Abstract
Background
Eighty percent (80%) of patients with Autoimmune hepatitis (AIH) respond to a combination of prednisolone and Azathioprine (AZA). Choice of treatment is limited for those who do not respond to this standard therapy. We evaluated the role of Mycophenolate mofetil (MMF) as a second line therapy in AIH.
Method
A retrospective observational study was carried out on all patients who received MMF for AIH.
Results
Twenty out of 117 patients with AIH received MMF due to AZA intolerance (18 patients) or refractory disease (2 patients). Median age of the study patients was 56 (18–79) years, Male, n = 3 (15%) and Female, n = 18 (85%). After a median follow-up period of 47 (5–83) months, 14 (73.6%) patients were still on MMF with biochemical remission, including 4 out of 5 patients with cirrhosis. One patient was lost to follow-up. Three patients were intolerant of MMF due to adverse events, and two had disease refractory to MMF. Both these patients with refractory disease to MMF were initially unresponsive to AZA therapy.
Conclusion
MMF is a safe second line agent in patients with autoimmune hepatitis including those with cirrhosis.
Keywords: autoimmune hepatitis, mycophenolate mofetil, azathioprine, non-responders
Abbreviations: AIH, autoimmune hepatitis; AZA, azathioprine; MMF, mycophenolate mofetil
Autoimmune hepatitis (AIH) is characterized by an immune-mediated injury to the hepatocytes that leads to inflammation, necrosis and fibrosis. It is more prevalent in females and can affect all age groups. AIH can encompass a broad spectrum of clinical presentations from the asymptomatic patient with abnormal liver function tests through to fulminant hepatic failure. The diagnosis is made on clinical, biochemical, immunological and histological parameters that comply with agreed international criteria.1,2 Eighty percent of patients respond to the standard therapy of combination corticosteroid and Azathioprine (AZA). Unfortunately, the treatment options for patients who fail to respond to standard therapy have historically been limited. But, the range of drugs for the treatment of AIH is broadening with clinicians looking to evaluate the role of alternative immunosuppressive and biological agents including Mycophenolate mofetil (MMF),3 budesonide,4 tacrolimus,5 cyclosporine6 and others.7,8
MMF is a potent immunosuppressant that blocks purine biosynthesis. It has been used extensively organ transplantation settings.9–11 Earlier observational studies have implied that MMF is a good second line agent for patients who fail to respond to, or who cannot tolerate AZA.12
The aim of the present study was to assess the indications and tolerability of second line therapy (MMF) in patients who did not tolerate azathioprine. The clinical efficacy of MMF for the treatment of AIH was also assessed.
Methods
A retrospective case note review was performed from the South West Liver Unit (Plymouth, United Kingdom) database identifying adult patients diagnosed with AIH from January 2000 to May 2010. Patients were diagnosed with AIH according to internationally agreed Hennes criteria.1,2,13 Patients included in the study were all patients with a diagnosis of AIH from the ages of 18 years onwards identified from the database. Patients were excluded from the study if they had co-existent liver diseases including: chronic hepatitis C infection, chronic hepatitis B infection, alcoholic liver disease as defined by an alcohol consumption >40 g alcohol/day in females and >60 g alcohol/day in males, fatty liver disease, previous liver transplantation, or hepatocellular carcinoma.
Biochemical parameters recorded included: bilirubin (μmol/L), alanine aminotransferase (ALT, IU/L), aspartate aminotransferase (AST, IU/L), albumin (g/dL), alkaline phosphatase (ALP, IU/L), full blood count, prothrombin time (seconds), Immunoglobulins (IgG, IgA, IgM), and autoimmune liver screen consisting of anti-nuclear antibodies (ANA), anti-smooth muscle antibodies (ASMA), and anti-mitochondrial antibodies (AMA). All patients were routinely screened for hepatitis B surface antigen (HBsAg) and hepatitis C antibody (HCVAb). If the later was positive, a hepatitis C RNA and genotype was requested. All patients underwent a percutaneous liver biopsy for the assessment of inflammatory activity and presence of fibrosis.
