesurfer 4.5 (surfer.nmr.mgh.harvard.edu) to yield cortical and subcortical surfaces, which were visually inspected for errors and corrected as necessary.
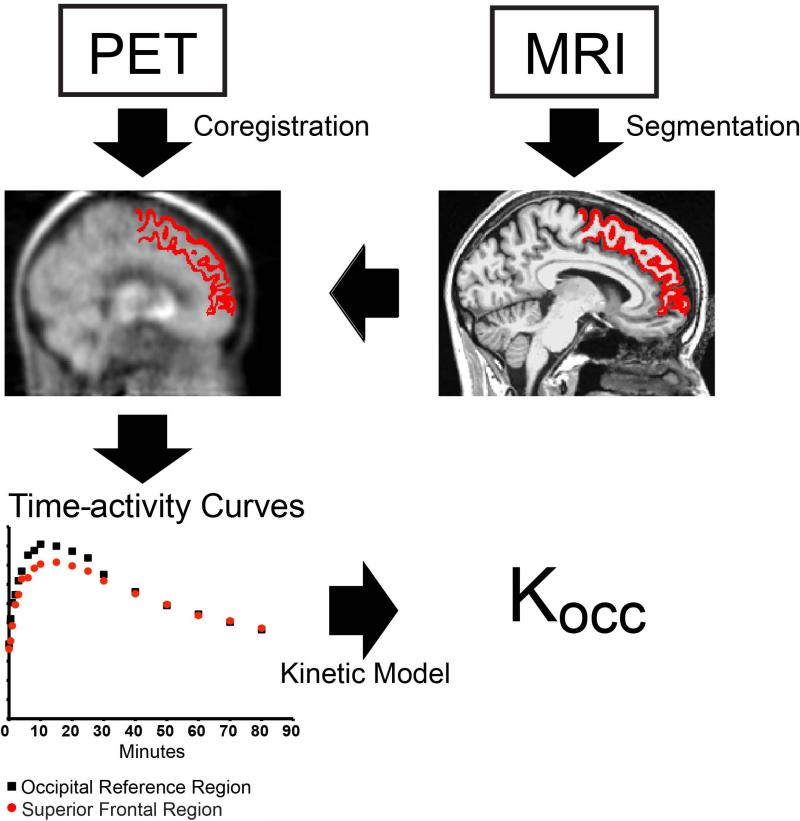
For PET image analysis, FDOPA and FMT PET sinograms were reconstructed by filtered back-projection into 18 volumes (time frames), each containing 128×128×63, 1.84×1.84×2.43 mm voxels, corrected for scatter, attenuation, and F-18 decay. Each frame was realigned, within-subject, to the total sum image of the FDOPA study. The FDOPA sum image was then coregistered to the T1-weighted MRI scan using a 12-degree affine transformation in SPM8 (www.fil.ion.ucl.ac.uk/spm). This transformation was then applied to each of the FDOPA and FMT volumes, resampling them to the 1×1×1mm FreeSurfer resolution using a 7th degree B-Spline interpolation. Mean time-activity curves (TAC) were extracted from the coregistered PET time series for each Freesurfer ROI as defined by the Desikan-Killany (2006) cortical and Fischl (2002) subcortical labeling systems. Occipital cortex TAC's were derived from hand-drawn volumes of 11,378 (SD 2284) mm3. These Freesurfer-defined ROI and occipital reference tissue TAC's were used to derive a normalized tissue uptake rate constant (Kocc) using Patlak analysis (Patlak and Blasberg, 1985). An overview of the method is shown in Figure 1. Since uptake within right and left ROIs was similar, Kocc were averaged across hemispheres.
Figure 1. Schematic representation of methods.
High-resolution T1-weighted MRI (upper right) was parcellated in Freesurfer 4.5 (middle right). 4D PET were coregistered within-subject to the MRI (middle left) and mean time-activity curve (TAC) extracted within each MRI-defined region (bottom left). The tissue-derived uptake rate constant (Kocc) was generated using Patlak analysis from the TAC for each region and that of the occipital reference region (bottom left). The region shown as an example (middle left and right) is the superior frontal cortex.
