Abstract
Transcatheter intra-arterial therapies play a vital role in treatment of HCC due to the unique tumor vasculature. Evolution of techniques and newer efficacious modalities of tumor destruction have made these techniques popular. Various types of intra-arterial therapeutic options are currently available. These constitute: bland embolization, trans-arterial chemotherapy, trans-arterial chemo embolization with or without drug-eluting beads and trans-arterial radio embolization, which are elaborated in this review.
Keywords: hepatocellular carcinoma (HCC), trans-arterial chemoembolization (TACE), trans-arterial radiotherapy (TART), drug eluting bead (DEB), trans-arterial embolization (TAE)
Abbreviations: AFP, alpha feto protein; CR, complete response; HAIC, hepatic artery infusion chemotherapy; HCC, hepatocellular carcinoma; LA, laser ablation; OLT, orthotopic liver transplant; PD, progressive disease; PEI, percutaneous ethanol injection; PR, partial response; PVT, portal vein thrombosis; RFA, ablation; SD, stable disease; TACE, trans-arterial chemoembolization; TAE, Trans-arterial embolization; TART, trans-arterial radiotherapy
Transcatheter intra-arterial therapies are widely used loco-regional palliative therapies for the management of intermediate and relatively advanced stage of hepatocellular carcinoma (HCC).1–4 This is largely so because, the majority of the patients present with advanced disease at the outset and this precludes the use of curative treatment options.5
The current article expands on the consensus guidelines discussed and drafted at the INASL task force on hepatocellular carcinoma convened at Puri from February 7,8 2013. Last decade has witnessed important developments in practised intra-arterial therapies. These constitute-bland embolization, trans-arterial chemotherapy, trans-arterial chemoembolization with or without drug-eluting beads and trans-arterial radioembolization.6 All these types of intra-arterial options have a common goal of producing local tumor destruction but the mechanism of achieving this goal varies.
Rationale of transcatheter intra-arterial therapies
The liver has a dual blood supply. The dominant supply (about 75%–80%) is from the portal vein while the remaining 20%–25% is supplied by the hepatic artery. During carcinogenesis, HCC becomes increasingly “arterialized” and the hepatic artery becomes its sole supplier resulting in neo-angiogenesis.7 This fact is utilized by the different intra-arterial therapies for administering cytotoxic drugs/embolizing agents to the tumor through its feeding hepatic artery leading to local tumor destruction and sparing the normal liver parenchyma.
Trans-arterial embolization [Bland embolization, (TAE)]
Trans-arterial embolization was introduced in the 1950s.7 Embolization can be categorized into bland embolization (TAE), trans-arterial chemoembolization (TACE) and trans-arterial radiotherapy (TART). TAE produces terminal arterial blockade resulting in ischemia and cytotoxic damage to the tumor. TACE refers to a combination of the delivery of chemotherapy followed by embolization of the feeding arterial supply producing twin advantages of action. TART uses internal radiation for destroying the tumor(s) followed by concomitant embolization of the feeding artery.8
Embolization aims at occluding the arterial supply of the malignant liver tumor using embolizing agents producing tumor hypoxia and resultant tumor necrosis. Two types of embolizing agents are in use-temporary embolizing agents like gelatin sponge (available as particles, cubes, pellets or powder form), autologous blood clot and degradable starch microspheres, and the permanent or semi-permanent types like polyvinyl alcohol (PVA particles) and steel coils.9 Gelatin sponge is the most commonly used temporary embolizing material, which is 1–2 mm in diameter. Recanalization of the embolized artery generally takes place within 2 weeks.10,11 PVA particles produce distal arterial obstruction due to their smaller size and cause semi permanent/permanent occlusion. Hence, in HCC they are mainly used as a second line agent for embolizing the collaterals formed as a consequence to repeated embolization with other agents. No consensus exists on the most effective embolizing agent.