Patients were initially commenced on prednisolone 30–40 mg daily for two weeks. The dose was then tapered at 5 mg per week until the dose was 15 mg. Further dose reduction was carried out at 2.5 mg per week with the intention to stop or maintain at the lowest dose. AZA was commenced for maintenance of remission. Treatment response was assessed based on clinical symptoms and biochemical parameters such as AST, ALT and IgG levels as defined by agreed criteria.13 These were assessed at clinic visits. Drug compliance and adverse events were checked during every clinic visit by direct questioning. A treatment responder was defined as a patient who developed improvement in their AST and ALT, below twice the upper limit normal 3 months after treatment commencement. Remission was defined as normalisation of ALT and or AST after treatment commencement. A relapse was defined as a rise in ALT and or AST following a response. A non-responder to treatment was defined as a patient who did not tolerate the treatment due to adverse event or a true non-responder whose disease was refractory to the therapy despite adequate compliance. Liver histology was performed in cases where there was uncertainty of the diagnosis based on immunological, biochemical, and clinical parameters. In patients in whom there was an absence of clarity surrounding the diagnosis of AIH, immunosuppressive treatment was withheld until histological confirmation was available. Liver histology was repeated if there was a failure to respond to standard therapy or if there was a suspicion of an evolving overlap syndrome with co-existent features of primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC). Histology specimens were reviewed by a specialist liver histopathologist and stains were performed with haematoxylin and eosin (H&E), whilst reticulin and HVG stains were performed to detect presence of liver fibrosis. The fibrosis severity was staged using the scoring system devised by Ishak and colleagues.14 The diagnosis was made after the identification of features present in AIH including portal interface hepatitis, lympho-plasmacytic infiltration of the portal tracts, extension of inflammation from the portal tracts to lobules (interface hepatitis) and hepatocyte apoptosis with varying stages of fibrosis.15
The rationale for discontinuing AZA needed to be clearly documented in the clinical records and this was decided by the treating physician. If AZA was stopped because of drug intolerance due to adverse reactions these events were documented in the patient records and defined. Following cessation of AZA, patients were commenced on MMF 500 mg twice daily for 2 weeks, and if they tolerated this dose, it was increased to the maximally tolerated level up to a maximum of 1 g twice daily. Patients were kept under close outpatient monitoring and were followed up 2–4 weekly on commencement of MMF. If biochemical parameters stabilised they were followed up on a monthly basis until established on a stable dose of corticosteroids and MMF. Once on a stable dose, patients were reviewed on a 3-6 monthly basis. Biochemical and immunological parameters were routinely checked at each outpatient visit. Patients who did not respond to azathioprine therapy were switched over to MMF by the treating physician.
The endpoints for the study were as follows: to determine what group of patients were prescribed MMF and the indication for MMF commencement; the secondary endpoints were to determine what proportion of patients had a treatment response to MMF who had failed to have one with AZA.
Statistical Analysis
Descriptive statistics were described as median with inter-quartile ranges (IQR). Serial tests were analysed using Wilcoxan log rank test. All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS 13.0 for Windows; SPSS Inc., Chicago, Illinois, USA).
Results
One hundred seventeen (117) patients with AIH were identified. All patients had treatment induction with a tapering course of prednisolone (median dose 30 mg (range 7.5–40)) daily and remission with AZA (1–2 mg/kg daily). AZA was discontinued in 20 (17%) patients within 4 months of commencement. The clinical and demographics of these patients are outlined in Table 1. The median age of these patients were 56 (18–79) years and were predominantly Caucasians except for one patient of Asian background. The majority of the patients were females (Female n = 17 (85%), Male n = 3 (15%). Hennes score was 6 (probable AIH) in 11 patients and ≥7 (definite AIH) in 9 patients. Original scoring system showed ‘definitive’ AIH in 11 patients and ‘probable’ AIH in 9 patients. Five patients had histologic evidence of cirrhosis. Overlap syndromes with other conditions such as PBC, PSC and autoimmune cholangiopathy were noted in one patient each, respectively.
Table 1.
Demographics of Patients Who Received MMF (n = 20), According to AZA Intolerance or Resistance.
| Variables | AZA intolerance n = 18 |
AZA refractory disease n = 2 |
|---|---|---|
| Number of patients (years) | 55.5 (18–79) | 29.5 (18–41) |
| Sex, Male:Female | 1:8 | 1:1 |
| Cirrhosis (no. of patients) | 4 | 1 |
| Overlap syndrome (no. of patients) | 2 | 1 |
Azathioprine Treatment Failure
Out of the 20 patients 18 were intolerant of AZA over a period of 4 (0–24) months. The majority of them suffered from gastrointestinal related events, some had leucopenia and flu-like illness (Table 2). Only two patients had disease refractory to AZA therapy despite compliance. All patients received MMF 0.5–1.0 g twice daily and were followed up regularly with clinical and biochemical parameters. After a follow-up period of 47 (5–83) months, 14 (73.6%) patients were still on MMF with adequate disease remission, including the majority of patients with cirrhosis. One patient was lost to follow-up while 3 were intolerant of MMF and two patients had disease refractory to MMF despite good compliance. In MMF responders, the median AST levels pre-MMF, at week 12, 52 and at the end of 2 years of MMF were 63, 31 (p = 0.0002), 25 (p = 0.002) and 30 (p = 0.0001) IU/L, respectively. The decline in AST and ALT was statistically significant compared to the pre-MMF levels (Figure 1). Similarly, ALP and GGT levels declined significantly during the same follow-up period. In addition, serum IgG levels showed a significant decline in MMF responders. Median IgG levels at pre-MMF, week 12, 52 and year 2 were 18.2, 14 (p < 0.005) 12.6 (p < 0.005), 11.6 (p < 0.005) and 12.5 (p < 0.005) g/L (Figure 2).
