Abstract
Chronic microangiopathy of portal venules results in idiopathic non-cirrhotic intrahepatic portal hypertension (NCIPH). Recent data suggest a role for vasoactive factors of portal venous origin in the pathogenesis of this ‘pure’ vasculopathy of the liver. Enteropathies (often silent), are an important ‘driver’ of this disease. NCIPH is under-recognized and often mis-labeled as cryptogenic cirrhosis. Liver biopsy is needed to prove the diagnosis of NCIPH. In these patients, with advancing disease and increased porto-systemic shunting, the portal venous vasoactive factors bypass the liver filter and contribute to the development of pulmonary vascular endothelial disorders—porto-pulmonary hypertension and hepato-pulmonary syndrome as well as mesangiocapillary glomerulonephritis. Prognosis in NCIPH patients is determined by presence, recognition and management of associated disorders. With better understanding of the pathogenesis of NCIPH, newer treatment options are being explored. Imbalance in ADAMTS 13 (a disintegrin and metalloprotease with thrombospondin type 1 motif, member 13): vWF (von-Willebrand factor) ratio is documented in NCIPH patients and may have a pathogenic role. Therapeutic interventions to correct this imbalance may prove to be important in the management of NCIPH.
Keywords: endothelial dysfunction, ADAMTS 13, von-Willebrand factor (vWF), primary haemostasis
Abbreviations: NCIPH, non-cirrhotic intrahepatic portal hypertension; CVID, common variable immunodeficiency; tTG, Tissue transglutaminase; IBD, inflammatory bowel disease; NRH, nodular regenerative hyperplasia; SOS, sinusoidal obstruction syndrome; PVT, portal vein thrombosis; OPV, obliterative portal venopathy; HVPG, hepatic venous pressure gradient; PPH, porto-pulmonary hypertension; HPS, hepato-pulmonary syndrome
The term “portal hypertension” is used to reflect a clinical condition secondary to increased pressure in the hepatic portal circulation. Clinically, it usually presents with symptoms or signs of gastroesophageal varices, ascites, encephalopathy, splenomegaly and/or hypersplenism. Portal hypertension can be classified in terms of the anatomic location of the causal resistance to portal blood flow as pre-hepatic (e.g. portal vein thrombosis), post-hepatic (e.g. Budd–Chiari syndrome) and intrahepatic. Intrahepatic portal hypertension can be further sub-classified as pre-sinusoidal (e.g. congenital hepatic fibrosis) or sinusoidal (sinusoidal obstruction syndrome/veno-occlusive disease). This review focuses on idiopathic non-cirrhotic intrahepatic portal hypertension (NCIPH), a cause of pre-sinusoidal intrahepatic portal hypertension.
NCIPH occurs as a consequence of increased resistance to flow within intrahepatic portal vein radicles. Based on vascular corrosion cast and morphometric studies, the site of narrowing or occlusion was localized to the 3rd/4th order portal vein radicles. Boyer1 and Wanless2 proposed that the pruning of portal vein radicles is a consequence of thrombosis, but this view is not universally accepted. Sarin et al3 have shown that there are two independent pressure gradients, one between intra-splenic and intrahepatic pressure and another between intrahepatic and wedged hepatic venous pressure indicating the likelihood of both pre-sinusoidal and peri-sinusoidal resistance to blood flow. Intra-variceal pressure is representative of portal pressure.3
Nomenclature
Various terminologies have been used in an attempt to define and characterize NCIPH—idiopathic portal hypertension,4–6 non-cirrhotic portal fibrosis,7,8 nodular regenerative hyperplasia (NRH),9 incomplete septal cirrhosis,10 partial nodular transformation of the liver, benign intrahepatic portal hypertension and hepatoportal sclerosis.11,12 Although their description differs in detail it is increasingly thought that they represent variations in appearance of a single pathological entity. For nomenclature we prefer the term NCIPH for clinical use, as it denotes the site of block as intrahepatic thereby excluding another important cause of idiopathic non-cirrhotic portal hypertension in India, namely extra-hepatic portal vein obstruction13
Definition
At present NCIPH remains a diagnosis of exclusion. Several lists of criteria have been proposed for establishing the diagnosis. The diagnosis is based on documentation of the combination of essential findings listed in Table 1, with inconstant supplementary elements which vary between series documented in Japan, India and South-east Asia.14,15
Table 1.
Criteria for Diagnosing Idiopathic Non-Cirrhotic Intrahepatic Portal Hypertension (NCIPH).
Essential criteria14
Variants–supplementary (non-essential) criteria.
|
Epidemiology
NCIPH has been reported from all parts of the world, though more so from developing countries where it is most commonly encountered in young males in their third to fourth decade.16–18 In contrast, in Japan and the West there is a female preponderance and a tendency to present around the fifth decade.19
The relative rarity of the disease in the West and an apparent decline in its incidence elsewhere as living standards and hygienic conditions improve support the notion that enteropathy plays a role in disease pathogenesis. Series of patients with NCIPH have shown a preponderance of those who belong to low socioeconomic groups.2,9–11,14–16 In India, Sarin et al,20 based on personal communications from multiple centers, estimated that NCIPH accounted for 23% of patients with portal hypertension in 1980's but only 5.6% more recently. This reduction in incidence accords with improving standards of hygiene and healthcare and reduction of diarrheal illnesses including tropical sprue. Nevertheless NCIPH remains an ongoing problem in India. The condition is almost certainly under-diagnosed. Krasinskas AM et al,21 reported that in 13 (81%) of 16 patients in whom unsuspected NCIPH was first diagnosed on histological examination of hepatectomy specimens following liver transplantation, cirrhosis had been the pre-operative diagnosis. Similarly, Radomski et al,22 reported that all four patients diagnosed with NCIPH in their liver transplantation series had been diagnosed as cirrhosis and only recognized as NCIPH on examination of the removed liver.
In a prospective southern Indian study spanning 2009–2010, Goel et al,23 diagnosed cryptogenic chronic liver disease in 203 of 583 consecutive portal hypertensive patients. Thirty nine of the 203 underwent liver biopsy which revealed NCIPH in 16 (41%).23 Of these 16 patients (10 males; age: 31, 20–59 years; median, range), 14 belonged to middle/lower socioeconomic class. 10 of these patients were from eastern India, 2 from southern India, 3 from northern India and 1 patient from Nepal.
Pathogenesis
Non-Cirrhotic Intrahepatic Portal Hypertension is a Microangiopathy Affecting the Small Portal Vein Radicles
Previous studies have demonstrated that small portal vein radicles are obliterated/thrombosed in patients with NCIPH. The pathological entities of nodular regenerative hyperplasia, incomplete septal cirrhosis and hepatoportal sclerosis are probably secondary to similar pathogenic process. In NCIPH, histological changes in the liver such as portal vein sclerosis and/or portal angiomatosis, perisnusoidal fibrosis and sinusoidal dilatation (as detailed below in section on Investigation), suggests obliterative portal venopathy (OPV).