Statistical analyses of the ROI Kocc values were conducted in SPSS (Version 21, IBM, Chicago). ROIs whose Kocc did not differ from zero by one-sample t test (p<0.05) were removed from further analysis. These regions of very low uptake were the frontal pole; rostral and caudal middle frontal gyrus; postcentral gyrus; superior and inferior parietal cortex; cingulate isthmus; precuneus and cuneus; pericalcarine cortex; and lingual gyrus.
The 26 retained ROIs were then ranked by Kocc and ROI/putamen ratios calculated for each ROI and radiotracer. Bivariate (Pearson) correlations were used to compare the ranks and Kocc values between FDOPA and FMT, and with anatomically similar regions reported in the literature. To explore autocorrelation in the PET dataset and to develop representative indices for comparison with clinical data, a factor analyses (by principal components) was conducted for each radiotracer on the 26 ROI Kocc values. Based on these PCA analyses and related correlation matrix, we generated an index of subcortical uptake by averaging highly correlated (r=0.6-0.9) Kocc values from amygdala, caudate nucleus, nucleus accumbens, putamen, pallidum, and brainstem, and a cortical index by averaging Kocc for the remaining ROIs with correlated uptake values (r=0.6-0.9). Disease duration and total UPDRS scores were then compared with these indices using partial correlations controlling for age.
Results
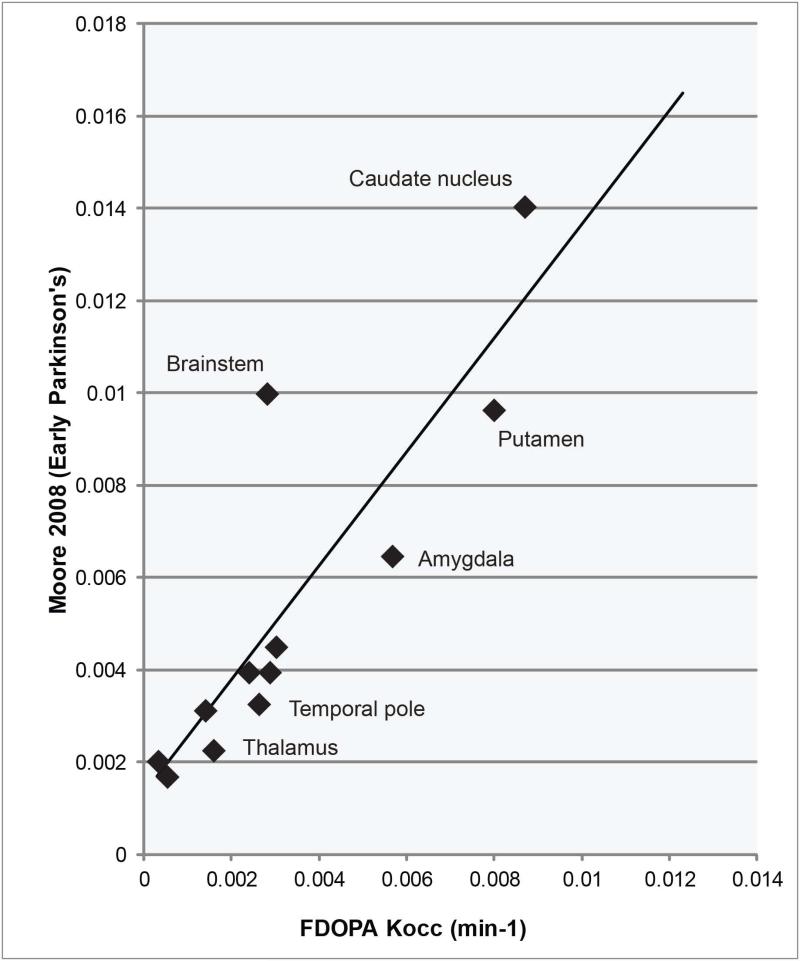
ROI's are ranked by Kocc in Figure 2. Both Kocc values and rankings were highly correlated between radiotracers (r = 0.98 and 0.90, respectively), with the exception of thalamic and brainstem uptake, which was higher for FMT than FDOPA. Regional uptake values were roughly proportionate to those described in previous studies, in spite of differences in region selection technique (Table 1). For example, Moore (2008) measured FDOPA Kocc within hand- refined regions analogous to 13 of the Freesurfer ROIs, deriving proportionate Kocc values (Figure 3; r = 0.87).
Figure 2. Ranking of regions by Kocc.