TAE has been previously used as an effective treatment for unresectable HCC. The survival benefit12 and marked anti tumoral effect13–15 on patients of unresectable HCC have been reported in many studies. A recent study showed a median survival of 21 months with the 1-, 2- and 3-year survival rates of 66%, 46% and 33% respectively.12
On comparing outcomes of TAE with TACE, a large meta-analysis (3 RCTs, 412 patients) demonstrated no survival difference between the two techniques9 However, another study found that compared to TAE, TACE significantly prolonged progression free survival and time to progression, but not the overall survival.16 Thus, though TAE is reported as efficacious, the ultimate outcome with TACE is better. Hence TAE is no longer recommended currently in the era when the procedure of TACE is available.
Trans-arterial chemoembolization (TACE)
TACE is a twin procedure of super-selective trans-arterial delivery of the chemotherapeutic agents through the feeding hepatic artery of the tumor followed by administration of the embolizing agents. (Figure 1) This provides a dual attack on the tumor, firstly by producing cytotoxic damage within the tumor by high concentration of the chemotherapeutic drugs and secondly by additional embolization which prevents the washout of the chemotherapeutic drugs from the tumor causing prolonged retention within the tumor site resulting in ischemic necrosis and enhanced tumor destruction.
Figure 1.
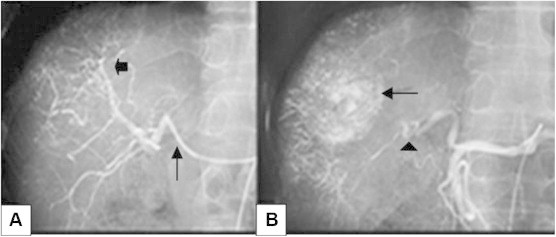
(A) Hepatic angiogram image showing super-selective cannulation of the hepatic artery (arrow) and neovascularity due to supply from the branches of the hepatic artery in the region of the tumor (small arrow) after contrast administration. The chemotherapeutic drug emulsion (doxorubicin 50 mg, cisplatin 100 mg, 10 ml of iodinated non-ionic contrast media and 20 ml of lipiodol) was injected followed by embolization of the feeding hepatic artery using gelatin sponge pledgets B) Post-TACE angiogram image depicting retained lipiodol in the tumor (black arrow) with embolized feeding artery (arrow head).
A. Indications and Contraindications of TACE
TACE was introduced in the late 1970s and since then this technique has come a long way.17,18 TACE is considered as the primary therapeutic option for unresectable HCC.5,19 A number of expert guidelines have commented on suitable candidates for TACE.20–22 The ideal candidates are patients of HCC with multi-nodular tumors with preserved liver function (Child-Pugh class A or B), without vascular invasion or extra-hepatic spread.14,23 These have been staged as BCLC B and C (with normal main portal vein) based on the Barcelona clinic liver cancer (BCLC) staging.24
TACE may also be offered to patients with inoperable small tumors (BCLC stage A), which are not amenable for local ablation due to technical limitations.25 It is also used as adjuvant therapy or as a means of down-staging the disease before liver transplantation, but whether these approaches provide ultimate survival benefit remains unclear26–29
Stringent selection criteria for TACE should be followed for favorable outcomes. Liver functional reserve is a crucial component and patients with Child-Pugh A or B7 without ascites should be encouraged, whilst those with Child-Pugh C status should be excluded since the ischemic insult can lead to severe adverse events.30 Contraindications of TACE are general contraindications to any intra-arterial procedure, allergic reaction to contrast media, pregnancy, poor liver function, presence of hepatofugal blood flow, main portal vein thrombosis, extra-hepatic metastases, WHO performance status more than 2 and end stage tumoral disease (Okuda III)10
Patients with thrombosis of the main portal vein (PVT) are considered a contraindication for TACE. This is due to the risk of aggravating hepatic insufficiency resulting from ischemia causing worst outcomes.23 However, some contradictory results of survival benefit have been reported31–34 PVT should therefore not be considered as an absolute contraindication for TACE in patients with preserved liver function.32,33,35 Better estimates of risk stratification in individual patients are needed.