Why Does the Microangiopathy Localize to Portal Venules in Non-Cirrhotic Intrahepatic Portal Hypertension?
The Splanchnic Circulation
The pathogenesis of NCIPH is not entirely clear but various associations give clues as to the likely components which interact in its causation. The association with inflammatory diseases of the intestine and confinement, atleast initially, of vascular disturbances to the portal circulation is consistent with an intestinal origin for perturbation of the afferent portal circulation to the liver.
Confluence of Portal Vein and Hepatic Artery
As the vascular occlusion is initially confined to the third and fourth radicles of the portal vein it may be surmised that a combination of mesenteric and systemic influences coincide. This would be explained if admixture of hepatic arterial and portal venous factors combine to fulfill the necessary preconditions.
Pro-Inflammatory Factors of Intestinal Origin may Drive Microvasculature Occlusion in Non-Cirrhotic Intrahepatic Portal Hypertension
We documented a 16–20% incidence of adult coeliac disease in two separate series of NCIPH patients from Britain and India respectively.24,25 In light of this we have postulated that the high incidence of NCIPH in India, and its predilection for lower social classes may be attributable to the high frequency of enteropathies of varying etiology encountered in this population. Dysfunction of the vascular endothelium has been documented during the development of portal hypertension.26 Pro-inflammatory cytokines are elevated in the sera of patients with active celiac disease.27 Pro-inflammatory stimuli emanating from damaged gut stimulate expression of ultra-large von-Willebrand Factor (ULvWF) in portal venous endothelium. In the normal state, von-Willebrand Factor (vWF) immunostaining is usually positive in large vessels but negative in the sinusoidal endothelial cells in the liver.28 On the occurrence of liver injury accompanied by a necroinflammatory process, the sinusoidal endothelial cell becomes positive for vWF, presumably in association with the capillarization of hepatic sinusoids.29
In an interesting in-vitro experiment, human umbilical vein endothelial cells were treated with interleukin 6 (IL-6), IL-8, or tumor necrosis factor alpha (TNF-α), and the formation of platelet-decorated ULvWF strings were quantitated. The results suggested that the inflammatory cytokines (IL-8 and TNF-α) stimulate ULvWF release and inhibit the ULvWF cleavage (IL-6), resulting in the accumulation of hyper-reactive ULvWF in plasma and on the surface of endothelial cells to induce platelet aggregation and adhesion on the vascular endothelium.30
Normal blood flow stretches the long strands of vWF, exposing sites which are cleaved by ADAMTS 13 (a disintegrin and metalloprotease with thrombospondin type 1 motif, member 13) thus releasing vWF into the circulation. In the absence of ADAMTS 13, there will be a tendency for ULvWF strings to remain anchored and interact with platelet glycoprotein 1bα, thereby activating intraplatelet signaling which has the potential to produce platelet thrombi.31
Prothrombotic Predisposition
We studied ADAMTS 13 in NCIPH which is free of various confounding variables that arise as a consequence of liver disease per se. On the basis of finding severe deficiency of ADAMTS 13 in NCIPH patients with an otherwise normally functioning liver, we propose that ADAMTS 13 deficiency in NCIPH is primary and permissive of the cause in producing the pruning of intrahepatic portal vein branches which characterizes NCIPH32–34(Figure 1).
Figure 1.
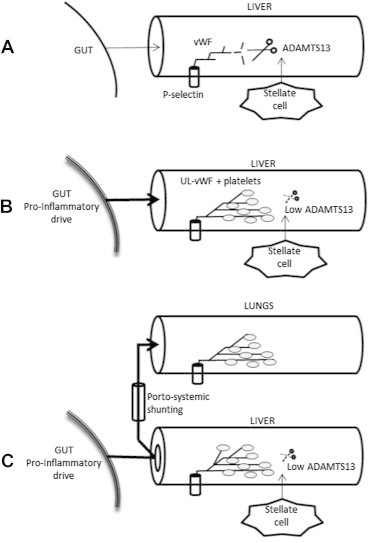
Proposed role of ADAMTS 13-ULvWF imbalance in pathogenesis of non-cirrhotic intrahepatic portal hypertension (NCIPH) and porto-pulmonary hypertension. A: Normal. Normal ADAMTS 13 production by the hepatic stellate cell leads to rapid cleavage of vWF from its p-selectin anchor; B: An increased pro-inflammatory drive of splanchnic origin leads to increased vWF production in venous endothelium which in the face of low ADAMTS 13 production results in ULvWF and platelet microthrombi; C: In the presence of low ADAMTS 13 production and increased splanchnic pro-inflammatory drive, porto-systemic shunting leads to build up of ULvWF and platelet rich thrombi in the pulmonary capillaries (Adapted from Ref 34). ADAMTS 13 :A disintegrin and metalloprotease with thrombospondin type 1 motif, member 13.
Hepatic End-Organ Status
In NCIPH patients, over time, with increasing porto-systemic shunting of portal venous blood, the liver is no longer able to restrict to the portal circulation the vasoactive factors originating within mesenteric blood. It may be speculated that these vasoactive factors, some of which have culminated in portal microangiopathy in NCIPH contribute to the development of porto-pulmonary hypertension,34 hepato-pulmonary syndrome and mesangiocapillary glomerulonephritis (see later).
Associated Disorders
Gut and Immune Disorders
An immunological aspect to the pathogenesis of NCIPH is suggested by its female preponderance in the west and its association with various immunological and autoimmune disorders. Though there has been a significant change over time in the detail of disorders associated with NCIPH there remains a consistent tendency for implicated factors to be of relevance to the immune system and gut.35,36 Earlier studies emphasized the importance of common variable immunodeficiency (CVID).37,38 Treatment with intravenous immunoglobulin replacement has largely resolved the issue, further supporting the notion that enteropathies associated with CVID were somehow promoting the vasculopathy of terminal portal venous radicles. Such a sequence points strongly to a gastrointestinal source for the initiating factors in NCIPH.
Accordingly we documented 16–20% incidence of adult coeliac disease in two separate series of NCIPH patients from Britain,24 and India25 respectively. In light of these findings it would appear logical to postulate that the high incidence of NCIPH reported from India in the past,16 may have been closely related to prevalent enteropathies including tropical sprue. Epidemiologically, such an association is also likely to account for the reported link with poverty.23 On screening, deficiency of serum vitamin B12 may be an indicator of the disease.39 This accords with the concept of a triad involving enteropathy (B12 malabsorption), portal hypertension and absence of hepatocyte damage (lack of leakage of B12 from the hepatocyte).