Regions were ranked by mean FMT Kocc (solid bars) with corresponding FDOPA Kocc.
Table 1.
Comparison of FMT and FDOPA Kocc to comparable regions in FDOPA studies
| Population: Radiotracer: | Current Study |
Moore 2003 Normal subjects FDOPA Mean Kocc (min−1) | Moore 2008 |
||
|---|---|---|---|---|---|
| Early Parkinson's FDOPA Mean Kocc (min−1) | Early Parkinson's FMT Mean Kocc (min−1) | Early Parkinson's FDOPA Mean Kocc (min−1) | Later Parkinson's FDOPA Mean Kocc (min−1) | ||
| Region | |||||
| Nucleus accumbens | 0.0123 | 0.0177 | 0.0112 | ||
| Caudate nucleus | 0.0087 | 0.0117 | 0.0128 | 0.0141 | 0.0072 |
| Putamen | 0.008 | 0.0127 | 0.0143 | 0.0095 | 0.0045 |
| Amygdala | 0.0057 | 0.0091 | 0.0049 | 0.0065 | 0.0054 |
| Pallidum | 0.0053 | 0.0091 | 0.0049 | ||
| Hippocampus | 0.003 | 0.0036 | 0.0034 | 0.0045 | 0.0038 |
| Entorhinal cortex | 0.0029 | 0.0036 | 0.0035 | 0.004 | 0.0039 |
| Brainstem | 0.0028 | 0.0056 | 0.007* | 0.01* | 0.065 |
| Temporal pole | 0.0026 | 0.0025 | 0.0031 | 0.0033 | 0.0027 |
| Anterior cingulate | 0.0024 | 0.0024 | 0.0032 | 0.004 | 0.0039 |
| Parahippocampal gyrus | 0.0016 | 0.0017 | |||
| Thalamus | 0.0016 | 0.0037 | 0.0023 | 0.0023 | 0.0023 |
| Orbitofrontal Cortex | 0.0014 | 0.0013 | 0.0029 | 0.0031 | 0.0027 |
| Superior Temporal | 0.0009 | 0.001 | 0.0015 | ||
| Fusiform gyrus | 0.0009 | 0.0008 | 0.0024 | ||
| Inferior Temporal | 0.0009 | 0.0006 | |||
| Middle Temporal | 0.0008 | 0.0005 | |||
| Pars triangularis | 0.0006 | 0.0006 | 0.0012 | ||
| Posterior cingulate | 0.0005 | 0.001 | 0.0028 | ||
| Pars opercularis | 0.0003 | 0.0007 | |||
| Superior frontal | 0.0005 | 0.0005 | 0.0017** | 0.0015** | |
| Pars orbitalis | 0.0004 | 0.0006 | |||
| Paracentral | 0.0003 | 0.0008 | |||
| Motor cortex | 0.0003 | 0.0007 | <0.002 | ||
| Medial Frontal | not different from 0 | not different from 0 | 0.0025 | 0.002 | |
Midbrain raphe.
Dorsolateral prefrontal
Figure 3. Studies comparison.
FDOPA Kocc values were correlated with those reported in previous investigations that used different methods of region selection. “Brainstem” Kocc is expected to differ because Moore et al selected the dorsal raphe, a region of high uptake, whereas the Freesurfer region encompasses the entire brainstem.
While subcortical uptake was higher for FMT, cortical Kocc was similar between radiotracers, yielding higher ROI/putamen ratios for FDOPA. For cortical ROIs, mean ROI/putamen Kocc ratio was 0.18 (SD 0.21) for FDOPA and 0.12 (SD 0.12) for FMT. FMT Kocc/FDOPA Kocc was greater than 1 in subcortical ROIs such as amygdala, thalamus, and brainstem, as well as anterior and posterior cingulate cortex, precentral gyrus, entorhinal cortex, and hippocampus; this ratio was less than or equal to 1 for the remainder of cortical ROIs.