Table 2.
Adverse Events and Reasons for AZA Discontinuation.
| Reason for AZA discontinuation | No. of patients reported (%)a |
|---|---|
| Gastrointestinal (nausea, vomiting or diarrhoea) | 9 (45) |
| Leucopenia | 5 (25) |
| Flu-like illness, myalgia | 4 (20) |
| Hair loss | 1 (5) |
| Non-responder | 2 (10) |
Some patients reported more than 1 adverse event.
Figure 1.
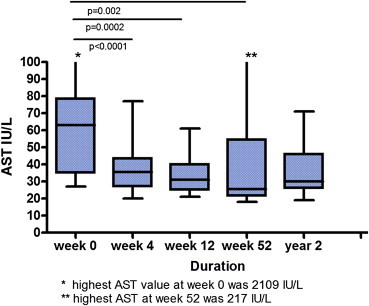
AST levels in MMF responders over a period of 2 years.
Figure 2.
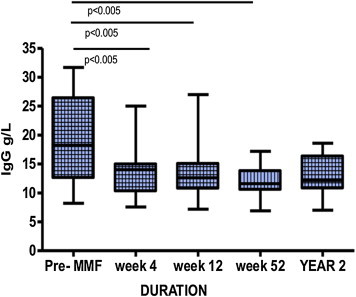
Serum IgG levels in MMF responders over a period of 2 years.
Mycophenolate Mofetil Treatment Failure
Non-response to MMF was noted in five (n = 5) patients. Three of these patients were intolerant of MMF due to adverse events, in spite of clinical and biochemical response. The most commonly reported adverse events were rash and hair loss. These occurred within the first year of treatment. MMF was stopped in a further two patients due to refractory disease (true non-responder), despite compliance. One of the true non-responder had AST elevation from 76 to 101 IU/L within 4 weeks of therapy and was cirrhotic. The other non-responder had a good initial response from AST 116 to 28 IU/L at 4 weeks but failed to maintain remission. The true non-responders were also non-responders to AZA.
Cirrhosis was noted in 5 patients with a median age of 52 years. All of who were females. Pre-MMF, week 12 and week 52 median AST and was 62, 32.5 and 27 and ALT was 56, 32.5, and 58 U/L, respectively. Similarly, IgG during the same periods of time was 17.2, 14.5 and 11.6 g/L. Four patients with cirrhosis who did not respond to AZA responded well to MMF therapy. One patient with no response to AZA and MMF eventually decompensated and required liver transplantation.
Current Treatment Regimens
Eight (57%) out of 14 patients were on MMF with low dose maintenance prednisolone. Four (28.5%) were on MMF monotherapy. One patient was taking MMF with prednisolone and ursodeoxycholic acid and one was on a combination of MMF and budesonide. There were no deaths in any of the treatment patients over the duration of the study. The current regimen for cirrhotic patients was MMF 1 g twice daily plus Prednisolone 7.5 mg a day.
Discussion
The combination of prednisolone and AZA is a well-established first line treatment for patients with AIH. Although the majority of patients respond well to this therapy, up to 20% of patients do not. The reasons for treatment failure include drug intolerance or refractory disease. Drug intolerance is usually due to adverse events or toxicity. Up to 0.3% of the population is homozygous for TPMT deficiency and thus, prone to AZA toxicity. The clinical features of TPMT deficiency include: skin rash, bone marrow toxicity, pancytopenia and an increased risk of opportunistic infections. Additional side effects include flu-like symptoms, nausea, vomiting, alopecia, hepatotoxicity and acute pancreatitis. In one series, approximately 20% of patients on AZA developed adverse reactions.16
A proportion of patients have refractory disease/drug intolerance where they do not respond to AZA. Evidence is not clear in terms of second line therapy for these patients. In the present study AZA was discontinued in 20 (17%) patients predominantly due to drug intolerance. This mostly occurred in the first 4 months after the commencement of AZA. Gastrointestinal and haematological side effects were the commonest causes of AZA intolerance. Only two patients had refractory disease.