No standardized protocol exists with regard to the choice of chemotherapeutic agent, dosage, dilution and the rate of injection.36 Doxorubicin, mitomycin and cisplatin are the common chemotherapeutic drugs used alone or in combination. Irrespective of the choice of the chemotherapeutic drug used, an emulsion of the drug is prepared with an iodized oil called lipiodol, which acts as a carrier and increases the intratumoral retention of the drugs causing a prolonged effect.37,38
Use of single or multiple chemotherapeutic drugs have been undertaken in the procedure of TACE.39,40 A recent three arm randomized trial has shown better response with less number of treatment sessions with the use of multiple drugs in TACE and with drug eluding beads as compared to TACE undertaken with a single drug.41
No standard embolizing agent, quantity or guidelines for re-treatment strategy have been recommended for TACE. More intense regimes of repeating TACE every 2 months has been shown to induce liver failure in high proportion of cases.27
A number of factors have been correlated with effective post TACE response viz; tumor diameter less than 5 cm, less than 50% replacement of liver by tumor tissue and unilobar tumor. Other prognostic factors include the alpha feto protein [AFP] level, differentiation of HCC, number of tumor nodules, portal vein thrombosis, presence of tumor capsule, and degree of lipiodol retention post-procedure.14,16,17,21,31,42 Large tumors with poor baseline liver function, have shown least benefit from TACE.
B. Role of TACE as a Combination Treatment
TACE has been successfully used in combination with various modalities for the treatment of relatively large tumors depicting better survival rates.43–46
Commonly tried combination modalities with improved results are, percutaneous ethanol injection (PEI), percutaneous acetic acid injection, radiofrequency ablation (RFA), laser ablation (LA), oral chemotherapy and targeted therapy.47–52
Repeated sessions of TACE can be used for downsizing the tumor and subsequently making the patient suitable for ablation. Complete response has been achieved in 90% of the large tumors subjected to repeated sessions of TACE followed by LA.53,54 Similar superior results have been achieved with combination with PEI, and RFA.55 Better quality of life scores have also been shown in patients treated with TACE with RFA than those treated with TACE alone.56 Combination of TACE and oral chemotherapeutic drug, sorafenib too is being tried with promising results.57
C. Role of TACE as an Adjuvant Therapy
TACE has multiple roles as a palliative treatment. TACE when performed prior to hepatic resection in patients with large tumors, produces reduction in tumor volume. It is safe, efficacious with high rates of pathological response.23 It destroys remnant cancer cells, decreases recurrence rate and prolongs survival.9,24 On the contrary, decreasing survival rates have also been reported, possibly due to hepatic and immunological damage occurring with TACE.25 Role of TACE as an adjuvant therapy thus remains quite controversial. TACE has also been used as adjuvant therapy for preventing postoperative recurrence. Cases where intrahepatic recurrence occurs following resection, TACE is successfully used as a palliative therapy.
TACE also has a role to play in HCC patients planned for orthotopic liver transplant (OLT). It provides a dual benefit - controlling tumor growth as well as producing tumor necrosis. This results in reduced chances of tumor dissemination during surgery. Moreover, down staging of the disease can be achieved making these patients suitable for OLT. TACE is thus the commonly used bridging modality in transplant patients. However, despite achieving tumor down staging, no significant advantage in survival and recurrence rate has been shown in the patients following OLT.26
D. Criteria for Successful and Failed TACE
Criteria for the success and failure of TACE relate to the technique and outcome of the procedure. Additionally superselective TACE with an attempt to deliver the drug very close to the tumor has a better outcome than whole-liver or lobar TACE. This minimizes embolization of non-targeted normal liver parenchyma.9,58 Superselective cannulation of the extra hepatic arterial feeders is even further difficult to negotiate because of the unusually long, tortuous and narrow caliber. Moreover, prolonged repeated attempts at cannulation may lead to spasm of the artery resulting in the procedure being futile.
Detailed assessment of the hepatic arterial anatomy on cross-sectional imaging (mutiphasic CT or MRI) is important. Presence of arterial anomalies, focal narrowing of the feeding artery at the origin or during its course, extreme tortuosity of the artery, narrow caliber, presence of multiple feeding arteries to the tumor can be picked up on imaging and such patients are poor candidates for the procedure of TACE.
Extra hepatic arterial supply is frequently observed in patients with large tumors near the liver surface or with an exophytic component or in contact with bare area of the liver or large tumors with direct invasion into the adjacent organs or extra capsular infiltration.59–64 Arterio-portal or arterio-venous shunting makes the procedure very difficult and risky. Attempts to block the shunting prior to delivery of chemotherapeutic drugs is important in order to prevent systemic dissemination.