However it must not be supposed that eradication of endemic enteropathies will eradicate the disease as milder and sub-clinical enteropathies may be able to promote the same hepatic vascular reaction. In a recent Indian study consecutive patients with portal hypertension having a clinical presentation compatible with cryptogenic cirrhosis or NCIPH (cases) and controls with hepatitis B or C related cirrhosis were prospectively enrolled.25 Tissue transglutaminase (tTG) antibody was positive in 66% of cases as compared to 29% in controls (P value < 0.001). Duodenal biopsy showed villous atrophy, crypt hyperplasia and lamina propria inflammation, significantly more commonly in cases as compared to controls; however most patients did not have raised intra-epithelial lymphocytes. 17 (43%) cases (including 5 NCIPH patients) as compared to 2 (18%) controls (P-value: 0.2) had this combination of tTG antibody positivity with duodenal villous atrophy. 13 of these 17 cases were followed up for >6 months (25, 11–59 months; median, range) on gluten free diet, with improvement in intestinal symptoms, tTG antibody titer and duodenal histology, but no improvement in liver disease severity. A longer duration on gluten free diet may be required for improvement in liver disease.
Furthermore, although the impact of CVID has declined as a cause of enteropathy, it appears that immunodeficiency continues to be an important aetiological factor as there has been a marked rise in instances of NCIPH that are associated with HIV (Human Immunodeficiency virus) infection.40–45 Enteropathy is a well established feature of HIV infection. Infection of hepatic stellate cells by HIV may contribute to the pathogenesis of portal venous and sinusoidal endothelial injury via stimulation of prothrombotic cytokines such as endothelin-l, inerleukins-l and 6 and platelet derived growth factor46 and protein S deficiency.47 Although untested it may also be that stellate cell infection by HIV may contribute by diminishing production of ADAMTS 13 (see later). NCIPH occurring in HIV infection has been associated with prolonged exposure to Didanosine or concomitant exposure to Stavudine or Tenofovir.44,48
Although typically primary biliary cirrhosis is accompanied by cholestasis which may be advanced prior to development of portal hypertension or cirrhosis, in a subgroup the illness presents with bleeding varices due to pre-sinusoidal portal hypertension in the absence of cholestasis or cirrhosis,49 thus supporting the hypothesis that in genetically predisposed individuals, inflammation of the portal area of the liver promotes NCIPH. There are similar reports linking NCIPH with other autoimmune disorders including, systemic lupus, scleroderma,50 Sjogren syndrome, rheumatoid arthritis51 autoimmune hepatitis15,52 and Felty's syndrome.53,54 Despite isolated case reports it is not clear that there is a significant association with the presence of anti-cardiolipin antibody.24,55
Seksik et al,56 described NCIPH in inflammatory bowel disease (IBD) patients treated with azathioprine. According to a recent review, IBD patients treated with Azathioprine have a cumulative incidence of nodular regenerative hyperplasia (NRH) of approximately 0.6% and 1.28% at 5 and 10 years respectively, whereas those treated with high-dose thioguanine (TG > 40 mg/day) have a frequency of NRH of upto 62%, which is higher in patients with elevated liver enzymes and/or thrombocytopenia than those without these abnormalities (frequency 76% vs. 33%). Conversely, low-dose TG therapy (<20 mg/day) is relatively safe, with no cases of NRH observed.57
Devarbhavi et al,58 described 14 patients who developed NRH, unrelated to Azathioprine in most cases, 3 months–11 years after orthotopic liver trasplantation (LT). A total of 10 patients developed NRH within 4 years (early onset), and 4 other patients developed the condition beyond 4 years of LT.
Although both the sinusoidal obstruction syndrome (SOS) and NCIPH may present as nodular regenerative hyperplasia it is important to differentiate between them. In a setting of carcinoma colon with liver metastases, NRH occurred in 22 of 146 treated patients (15%; 20 of these patients received Oxaliplatin). Patients treated by Oxaliplatin more often had NRH compared with Oxaliplatin-naïve patients (22 vs. 4%). Although operative mortality was nil, the presence of NRH was associated with increased postoperative hepatic morbidity (50 vs. 29%). Careful histological review suggested that the causative injury was to the endothelium lining hepatic sinusoids (SOS) rather than to portal venules (NCIPH).59,60
Toxins
Prolonged exposure to several toxins especially arsenic has also been incriminated as a cause of NCIPH.61 Clinical and laboratory investigations were carried out on 248 patients with chronic arsenic toxicity due to drinking of arsenic contaminated water (0.05–3.2 mg/l) in 6 districts of West Bengal. Portal hypertension was found in 33.3% patients. Liver biopsy in 69 patients showed non-cirrhotic portal fibrosis in 63.62
Pro-Thrombotic Factors
A role of prothrombotic disorders in the pathogenesis of NCIPH is supported by autopsy studies showing high prevalence of portal vein thrombosis (PVT) and studies from the West indicating association with prothrombotic states in 30–50% of the patients.15,63
Hematologic Disorders
Potentially prothrombotic myeloproliferative disorders have been reported by Wanless et al in patients with NCIPH.64
Genetic Disorders
Finally, support for the notion of genetic predisposition to development of NCIPH has come from reports on the familial occurrence as reported by Sarin et al65 and a family we documented in which several members developed porto-pulmonary hypertension in association with NCIPH.66
Clinical presentation
History
The typical patient with NCIPH presents in early adulthood with a complication of portal hypertension, usually bleeding varices.14–16 Ascites is uncommon except transiently after gastrointestinal bleeding. Not infrequently the patient will have presented to a hematology clinic following detection of thrombocytopenia or anemia with splenomegaly, in the absence of any other symptoms or signs to implicate liver disease.
Clinical Examination
Examination Reveals moderate splenomegaly but no hepatomegaly, indeed the liver may appear reduced in size. A series of 16 liver explants, of which 15 had NRH and 9 had incomplete septal cirrhosis, weighed a mean of 1,100 g.21 In a more recent survey involving 55 patients, ultrasonography showed a shrunken liver in fifty-eight percent.67
Excluding cirrhosis is difficult in the absence of liver histology. Blood tests most frequently reveal thrombocytopenia but otherwise are unremarkable with normal or near normal liver function tests. Laboratory testing for diagnosis of other known causes of liver disease is unyielding. Both, hepato-pulmonary syndrome21 and porto-pulmonary hypertension,34,68 may arise as complications of NCIPH.
Investigation
Imaging
NCIPH is defined by the combination of pre-sinusoidal portal hypertension with a patent extra-hepatic portal vein and the absence of cirrhosis. At present, available conventional imaging tests i.e.ultrasonography, computerized tomogram and magnetic resonance imaging are limited in their ability to visualize small intrahepatic portal vein radicles. Subjective assessment of the size of liver on ultrasonography may reveal a shrunken liver in majority of these patients. In a recent study, Goel et al reported that 30 of the 45 patients with NCIPH had shrunken liver.39 Few studies have explored the role of Role of Tc99m sulfur colloid scintigraphy69 and fibroscan70 in NCIPH, but their role in differentiating NCIPH from cirrhosis remains uncertain.
Pathology of the Liver
Grossly, the liver may be normal, enlarged or atrophic and/or nodular. The surface may appear normal or may show wrinkling or nodularity, depending on the stage of the disease.