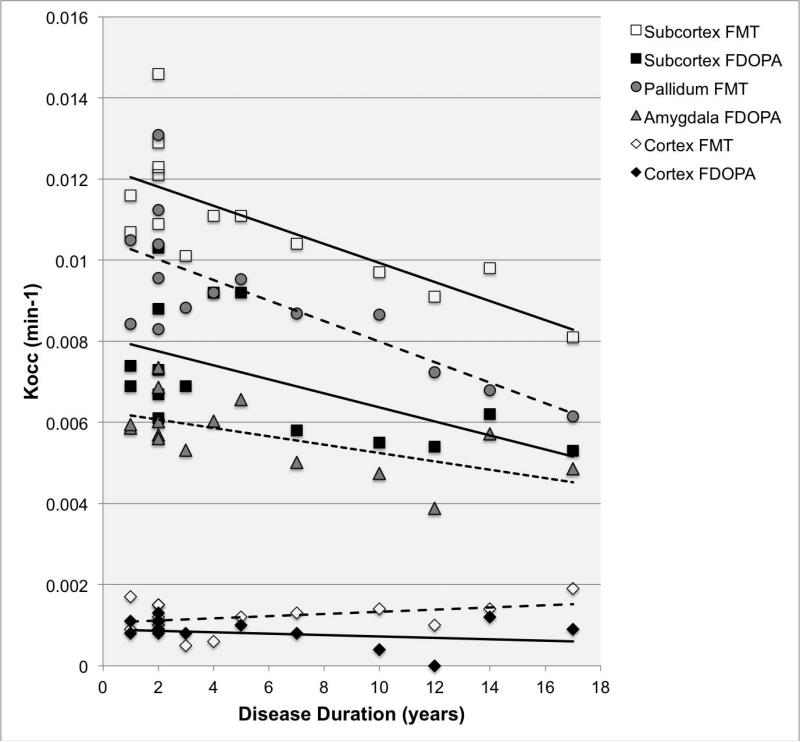
The exploratory factor analysis of 26 regional Kocc values yielded a principal component that was correlated with cerebral cortical and hippocampal uptake (r= 0.65-0.88), and explained 40-42% of the variance, a subcortical component correlated with nucleus accumbens, caudate nucleus, putamen, amygdala, pallidum, and brainstem uptake (r= 0.41-0.85) that explained 20-22% of the variance, and minor components. Based on these analyses, cortical and subcortical indices were generated (see methods). Partial correlations, controlling for age, showed that the subcortical index declined with disease duration (FMT partial correlation coefficient, pr= - 0.86; FDOPA pr= - 0.70; 2-tailed P<0.01), but that the cortical index did not (Figure 4). Kocc within individual extrastriatal, sub-cortical structures such as the amygdala (FDOPA pr= - 0.69; 2-tailed P<0.01) and pallidum (FMT pr= - 0.90; 2-tailed P<0.001) were also related to disease duration. UPDRS total scores were inversely correlated with the subcortical index (FMT pr= -0.63; FDOPA pr= - 0.57; 2-tailed P<0.05), but not with the cortical index.
Figure 4. Kocc versus duration disease.
Mean Kocc as a function of disease duration for cortical versus subcortical structures; cortical and subcortical indices were derived by averaging Kocc within the relevant regions.
Discussion
This is one of the first human studies to compare extrastriatal uptake between FMT and FDOPA. As was seen in a similar study of three non-human primates (Brown et al., 1999a), regional uptake rate constants were higher for FMT in regions of high/specific uptake, but similar or indistinguishable between tracers in regions of low uptake. Unfortunately the study design, which did not include control subjects, precludes comment on the relative sensitivity of the two tracers. Regional uptake values and ranks were highly correlated between the radiotracers and largely in agreement with previous FDOPA rankings in Parkinson's and normal subjects, in spite of differences in ROI selection technique (Table 1, Figure 3; Moore et al., 2008; Moore et al., 2003). The narrow Freesurfer-defined ROI's (Figure 1) generated lower FDOPA Kocc values than reported in some previous investigations (Moore et al., 2008) but similar to others (Kaasinen et al., 2001; Nagano et al., 2000).
In (non-PD) post-mortem brains, concentrations of dopamine and noradrenaline are highest in hippocampus, followed by anterior cingulate, entorhinal, and dorsolateral prefrontal cortex; concentrations in PD brains are 2-3 fold lower (Scatton B et al., 1983). It is encouraging that the order of ROI's ranked by Kocc in the present study is roughly commensurate with relative catecholamine concentrations reported by Scatton et al. The amygdala, entorhinal cortex, and hippocampal formation participate in an allocortical loop that funnels sensory information into limbic circuits and projects to the ventral striatum; pathologic involvement of brain structures in this pathway is thought to underlie emotional symptoms of PD (Braak et al., 1995). Therefore, a reliable system for quantifying AAAD activity in these regions holds promise for the study of non-motor symptoms in vivo.