MMF is a pro-drug that is converted in the liver to its active metabolite mycophenolic acid, a selective and reversible inhibitor of inosine monophosphate dehydrogenase, a rate-limiting enzyme in purine biosynthesis. MMF exerts selective anti-proliferative effects on T- and B-lymphocytes by inhibiting DNA synthesis. This drug is used extensively in transplantation medicine. The role of MMF in AIH was questioned following a publication from the Mayo clinic comparing empirical MMF with high dose corticosteroids in patients with refractory disease. This case series showed that MMF failed to induce biochemical remission (35% vs.0%, p = 0.1), nor did it prevent fibrosis progression or allow corticosteroid withdrawal.17 Yet a number of studies have contradicted these findings and suggested that MMF could have a role for some patients. Richardson et al18 showed that 5 of 7 patients with active disease responded to MMF, with a significant improvement in liver histology and good tolerability.
In a retrospective longitudinal study by Hlivko et al, 29 AIH patients (treatment naïve and previously failed therapy) received MMF. Remission was achieved in16 patients (55%) and 10 (34%) were intolerant of MMF due to adverse events.12 Similarly, MMF was prescribed for 15 patients intolerant of standard therapy where 12 patients received it in addition to prednisolone and 3 received MMF monotherapy. Follow-up over 3.5 years showed a good reduction in ALT levels from 92 to 61 (p = 0.03), inflammatory scores (2.59–1.14, p = 0.02) and Ishak fibrosis stage (4.1–2.5, p = 0.02). Furthermore, the drug was well tolerated.19 In a small case series, MMF was given to 5 patients refractory to AZA and steroids, where it was able to induce and maintain remission safely.20 Similar results have been observed from other patient series.21,22 In a multi-centre retrospective study of 16 patients who failed previous standard therapy received MMF. Eight (50%) had complete response, 2 (12.5%) had partial response, 4 (25%) had no response and 2 (12.5%) relapsed after 26.5 months follow-up.23 Zachou and colleagues performed a prospective study in 59 patients assessing the efficacy of MMF as first line therapy in patients with AIH. Eighty eight percent (88%) of them responded to the combination of tapering dose of prednisolone and MMF 1–2 g/day within 3 months and the remaining patients responded eventually. Interestingly, there were no non-responders. Prednisolone was completely withdrawn in 34 out of 59 patients (57.6%) over a period of 8 months.3
In our study the response to MMF was 74% (14 patients). MMF treatment failure was observed in 5 (26.3%) patients in whom drug intolerance was noted in three patients. Skin rash and hair loss were the commonest MMF related adverse events in our study population. Two patients did not respond to MMF. Interestingly, these 2 patients did not respond to AZA probably due to severe AIH or non-compliance. This is in line with the recent multi-centre study from Hennes et al where 36 patients received MMF as a second line treatment for steroid refractory AIH. After a follow-up of 15 months, 14 (39%) patients achieved remission, but 22 (61%) achieved an insufficient response. The majority of patients with disease refractory to AZA had a poor response to MMF.24 This area needs to be studied to identify patients who may not respond to MMF. Moreover, it may facilitate our understanding of the pathogenesis of AIH.
An interesting observation of our study was in patients with cirrhosis. Although, the numbers are small, 4 out of 5 patients tolerated MMF with disease remission. There was no significant adverse reactions and worsening of liver function in this high risk patients. This positive response to MMF should be confirmed in a large multi-centre study.
There are some limitations to our study. Firstly, the study involved a small number of patients with no control arm for comparison. Secondly, presence of slightly higher number of probable AIH could be explained by the fact that 5 patients had cirrhosis where classical findings of AIH may not persist and 3 patients had Overlap syndrome, which likely to confound the AIH scoring system. Thirdly, most patients were on a combination of MMF and prednisolone, and although the dose of corticosteroid was modest its role in disease remission should not be disregarded. Finally, we did not have sufficient post treatment liver biopsies to demonstrate histological remission. In summary, our study supports the efficacy, tolerability and safety of MMF in patients with AIH who were intolerant to azathioprine. However, in patients with refractory disease on AZA do not respond to MMF therapy. An alternative immunosuppressive agent should be considered in these patients. More studies are required to identify patients with refractory disease who may not respond to conventional immunosuppressants.
Conflicts of interest
All authors have none to declare.