A variety of morphological patterns can be seen on histological examination of the liver, which include NRH characterized by diffuse parenchymal nodularity due to alternating hyperplastic and atrophic hepatocytes without fibrosis (Figure 2a and b); incomplete septal cirrhosis in which there is slender septal fibrosis (Figure 3) demarcating the liver parenchyma into inconspicuous nodules and approximation of portal tracts with each other and with central veins; partial nodular transformation in which there are grossly visible nodules of hyperplastic hepatocytes located in the perihilar region of the liver; and obliterative portal venopathy (OPV) characterized by portal vein sclerosis and/or aberrant portal vein branches/angiomatosis (Figures 4 and 2b), sinusoidal dilatation and perisinusoidal fibrosis.21,71–73
Figure 2.
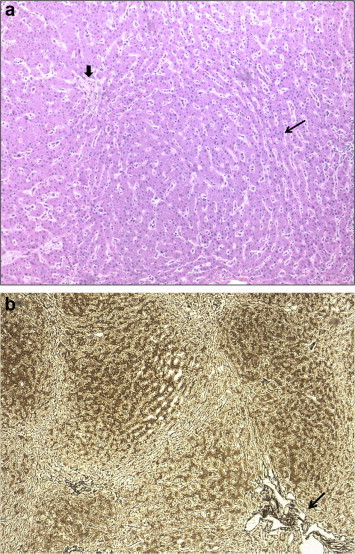
a. Nodular regenerative hyperplasia (NRH) showing hyperplastic hepatocytes surrounded by atrophic hepatocytes (long arrow) and hypoplastic portal tract (short arrow), H&E 100x. b. Nodular regenerative hyperplasia (NRH) showing parenchymal nodularity without fibrosis and portal angiomatosis (arrow), reticulin stain 50x.
Figure 3.
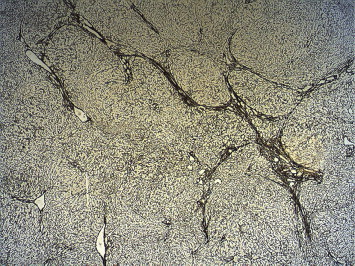
Thin septa extending from the portal tracts, reticulin stain 25x.
Figure 4.
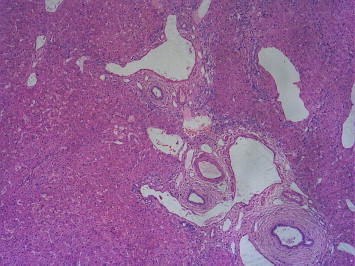
Aberrant and dilated portal venules herniating into the parenchyma, H&E 50x.
The whole liver can show heterogenous morphology and the findings can be focal and vary in severity. This should be kept in mind while evaluating liver biopsy samples or explant livers. Liver biopsy is essential to exclude cirrhosis. Histological changes of OPV, perisinusoidal and portal/periportal fibrosis and NRH have been reported in liver biopsies which are of adequate length (>1 cm) or multiple cores with adequate number of portal tracts (>5).15,18 In per operative cases both wedge and needle biopsies are advisable, since subcapsular nodularity can be mistaken for cirrhosis.15 In advanced stages of the disease, thrombosis of the medium and large portal vein branches has been reported.73
These morphological patterns could represent a histological spectrum of different stages of a single clinical entity, resulting in non-cirrhotic intrahepatic portal hypertension.21,72
Hepatic Venous Pressure Studies
NCIPH being a pre-sinusoidal portal hypertension, is expected to have normal hepatic venous pressure gradient (HVPG :difference between wedged hepatic and free hepatic pressure). Sarin et al,3 showed two pressure gradients in these patients, one being between intrahepatic and wedged hepatic, thus suggesting a peri-sinusoidal component to portal hypertension. In our center, we found that up to 1/3rd of patients with NCIPH have normal HVPG.67,74 This suggests that in a majority, may be with advancing disease, there is a peri-sinusoidal component contributing to portal hypertension in these patients.
Prognosis/natural history
Although many published series report that overall survival of NCIPH patients is excellent,20 there are caveats to that assertion as with longer follow up there is evidence of significant morbidity impacting survival.21 The studies with highest number of patients consist largely of patients who have presented at a relatively early age with bleeding varices. Given that many of the patients are relatively young, largely Child's class A status and that their follow up was relatively short it is not surprising that no excess deaths are recorded for several years. In a more recent series, active celiac disease diagnosed late in the natural history contributed significantly to an excess of early deaths.24 Schouten et al,44 reported in a cohort of 62 patients followed up for a median of 90 months (range 24–310) overall survival were 100% (95% CI 95–100%), 78% (95% CI 67–89%) and 56% (95% CI 40–72%) at 1, 5 and 10 years respectively which was significantly decreased as compared to general population (P < 0.001). It is mandatory therefore that patients are monitored for extra-hepatic factors that may not be directly associated with their liver disease including disorders affecting the splanchnic circulation. Although by definition NCIPH occurs in the absence of PVT it is well documented that as the disease progresses that there is a high incidence of PVT. From this it is tempting to postulate that the factors which have contributed to NCIPH may have also contributed to PVT. Indications for liver transplantation include decompensated liver disease, porto-pulmonary hypertension (PPH) and hepato-pulmonary syndrome.75
Complications
Hepato-pulmonary Syndrome (HPS)
Orthostatic hypoxemia due to dilatation of intrapulmonary capillaries may occur late in many liver diseases including NCIPH. In a series of 15 NCIPH patients undergoing liver transplantation, the indication for transplantation was hepato-pulmonary syndrome in 3 patients.21
Porto-Pulmonary Hypertension (PPH)
In a patient with NCIPH and severe PPH, we documented significant reduction in pulmonary artery pressures following a period of weekly transfusions of fresh frozen plasma as a source of replacement ADAMTS 13.34 Subsequent infusion of platelet concentrate resulted in an immediate and almost fatal exacerbation of pulmonary hypertension. We postulated that increasing porto-systemic shunting complicating NCIPH in this patient transferred the pathological events of portal venous occlusion to the pulmonary vessels and as a consequence of severe ADAMTS 13 deficiency involved occlusion of pulmonary arterioles by platelets.