In a study of normal subjects, Brown et al (1999b) calculated regional FDOPA uptake as a percentage of putamen uptake: Amygdala 35% (current study FDOPA, FMT both 80%); hippocampus 30% (current study, FDOPA 22%, FMT 32%); frontal and temporal cortices 10-15% (current study FDOPA 10-25%, FMT 4-9%); and anterior cingulate 20% (current study FDOPA 23%, FMT 21%). A comparison of these ratios between normal and PD subjects suggests that amygdala Kocc is preserved relative to putamen in early PD. This is consistent with previous reports of increased FDOPA uptake in the amygdala, as well as pallidum, dorsolateral prefrontal, and anterior cingulate cortex, in early Parkinson's disease (Brooks and Piccini, 2006; Bruck et al., 2005; Kaasinen et al., 2001; Rakshi et al., 1999).
Although FMT Kocc exceeded FDOPA Kocc in regions of high/specific uptake, Kocc was similar between tracers in the cerebral cortex. This effect probably occurs because in regions of very low uptake, the ROI TAC resembles that of the reference region (See Figure 1 for example), producing a “floor effect” for the accurate measurement of Kocc. Nonetheless, we did eliminate regions for which measurement error rendered Kocc indistinguishable from zero. Therefore, the relatively high FDOPA Kocc in cortical regions may reflect differences in the metabolism and trapping of the radiotracers, as well as effects of COMT inhibition on FDOPA trapping. The uptake rate constant reflects the amount of tracer accumulated in regions of specific trapping relative to the cumulative exposure of these regions to the tracer circulating in the plasma; when using a tissue input function for the Patlak analysis, the presence of circulating metabolite in the reference region leads to an overestimate of the amount of tracer available for trapping, lowering Kocc. Tolcapone reduces circulating metabolites, thus increasing FDOPA Kocc, (but not Ki, which is calculated using a metabolite-corrected input function) (Doudet et al., 1997; Ruottinen et al., 1997). In previous work, we estimated peripheral COMT inhibition in the current study to be as high as 80% (Gallagher et al., 2011). COMT is highly expressed in frontal cortex, and its inhibition has been shown to increase synaptic dopamine in these regions, thus influencing dopamine-dependent executive functions (Apud and Weinberger, 2007).
The factor analysis and related correlation matrices suggested that regional Kocc values fell primarily into cortical and subcortical components that differed in their relationship to disease duration and severity. In the brain, cells that synthesize dopamine are located in the substantia nigra (SN), ventral tegemental area (VTA), and neurohypophyseal system. The VTA sends projections through several pathways to cortical and subcortical structures: Mesocortical projections to the prefrontal cortex and insula; mesolimbic projections to the nucleus accumbens, amygdala, hippocampus, entorhinal cortex, anterior cingulate cortex, and claustrum; mesostriatal projections to the anteromedial striatum; mesodiencephalic projections to several thalamic nuclei as well as hypothalmaus; and mesorhombencephalic projections to superior colliculus, reticular formation, periaqueductal grey, and spinal cord (Oades and Halliday, 1987). Although the VTA is relatively preserved in PD in comparison to the SN, pathological studies have shown both pathways to be affected (Braak et al., 1995). Differential vulnerability of SN versus VTA dopaminergic projections may partially explain why cortical and subcortical uptakes were not well correlated.
We observed that disease duration and severity (UPDRS scores) were related to subcortical uptake of both tracers, but showed little relationship to cortical uptake. Both pathologic and imaging investigations have suggested that extrastriatal catecholamine levels are lower than normal in PD (Moore et al., 2008; Scatton B et al., 1983). Longitudinal studies have shown progressive decline in FDOPA uptake within extrastriatal structures (locus ceruleus, pallidum, hypothalamus, midbrain raphe) over 3 years in PD (Pavese et al., 2011) —however, decline in cortical regions was not described. In PD patients treated with L-dopa, there is a progressive decline in D2 receptor availability in anterior cingulate, dorsolateral prefrontal, and temporal cortex, as well as thalamic regions, with disease progression (Kaasinen et al., 2003). Based on these previous investigations as well as findings from the present study, we hypothesize that progressive decline in uptake of dopamine radiotracers will be detected in extrastriatal regions of high specific uptake (such as amygdala) with disease progression, but that decline in cortical regions will not be detectable.