References
- 1.Alvarez F., Berg P.A., Bianchi F.B. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 2.Hennes E.M., Zeniya M., Czaja A.J. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 3.Zachou K., Gatselis N., Papadamou G. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naive patients. J Hepatol. 2011;55:636–646. doi: 10.1016/j.jhep.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Manns M.P., Woynarowski M., Kreisel W. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139:1198–1206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 5.Aqel B.A., Machicao V., Rosser B. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805–809. doi: 10.1097/01.mcg.0000139050.67178.be. [DOI] [PubMed] [Google Scholar]
- 6.Malekzadeh R., Nasseri-Moghaddam S., Kaviani M.J. Cyclosporin A is a promising alternative to corticosteroids in autoimmune hepatitis. Dig Dis Sci. 2001;46:1321–1327. doi: 10.1023/a:1010683817344. [DOI] [PubMed] [Google Scholar]
- 7.Jothimani D., Cramp M.E., Mitchell J.D. Treatment of autoimmune hepatitis: a review of current and evolving therapies. J Gastroenterol Hepatol. 2011;26:619–627. doi: 10.1111/j.1440-1746.2010.06579.x. [DOI] [PubMed] [Google Scholar]
- 8.Yeoman A.D., Longhi M.S., Heneghan M.A. Review article: the modern management of autoimmune hepatitis. Aliment Pharmacol Ther. 2010;31:771–787. doi: 10.1111/j.1365-2036.2010.04241.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmeding M., Kiessling A., Neuhaus R. Mycophenolate mofetil monotherapy in liver transplantation: 5-year follow-up of a prospective randomized trial. Transplantation. 2011;92:923–929. doi: 10.1097/TP.0b013e31822d880d. [DOI] [PubMed] [Google Scholar]
- 10.Klupp J., Pfitzmann R., Langrehr J.M. Indications of mycophenolate mofetil in liver transplantation. Transplantation. 2005;80:S142–S146. doi: 10.1097/01.tp.0000187133.53916.8f. [DOI] [PubMed] [Google Scholar]
- 11.Manzia T.M., De Liguori Carino N., Orlando G. Use of mycophenolate mofetil in liver transplantation: a literature review. Transpl Proc. 2005;37:2616–2617. doi: 10.1016/j.transproceed.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 12.Hlivko J.T., Shiffman M.L., Stravitz R.T. A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6:1036–1040. doi: 10.1016/j.cgh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Manns M.P., Czaja A.J., Gorham J.D. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 14.Ishak K.G. Chronic hepatitis: morphology and nomenclature. Mod Pathol. 1994;7:690–713. [PubMed] [Google Scholar]
- 15.Gleeson D., Heneghan M.A. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611–1629. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 16.Hindorf U., Jahed K., Bergquist A. Characterisation and utility of thiopurine methyltransferase and thiopurine metabolite measurements in autoimmune hepatitis. J Hepatol. 2010;52:106–111. doi: 10.1016/j.jhep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Czaja A.J., Carpenter H.A. Empiric therapy of autoimmune hepatitis with mycophenolate mofetil: comparison with conventional treatment for refractory disease. J Clin Gastroenterol. 2005;39:819–825. doi: 10.1097/01.mcg.0000177260.72692.e8. [DOI] [PubMed] [Google Scholar]
- 18.Richardson Paul D., James P.D., Ryder S.D. Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol. 2000;33(3):371–375. doi: 10.1016/s0168-8278(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 19.Inductivo-Yu I., Adams A., Gish R.G. Mycophenolate mofetil in autoimmune hepatitis patients not responsive or intolerant to standard immunosuppressive therapy. Clin Gastroenterol Hepatol. 2007;5:799–802. doi: 10.1016/j.cgh.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Devlin S.M., Swain M.G., Urbanski S.J. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory to standard therapy. Can J Gastroenterol. 2004;18:321–326. doi: 10.1155/2004/504591. [DOI] [PubMed] [Google Scholar]
- 21.Brunt E.M., Di Bisceglie A.M. Histological changes after the use of mycophenolate mofetil in autoimmune hepatitis. Hum Pathol. 2004;35:509–512. doi: 10.1016/j.humpath.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Wolf D.C., Bojito L., Facciuto M. Mycophenolate mofetil for autoimmune hepatitis: a single practice experience. Dig Dis Sci. 2009;54:2519–2522. doi: 10.1007/s10620-008-0632-0. [DOI] [PubMed] [Google Scholar]
- 23.Chatur N., Ramji A., Bain V.G. Transplant immunosuppressive agents in non-transplant chronic autoimmune hepatitis: the Canadian association for the study of liver (CASL) experience with mycophenolate mofetil and tacrolimus. Liver Int. 2005;25:723–727. doi: 10.1111/j.1478-3231.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 24.Hennes E.M., Oo Y.H., Schramm C. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103:3063–3070. doi: 10.1111/j.1572-0241.2008.02180.x. [DOI] [PubMed] [Google Scholar]