Cryptogenic Cirrhosis
It is uncertain whether the evolution of NCIPH may in some cases progress to cirrhosis.76 In many areas of the world a majority of cases of cryptogenic cirrhosis is believed to be a consequence of non alcoholic steatohepatitis. However there is not the same prevalence of obesity in areas of India where NCIPH is most common suggesting an alternative pathogenesis. It is possible that continuation of the vascular injury that has led to NCIPH could culminate in cryptogenic cirrhosis. Clinically, NCIPH can mimic cirrhosis in every aspect, and these patients are clumped together as ‘cryptogenic’ cirrhosis prior to liver biopsy. Only when the liver biopsy shows absence of fibrosis encircling hepatocyte nodules is the diagnosis of NCIPH confirmed. Thus clinico-epidemiological studies tend to under-estimate the prevalence of NCIPH. In a prospective study of etiology of portal hypertension conducted, only 20% patients with cryptogenic chronic liver disease underwent biopsy.23 In a retrospective analysis (during 2005–2007), of the 62 patients with cryptogenic chronic liver disease who underwent liver biopsy at our centre, 30 (48%) had NCIPH.18 Finding of a tendency for Indian patients with cryptogenic cirrhosis of Child's class A to have low plasma ADAMTS 13 levels raises the intriguing possibility that NCIPH and cryptogenic cirrhosis in India are pathogenetically related.33
Nephropathy
Of NCIPH patients who were treated by meso-caval shunt surgery for bleeding varices a high proportion developed glomerulonephritis (mesangiocapillary glomerulonephritis—MCGN),77 and similar findings occurred in those in whom shunt surgery had been performed prophylactically to prevent variceal bleeding.78 In contrast, patients who underwent meso-caval shunting for variceal bleeding caused by extra-hepatic portal vein thrombosis did not develop nephropathy. The implications again point to the presence in mesenteric blood, emerging from the intestine, of pro-inflammatory factors which when confined to the mesenteric circulation result in NCIPH but which on spill-over into the systemic circulation may result in PPH, HPS or MCGN.
Hepatocellular Carcinoma
Hepatocellular carcinoma has been rarely associated with NRH.79 The possibilities of NRH being primary or secondary to the HCC need further studies.
Treatment
It is very likely that progression of NCIPH is to some extent dependent on the strength of the intestinal pro-inflammatory drive. It can be surmised that the widely reported reduction of incidence of NCIPH in India in recent years,80 is to some extent related to the reduction seen in the incidence of chronic enteropathies such as tropical sprue. In a Caucasian population, Eapen et al reported a significantly worse prognosis of NCIPH in those with untreated adult coeliac disease.24 A major facet in patient management must therefore be detection and treatment of any identifiable source of a pro-inflammatory drive within the mesenteric circulation.
The commonest complication is of bleeding oesophageal varices. A single controlled trial involving a 2 year follow up of 101 patients showed no significant difference in the rate of rebleeding between patients treated with propranolol or sclerotherapy.81 Pal et al,78 reported on 41 patients with NCIPH in whom prophylactic proximal lienorenal shunt was performed and concluded that the morbidity was sufficient for them to advise against the practice. Though there were no operative deaths and no one died of bleeding there was an excess of delayed morbidity in 47% including a death from chronic renal failure, porto-systemic encephalopathy (7 patients), glomerulonephritis (4), HPS (2) and ascites (5). Thus only 20 (53%) patients were symptom-free on follow up.78 Although successful in reducing portal pressure and risk of rebleeding, we therefore believe that porto-systemic shunt surgery should be avoided unless totally necessary as it may have systemic complications.
Prevention and management of variceal bleeding can probably be performed according to the same guidelines as for cirrhosis: beta-blockers and/or endoscopic therapy for the prevention of bleeding82; endoscopic therapy associated with vasoactive drugs for the treatment of acute variceal bleeding. In NCIPH patients, transjugular intrahepatic portosystemic shunt should be held in reserve as it has been reported to be associated with liver failure and excess mortality.24
Although not reported in the literature we are aware of cases in which unanticipated fatal cardiopulmonary collapse has complicated treatment by portosystemic shunting in patients with NCIPH. We postulate that these cases have arisen as a consequence of transfusion of platelets or colloid in patients with pre-existing but unidentified porto-pulmonary hypertension.34 We envisage a scenario in which if on pre-operative assessment of a patient with any liver disease, porto-pulmonary hypertension is found, attention to their ADAMTS 13: ULvWF ratio becomes a necessary step in preparing for surgery. Platelet transfusion in such patients should either be deferred until ADAMTS 13 deficiency has been excluded or reversed by infusion of fresh frozen plasma. Should urgency preclude such management, platelet transfusion should be monitored with sensitive measures to detect early signs of rapidly increasing acute pulmonary vascular resistance.
Liver transplantation
Various studies have documented excellent prognosis in patients with NCIPH, but transplant units, especially from this part of the world, would encounter patients with NCIPH. In a series from N. India, of the 402 transplanted patients; 87 had a pre-transplant diagnosis of cryptogenic cirrhosis and 10 of these patients were diagnosed as NCIPH after explant biopsy.83 At our center 20 of the 54 (39%) patients, who underwent liver transplant during 1999–2013, were labeled as cryptogenic liver disease at the time of liver transplant. On histopathological examination of the explanted liver, 3 patients had NCIPH.
During liver transplantation, pre-existing deficiency due to end-stage liver disease is compounded by the interval during which restoration of normal levels of ADAMTS 13 by the implanted liver lags behind the recovery to normal of vWF function. During this window period, a hyper-coagulable state may pertain. ADAMTS 13 deficiency is not only associated with NCIPH but is also a significant complication of advanced liver disease of any cause and may contribute to the risks of surgery including liver transplantation.84 In a recent analysis, platelet transfusion was the only statistically significant factor that could be identified which was associated with excess mortality following liver transplantation.76 On autopsy evidence, the excess was accounted for by pulmonary ‘hepatisation’ and right heart failure indicative of platelet driven occlusion of the pulmonary microvasculature.85 We have proposed that this scenario is a consequence of ADAMTS 13 deficiency, and if so, may be prevented by adequate correction of ADAMTS 13 blood levels prior to any platelet transfusion in such patients.34
Conflicts of interest
All authors have none to declare.