Neither FMT nor FDOPA provided advantage for the study of cortical regions; however, FMT may have advantages for quantification of AAAD activity in extrastriatal regions of relatively high specific uptake such as amygdala, pallidum, thalamus, anterior cingulate cortex, entorhinal cortex, and hippocampus. The cognitive and emotional symptoms of PD may be related to radiotracer loss within these allocortical structures. A larger, longitudinal study will be needed to definitively investigate these hypotheses.
Acknowledgements/Research support
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development Service. It was also funded by the University of Wisconsin Institute for Clinical and Translational Research, funded through a National Institutes of Health Clinical and Translational Science Award, [grant number 1UL1RR025011]. This work was supported with use of facilities at the William S. Middleton Memorial Veterans Hospital Geriatric Research Education and Clinical Center and the Waisman Laboratory for Brain Imaging and Behavior, Madison, WI, USA.
References
- Apud JA, Weinberger DR. Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs. 2007;21(7):535–557. doi: 10.2165/00023210-200721070-00002. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, Schultz C, de Vos RA, Jansen EN. Nigral and extranigral pathology in Parkinson's disease. J Neural Transm Suppl. 1995;46:15–31. [PubMed] [Google Scholar]
- Brooks DJ, Piccini P. Imaging in Parkinson's disease: the role of monoamines in behavior. Biol Psychiatry. 2006;59(10):908–918. doi: 10.1016/j.biopsych.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Brown WD, DeJesus OT, Pyzalski RW, Malischke L, Roberts AD, Shelton SE, Uno H, Houser WD, Nickles RJ, Holden JE. Localization of trapping of 6-[F-18]fluoro-L-mtyrosine, an aromatic L-amino acid decarboxylase tracer for PET. Synapse. 1999a;34(2):111–123. doi: 10.1002/(SICI)1098-2396(199911)34:2<111::AID-SYN4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Brown WD, Taylor MD, Roberts AD, Oakes TR, Schueller MJ, Holden JE, Malischke LM, DeJesus OT, Nickles RJ. FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology. 1999b;53(6):1212–1218. doi: 10.1212/wnl.53.6.1212. [DOI] [PubMed] [Google Scholar]
- Bruck A, Aalto S, Nurmi E, Bergman J, Rinne JO. Cortical 6-[18F]fluoro-L-dopa uptake and frontal cognitive functions in early Parkinson's disease. Neurobiol Aging. 2005;26(6):891–898. doi: 10.1016/j.neurobiolaging.2004.07.014. [DOI] [PubMed] [Google Scholar]
- DeJesus OT. Positron-labeled DOPA analogs to image dopamine terminals. Drug Development Research. 2003;59(2):249–260. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Doudet DJ, Chan GL, Holden JE, Morrison KS, Wyatt RJ, Ruth TJ. Effects of catechol-O-methyltransferase inhibition on the rates of uptake and reversibility of 6-fluoro-L-Dopa trapping in MPTP-induced parkinsonism in monkeys. Neuropharmacology. 1997;36(3):363–371. doi: 10.1016/s0028-3908(97)00017-8. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Swaminathan S, DeJesus OT, Sievert M, Ruoho AE, Murali D, Rommelfanger SG, Holden JE. Affinities of dopamine analogs for monoamine granular and plasma membrane transporters: implications for PET dopamine studies. Life Sci. 1997;60(26):2399–2406. doi: 10.1016/s0024-3205(97)00300-7. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Members UP. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinsons Disease. McMillan Healthcare Information; Florham Park, N.J.: 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gallagher CL, Christian BT, Holden JE, Dejesus OT, Nickles RJ, Buyan-Dent L, Bendlin BB, Harding SJ, Stone CK, Mueller B, Johnson SC. A within-subject comparison of 6-[18F]fluoro-m-tyrosine and 6-[18F]fluoro-L-dopa in Parkinson's disease. Mov Disord. 2011;26(11):2032–2038. doi: 10.1002/mds.