References
- 1.Boyer J.L., Hales M.R., Klatskin G. “Idiopathic” portal hypertension due to occlusion of intrahepatic portal veins by organized thrombi. A study based on postmortem vinylite-injection corrosion and dissection of the intrahepatic vasculature in 4 cases. Medicine (Baltimore) 1974;53:77–91. doi: 10.1097/00005792-197401000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Wanless I.R., Godwin T.A., Allen F., Feder A. Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore) 1980;59:367–377. [PubMed] [Google Scholar]
- 3.Sarin S.K., Sethi K.K., Nanda R. Measurement and correlation of wedged hepatic, intrahepatic, intrasplenic and intravariceal pressure in patients with cirrhosis of liver and non-cirrhotic portal fibrosis. Gut. 1987;28:260–266. doi: 10.1136/gut.28.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okuda K., Nakashima T., Okudaira M. Liver pathology of idiopathic portal hypertension. Comparison with non-cirrhotic portal fibrosis of India. The Japan idiopathic portal hypertension study. Liver. 1982;2:176–192. doi: 10.1111/j.1600-0676.1982.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 5.Okuda K., Kono K., Ohnishi K. Clinical study of eighty-six cases of idiopathic portal hypertension and comparison with cirrhosis with splenomegaly. Gastroenterology. 1984;86:600–610. [PubMed] [Google Scholar]
- 6.Boyer J.L., Sen Gupta K.P., Biswas S.K. Idiopathic portal hypertension. Comparison with the portal hypertension of cirrhosis and extrahepatic portal vein obstruction. Ann Intern Med. 1967;66:41–68. doi: 10.7326/0003-4819-66-1-41. [DOI] [PubMed] [Google Scholar]
- 7.Basu A.K., Boyer J., Bhattacharya R., Mallik K.C., Sen Gupta K.P. Non-cirrhotic portal fibrosis with portal hypertension: a new syndrome. I. Clinical and function studies and results of operations. Indian J Med Res. 1967;55:336–350. [PubMed] [Google Scholar]
- 8.Sama S.K., Bhargava S., Nath N.G. Noncirrhotic portal fibrosis. Am J Med. 1971;51:160–169. doi: 10.1016/0002-9343(71)90234-8. [DOI] [PubMed] [Google Scholar]
- 9.Wanless I.R. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787–797. doi: 10.1002/hep.1840110512. [DOI] [PubMed] [Google Scholar]
- 10.Sciot R., Staessen D., Van Damme B. Incomplete septal cirrhosis: histopathological aspects. Histopathology. 1988;13:593–603. doi: 10.1111/j.1365-2559.1988.tb02091.x. [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen W.P., Edmondson H.A., Peters R.L., Redeker A.G., Reynolds T.B. Extra- and intrahepatic portal hypertension without cirrhosis (hepatoportal sclerosis) Ann Surg. 1965;162:602–620. doi: 10.1097/00000658-196510000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibarrola C., Colina F. Clinicopathological features of nine cases of non-cirrhotic portal hypertension: current definitions and criteria are inadequate. Histopathology. 2003;42:251–264. doi: 10.1046/j.1365-2559.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- 13.Simon E.G., Joseph A.J., George B. Aetiology of paediatric portal hypertension—experience of a tertiary care centre in South India. Trop Doct. 2009;39:42–44. doi: 10.1258/td.2008.080050. [DOI] [PubMed] [Google Scholar]
- 14.Schouten J.N.L., Garcia-Pagan J.C., Valla D.C., Janssen H.L.A. Idiopathic noncirrhotic portal hypertension. Hepatology. 2011;54:1071–1081. doi: 10.1002/hep.24422. [DOI] [PubMed] [Google Scholar]
- 15.Cazals-Hatem D., Hillaire S., Rudler M. Obliterative portal venopathy: portal hypertension is not always present at diagnosis. J Hepatol. 2011;54:455–461. doi: 10.1016/j.jhep.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Dhiman R.K., Chawla Y., Vasishta R.K. Non-cirrhotic portal fibrosis (idiopathic portal hypertension): experience with 151 patients and a review of the literature. J Gastroenterol Hepatol. 2002;17:6–16. doi: 10.1046/j.1440-1746.2002.02596.x. [DOI] [PubMed] [Google Scholar]
- 17.Chawla Y., Dhiman R.K. Intrahepatic portal venopathy and related disorders of the liver. Semin Liver Dis. 2008;28:270–281. doi: 10.1055/s-0028-1085095. [DOI] [PubMed] [Google Scholar]
- 18.Madhu K., Avinash B., Ramakrishna B. Idiopathic non-cirrhotic intrahepatic portal hypertension: common cause of cryptogenic intrahepatic portal hypertension in a Southern Indian tertiary hospital. Indian J Gastroenterol. 2009;28:83–87. doi: 10.1007/s12664-009-0030-3. [DOI] [PubMed] [Google Scholar]
- 19.Aoki H., Hasumi A., Yoshida K. A questionnaire study on treatment of idiopathic portal hypertension and extrahepatic portal obstruction. In: Kameda H., editor. Annual Report on Portal Portal Hemodynamics Abnormalities (In Japanese) Japan Ministry of Health and Welfare; Tokyo: 1988. pp. 179–189. [Google Scholar]
- 20.Sarin S.K., Kumar A., Chawla Y.K. Noncirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int. 2007;1:398–413. doi: 10.1007/s12072-007-9010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krasinskas A.M., Eghtesad B., Kamath P.S., Demetris A.J., Abraham S.C. Liver transplantation for severe intrahepatic noncirrhotic portal hypertension. Liver Transpl. 2005;11:627–634. doi: 10.1002/lt.20431. [DOI] [PubMed] [Google Scholar]
- 22.Radomski J.S., Chojnacki K.A., Moritz M.J. Results of liver transplantation for nodular regenerative hyperplasia. Am Surg. 2000;66:1067–1070. [PubMed] [Google Scholar]
- 23.Goel A., Madhu K., Zachariah U. A study of aetiology of portal hypertension in adults (including the elderly) at a tertiary centre in southern India. Indian J Med Res. 2013;137:922–927. [PMC free article] [PubMed] [Google Scholar]
- 24.Eapen C.E., Nightingale P., Hubscher S.G. Non-cirrhotic intrahepatic portal hypertension: associated gut diseases and prognostic factors. Dig Dis Sci. 2011;56:227–235. doi: 10.1007/s10620-010-1278-2. [DOI] [PubMed] [Google Scholar]
- 25.Maiwall R., Pulimood A.B., Ramakrishna B. Celiac disease is associated with non-cirrhotic intrahepatic portal hypertension and cryptogenic chronic liver disease in India. Indian J Gastroenterol. 2010;29(suppl 1):A96. [Google Scholar]
- 26.Sato Y., Nakanuma Y. Role of endothelial-mesenchymal transition in idiopathic portal hypertension. Histol Histopathol. 2013;28:145–154. doi: 10.14670/HH-28.145. [DOI] [PubMed] [Google Scholar]
- 27.Manavalan J.S., Hernandez L., Shah J.G. Serum cytokine elevations in celiac disease association with disease presentation. Hum Immunol. 2010;71:50–57. doi: 10.1016/j.humimm.2009.09.351. [DOI] [PubMed] [Google Scholar]
- 28.Hollestelle M.J., Thinnes T., Crain K. Tissue distribution of factor VIII gene expression in vivo–a closer look. Thromb Haemost. 2001;86:855–861. [PubMed] [Google Scholar]
- 29.Urashima S., Tsutsumi M., Nakase K., Wang J.S., Takada A. Studies on capillarization of the hepatic sinusoids in alcoholic liver disease. Alcohol Alcohol Suppl. 1993;1B:77–84. doi: 10.1093/alcalc/28.supplement_1b.77. [DOI] [PubMed] [Google Scholar]
- 30.Bernardo A., Ball C., Nolasco L., Moake J.F., Dong J.F. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–106. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 31.Uemura M., Fujimura Y., Ko S., Matsumoto M., Nakajima Y., Fukui H. Pivotal role of ADAMTS13 function in liver diseases. Int J Hematol. 2010;91:20–29. doi: 10.1007/s12185-009-0481-4. [DOI] [PubMed] [Google Scholar]
- 32.Mackie I., Eapen C.E., Neil D. Idiopathic non-cirrhotic intrahepatic portal hypertension (NCIPH) is associated with sustained ADAMTS13 deficiency. Dig Dis Sci. 2011;56:2456–2465. doi: 10.1007/s10620-011-1729-4. [DOI] [PubMed] [Google Scholar]
- 33.Goel A., Alagammai P.L., Nair S.C. ADAMTS13 deficiency, despite well compensated liver functions in patients with noncirrhotic portal hypertension. Indian J Gastroenterol. 2014 doi: 10.1007/s12664-014-0460-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 34.Elias J.E., Mackie I., Eapen C.E., Chu P., Shaw J.C., Elias E. Porto-pulmonary hypertension exacerbated by platelet transfusion in a patient with ADAMTS13 deficiency. J Hepatol. 2013;58:827–830. doi: 10.1016/j.jhep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Lal B.K., Stanley A. Nodular regenerative hyperplasia related portal hypertension in a patient with hypogammaglobulinaemia. World J Gastroenterol. 2013;19:3502–3504. doi: 10.3748/wjg.v19.i22.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuss I.J., Friend J., Yang Z. Nodular regenerative hyperplasia in common variable immunodeficiency. J Clin Immunol. 2013;33:748–758. doi: 10.1007/s10875-013-9873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal S., Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol. 2013;11:1050–1063. doi: 10.1016/j.cgh.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malamut G., Ziol M., Suarez F. Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol. 2008;48:74–82. doi: 10.1016/j.jhep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Goel A., Ramakrishna B., Muliyil J. Use of serum vitamin B12 level as a marker to differentiate idiopathic noncirrhotic intrahepatic portal hypertension from cryptogenic cirrhosis. Dig Dis Sci. 2013;58:179–187. doi: 10.1007/s10620-012-2361-7. [DOI] [PubMed] [Google Scholar]
- 40.Maida I., Garcia-Gasco P., Sotgiu G. Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors, and outcome. Antivir Ther. 2008;13:103–107. [PubMed] [Google Scholar]
- 41.Vispo E., Moreno A., Maida I. Noncirrhotic portal hypertension in HIV-infected patients: unique clinical and pathological findings. AIDS. 2010;24:1171–1176. doi: 10.1097/QAD.0b013e3283389e26. [DOI] [PubMed] [Google Scholar]
- 42.Chang P.E., Miquel R., Blanco J.L. Idiopathic portal hypertension in patients with HIV infection treated with highly active antiretroviral therapy. Am J Gastroenterol. 2009;104:1707–1714. doi: 10.1038/ajg.2009.165. [DOI] [PubMed] [Google Scholar]
- 43.Cachay E.R., Peterson M.R., Goicoechea M., Mathews W.C. Didanosine exposure and noncirrhotic portal hypertension in a HIV clinic in North America: a follow-up study. Br J Med Med Res. 2011;1:346–355. doi: 10.9734/bjmmr/2011/554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schouten J.N.L., Nevens F., Hansen B. Idiopathic noncirrhotic portal hypertension is associated with poor survival: results of a long-term cohort study. Aliment Pharmacol Ther. 2012;35:1424–1433. doi: 10.1111/j.1365-2036.2012.05112.x. [DOI] [PubMed] [Google Scholar]
- 45.Schiano T.D., Kotler D.P., Ferran E., Fiel M.I. Hepatoportal sclerosis as a cause of noncirrhotic portal hypertension in patients with HIV. Am J Gastroenterol. 2007;102:2536–2540. doi: 10.1111/j.1572-0241.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 46.Tuyama A., Hong F., Saiman Y. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–622. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mallet V.O., Varthaman A., Lasne D. Acquired protein S deficiency leads to obliterative portal venopathy and to compensatory nodular regenerative hyperplasia in HIV-infected patients. AIDS. 2009;23:1511–1518. doi: 10.1097/QAD.0b013e32832bfa51. [DOI] [PubMed] [Google Scholar]
- 48.Cotte L., Bénet T., Billioud C. The role of nucleoside and nucleotide analogues in nodular regenerative hyperplasia in HIV-infected patients: a case control study. J Hepatol. 2011;54:489–499. doi: 10.1016/j.jhep.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Abraham S.C., Kamath P.S., Eghtesad B., Demetris A.J., Krasinskas A.M. Liver transplantation in precirrhotic biliary tract disease: portal hypertension is frequently associated with nodular regenerative hyperplasia and obliterative portal venopathy. Am J Surg Pathol. 2006;30:1454–1461. doi: 10.1097/01.pas.0000213286.65907.ea. [DOI] [PubMed] [Google Scholar]
- 50.Tsuneyama K., Harada K., Katayanagi K. Overlap of idiopathic portal hypertension and scleroderma: report of two autopsy cases and a review of literature. J Gastroenterol Hepatol. 2002;17:217–223. doi: 10.1046/j.1440-1746.2002.02587.x. [DOI] [PubMed] [Google Scholar]
- 51.Sasajima T., Suzuki T., Mori K. A case of idiopathic portal hypertension associated with rheumatoid arthritis. Mod Rheumatol. 2006;16:92–96. doi: 10.1007/s10165-006-0456-8. [DOI] [PubMed] [Google Scholar]
- 52.Saito K., Nakanuma Y., Takegoshi K. Non-specific immunological abnormalities and association of autoimmune diseases in idiopathic portal hypertension. A study by questionnaire. Hepatogastroenterology. 1993;40:163–166. [PubMed] [Google Scholar]
- 53.Blendis L.M., Lovell D., Barnes C.G., Ritland S., Cattan D., Vesin P. Oesophageal variceal bleeding in Felty's syndrome associated with nodular regenerative hyperplasia. Ann Rheum Dis. 1978;37:183–186. doi: 10.1136/ard.37.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moots R.J., Elias E., Hubscher S., Salmon M., Emery P. Liver disease in twins with Felty's syndrome. Ann Rheum Dis. 1994;53:202–205. doi: 10.1136/ard.53.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Austin A., Campbell E., Lane P. Nodular regenerative hyperplasia of the liver and coeliac disease: potential role of IgA anticardiolipin antibody. Gut. 2004;53:1032–1034. doi: 10.1136/gut.2003.036806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seksik P., Mary J.Y., Beaugerie L. Incidence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with azathioprine. Inflamm Bowel Dis. 2011;17:565–572. doi: 10.1002/ibd.21330. [DOI] [PubMed] [Google Scholar]