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, K NA, Hietala J, Sonninen P, Rinne JO. Extrastriatal dopamine D(2) receptors in Parkinson's disease: a longitudinal study. J Neural Transm. 2003;110(6):591–601. doi: 10.1007/s00702-003-0816-x. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Bruck A, Eskola O, Bergman J, Solin O, Rinne JO. Increased frontal [(18)F]fluorodopa uptake in early Parkinson's disease: sex differences in the prefrontal cortex. Brain. 2001;124(Pt 6):1125–1130. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- Keating GM, Lyseng-Williamson KA. Tolcapone: a review of its use in the management of Parkinson's disease. CNS Drugs. 2005;19(2):165–184. doi: 10.2165/00023210-200519020-00006. [DOI] [PubMed] [Google Scholar]
- Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson's disease: an 18F-dopa PET study. Neurobiol Dis. 2008;29(3):381–390. doi: 10.1016/j.nbd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Moore RY, Whone AL, McGowan S, Brooks DJ. Monoamine neuron innervation of the normal human brain: an 18F-DOPA PET study. Brain Res. 2003;982(2):137–145. doi: 10.1016/s0006-8993(03)02721-5. [DOI] [PubMed] [Google Scholar]
- Nagano AS, Ito K, Kato T, Arahata Y, Kachi T, Hatano K, Kawasumi Y, Nakamura A, Yamada T, Abe Y, Ishigaki T. Extrastriatal mean regional uptake of fluorine-18-FDOPA in the normal aged brain--an approach using MRI-aided spatial normalization. Neuroimage. 2000;11(6 Pt 1):760–766. doi: 10.1006/nimg.2000.0584. [DOI] [PubMed] [Google Scholar]
- Namavari M, Satyamurthy N, Phelps ME, Barrio JR. Synthesis of 6-[18F] and 4-[18F]fluoro-L-m-tyrosines via regioselective radiofluorodestannylation. Appl Radiat Isot. 1993;44(3):527–536. doi: 10.1016/0969-8043(93)90165-7. [DOI] [PubMed] [Google Scholar]
- Nickles R, Daube M, Ruth T. An Oxygen-18 Gas Target for the production of [F-18] F2. Int J Appl Rad Isotopes. 1984;35:117–123. [Google Scholar]
- Nurmi E, Ruottinen HM, Bergman J, Haaparanta M, Solin O, Sonninen P, Rinne JO. Rate of progression in Parkinson's disease: a 6-[18F]fluoro-L-dopa PET study. Mov Disord. 2001;16(4):608–615. doi: 10.1002/mds.1139. [DOI] [PubMed] [Google Scholar]
- Oades R, Halliday G. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, Brooks DJ. Progression of monoaminergic dysfunction in Parkinson's disease: a longitudinal 18F-dopa PET study. Neuroimage. 2011;56(3):1463–1468. doi: 10.1016/j.neuroimage.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, Dagher A, Jenkins IH, Friston KJ, Brooks DJ. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease A 3D [(18)F]dopa-PET study. Brain. 1999;122(Pt 9):1637–1650. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M, Solin O. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]fluorodopa positron emission tomographic study. Arch Neurol. 2000;57(4):470–475. doi: 10.1001/archneur.57.4.470. [DOI] [PubMed] [Google Scholar]
- Ruottinen HM, Rinne JO, Oikonen VJ, Bergman JR, Haaparanta MT, Solin OH, Ruotsalainen UH, Rinne UK. Striatal 6-[18F]fluorodopa accumulation after combined inhibition of peripheral catechol-O-methyltransferase and monoamine oxidase type B: differing response in relation to presynaptic dopaminergic dysfunction. Synapse. 1997;27(4):336–346. doi: 10.1002/(SICI)1098-2396(199712)27:4<336::AID-SYN7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Scatton B, F J-A, L R, B D, Y A Reduction of Cortical Dopamine, Noradrenaline, Serotonin, and Their Metabolites in Parkinson's Disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Sossi V, de La Fuente-Fernandez R, Holden JE, Doudet DJ, McKenzie J, Stoessl AJ, Ruth TJ. Increase in dopamine turnover occurs early in Parkinson's disease: evidence from a new modeling approach to PET 18 F-fluorodopa data. J Cereb Blood Flow Metab. 2002;22(2):232–239. doi: 10.1097/00004647-200202000-00011. [DOI] [PubMed] [Google Scholar]