- 57.Musumba C.O. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther. 2013;38:1025–1037. doi: 10.1111/apt.12490. [DOI] [PubMed] [Google Scholar]
- 58.Devarbhavi H., Abraham S., Kamath P.S. Significance of nodular regenerative hyperplasia occurring de novo following liver transplantation. Liver Transpl. 2007;13:1552–1556. doi: 10.1002/lt.21142. [DOI] [PubMed] [Google Scholar]
- 59.Morris-Stiff G., White A.D., Gomez D. Nodular regenerative hyperplasia (NRH) complicating oxaliplatin chemotherapy in patients undergoing resection of colorectal liver metastases. Eur J Surg Oncol. 2014;40:1016–1020. doi: 10.1016/j.ejso.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Rubbia-Brandt L., Lauwers G.Y., Wang H. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430–439. doi: 10.1111/j.1365-2559.2010.03511.x. [DOI] [PubMed] [Google Scholar]
- 61.Dutta D.V., Mitra S.K., Chhuttani P.N., Chakravarti R.N. Chronic oral arsenic intoxication as a possible aetiological factor in idiopathic portal hypertension [non cirrhotic portal fibrosis] in India. Gut. 1979;20:378–384. doi: 10.1136/gut.20.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santra A., Das Gupta J., De B.K., Roy B., GuhaMazumder D.N. Hepatic manifestations in chronic arsenic toxicity. Indian J Gastroenterol. 1999;18(4):152–155. [PubMed] [Google Scholar]
- 63.Hillaire S., Bonte E., Denninger M.H. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002;51:275–280. doi: 10.1136/gut.51.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wanless I.R., Peterson P., Das A., Boitnott J.K., Moore G.W., Bernier V. Hepatic vascular disease and portal hypertension in polycythemia vera and agnogenic myeloid metaplasia: a clinicopathological study of 145 patients examined at autopsy. Hepatology. 1990;12:1166–1174. doi: 10.1002/hep.1840120515. [DOI] [PubMed] [Google Scholar]
- 65.Sarin S.K., Mehra N.K., Agarwal A., Malhotra V., Anand B.S., Taneja V. Familial aggregation in noncirrhotic portal fibrosis: a report of four families. Am J Gastroenterol. 1987;82:1130–1133. [PubMed] [Google Scholar]
- 66.Talbot-Smith A., Syn W.K., MacQuillan G., Neil D., Elias E., Ryan P. Familial idiopathic pulmonary fibrosis in association with bone marrow hypoplasia and hepatic nodular regenerative hyperplasia: a new “trimorphic” syndrome. Thorax. 2009;64:440–443. doi: 10.1136/thx.2008.099796. [DOI] [PubMed] [Google Scholar]
- 67.Iyer V.H., Shyamkumar N.K., Surendrababu N.R.S. Hepatic venous pressure gradient in non-cirrhotic intrahepatic portal hypertension. J Clin Exp Hepatol. 2011;1:45. [Google Scholar]
- 68.Yutani C., Imakita M., Ishibashi-Ueda H., Okubo S., Naito M., Kunieda T. Nodular regenerative hyperplasia of the liver associated with primary pulmonary hypertension. Hum Pathol. 1988;19:726–731. doi: 10.1016/s0046-8177(88)80180-1. [DOI] [PubMed] [Google Scholar]
- 69.Chakraborty D., Sunil H.V., Mittal B.R., Bhattacharya A., Singh B., Chawla Y. Role of Tc99m sulfur colloid scintigraphy in differentiating non-cirrhotic portal fibrosis from cirrhosis liver. Indian J Nucl Med. 2010;25:39–42. doi: 10.4103/0972-3919.78247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scourfield A., Waters L., Holmes P. Non-cirrhotic portal hypertension in HIV-infected individuals. Int J STD AIDS. 2011;22(6):324–328. doi: 10.1258/ijsa.2010.010396. [DOI] [PubMed] [Google Scholar]
- 71.Nakanuma Y., Hoso M., Sasaki M. Histopathology of the liver in non-cirrhotic portal hypertension of unknown aetiology. Histopathology. 1996;28:195–204. doi: 10.1046/j.1365-2559.1996.d01-412.x. [DOI] [PubMed] [Google Scholar]
- 72.Nakanuma Y., Tsuneyama K., Ohbu M., Katayanagi K. Pathology and pathogenesis of idiopathic portal hypertension with an emphasis on the liver. Pathol Res Pract. 2001;197:65–76. doi: 10.1078/0344-0338-5710012. [DOI] [PubMed] [Google Scholar]
- 73.Roskams T., Baptista A., Bianchi L. Histopathology of portal hypertension: a practical guideline. Histopathology. 2003;42:2–13. doi: 10.1046/j.1365-2559.2003.01464.x. [DOI] [PubMed] [Google Scholar]
- 74.Goel A., Ramakrishna B., Madhu K. Idiopathic noncirrhotic intrahepatic portal hypertension is an ongoing problem in India. Hepatology. 2011;54:2274. doi: 10.1002/hep.24750. [DOI] [PubMed] [Google Scholar]
- 75.Madhu K., Ramakrishna B., Zachariah U., Eapen C.E., Kurian G. Non-cirrhotic intrahepatic portal hypertension. Gut. 2008;57:1529. doi: 10.1136/gut.2008.165480. [DOI] [PubMed] [Google Scholar]
- 76.Bernard P.H., Le Bail B., Cransac M. Progression from idiopathic portal hypertension to incomplete septal cirrhosis with liver failure requiring liver transplantation. J Hepatol. 1995;22:495–499. doi: 10.1016/0168-8278(95)80115-4. [DOI] [PubMed] [Google Scholar]
- 77.Dash S.C., Bhuyan U.N., Dinda A.K. Increased incidence of glomerulonephritis following spleno-renal shunt surgery in non-cirrhotic portal fibrosis. Kidney Int. 1997;52:482–485. doi: 10.1038/ki.1997.357. [DOI] [PubMed] [Google Scholar]
- 78.Pal S., Radhakrishna P., Sahni P., Pande G.K., Nundy S., Chattopadhyay T.K. Prophylactic surgery in non-cirrhotic portal fibrosis: is it worthwhile? Indian J Gastroenterol. 2005;24:239–242. [PubMed] [Google Scholar]
- 79.Nzeako U.C., Goodman Z.D., Ishak K.G. Hepatocellular carcinoma and nodular regenerative hyperplasia: possible pathogenetic relationship. Am J Gastroenterol. 1996;91:879–884. [PubMed] [Google Scholar]
- 80.Dhiman R.K. Spectrum of idiopathic noncirrhotic portal hypertension. J Clin Exp Hepatol. 2011;1:55–56. doi: 10.1016/S0973-6883(11)60122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarin S.K., Gupta N., Jha S.K. Equal efficacy of endoscopic variceal ligation and propranolol in preventing variceal bleeding in patients with noncirrhotic portal hypertension. Gastroenterology. 2010;139:1238–1245. doi: 10.1053/j.gastro.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 82.deFranchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Nayak N.C., Jain D., Saigal S., Soin A.S. Non-cirrhotic portal fibrosis: one disease with many names? An analysis from morphological study of native explant livers with end stage chronic liver disease. J Clin Pathol. 2011;64:592–598. doi: 10.1136/jcp.2010.087395. [DOI] [PubMed] [Google Scholar]
- 84.Pereboom I.T., de Boer M.T., Haagsma E.B., Hendriks H.G., Lisman T., Porte R.J. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108:1083–1091. doi: 10.1213/ane.0b013e3181948a59. [DOI] [PubMed] [Google Scholar]
- 85.Sankey E.A., Crow J., Mallett S.V. Pulmonary platelet aggregates: possible cause of sudden peroperative death in adults undergoing liver transplantation. J Clin Pathol. 1993;46:222–227. doi: 10.1136/jcp.46.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]