Outcome of TACE is gauged by determining the local tumor response on multiphasic CT or MRI performed at four weeks post TACE. Tumor response assessment was earlier reported on the basis of the WHO criteria,65 RECIST66 followed by the EASL criteria.67 Recently, the modified RECIST (mRECIST)68 criteria has been recommended. The mRECIST and EASL criteria have shown to have independent correlation with the survival69,70 Based on these criteria, the local tumor response has been categorized into complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).47 Figure 2 depicts two malignant masses evaluated by CT showing CR in one mass and PR in the other mass subsequent to the treatment of TACE.
Figure 2.
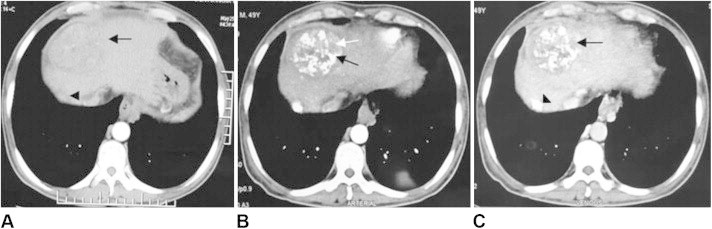
Pre TACE arterial phase CT image of the liver depicting two enhancing tumors, a large tumor in segment 8 (arrow) and a small tumor in segment 7 (arrow head). Patient was then subjected to TACE. Post TACE CT was done at 4 weeks. B) Arterial phase image showing inhomogenous dense lipiodol deposition in segment 8 (black arrow) and small areas of enhancement within the tumor (white arrow) which shows washout in the venous phase [arrow, (C)] suggestive of residual disease. The small segment 7 tumor shows complete coverage with dense lipiodol with no areas of enhancement in arterial and venous phase images B and C (arrow head), suggestive of complete response.
In properly selected patients, TACE is a relatively safe procedure with few minor complications, Post-embolization syndrome is the most frequently encountered complication seen in about 80–90% of the cases. This syndrome consists of fever, abdominal pain, nausea, vomiting, leukocytosis, and increased liver enzymes. It is self-limiting and treated symptomatically in most patients, but may progress to liver failure in very few. Treatment-induced renal dysfunction is also known.
E. Survival with TACE
Outcome of TACE has been studied extensively. A meta-analysis of nine RCTs have shown improved survival following TACE15 Significant tumor response is noted in 17%–61.9% cases, and poor complete tumor response 0–4.8% as the tumor cells remain viable following TACE.71,72 Limited effect of TACE occurs in cases of capsular invasion, extra capsular growth or vascular invasion.
Tumor size more than 5 cm is considered as a negative predictive factor affecting overall survival after TACE.73 Several RCTs, systematic reviews and meta-analysis have demonstrated a survival benefit post TACE in comparison with supportive care despite tumor size.14,16−18,25,31,74,75 A survival advantage of 82% and 63% for chemoembolization has been shown in comparison to 63% and 27% for supportive care (P = 0·009) at 1 and 2 years respectively.17 Marked tumor response, with significant survival benefit in the chemoembolization group has been documented.31
The largest cohort study of TACE for unresectable HCC on 8510 patients depicted a median survival of 34 months and a survival benefit at 1, 3, 5 and 7 year as 82%, 47%, 26% and 16%, respectively with negligible procedure-related mortality (0.5%).19 A solitary study from India76 in which TACE was done for relatively larger tumor size [upto16 cm (Mean size 6.6 ± 3.92 cm], the cumulative survival rate at 1,2,3 years was 66%,47% and 36.4% respectively(Table 1). The degree of liver damage, TNM stage and alpha-fetoprotein values were independent predictors of patient survival.
Table 1.
Cumulative Survival Following Trans-arterial Chemo-embolization in Advanced Hepatocellular Carcinoma.
| Study |
Number of patients |
Survival (%) |
||
|---|---|---|---|---|
| 1 year | 2 years | |||
| TACE {Gelfoam powder, doxorubicin [50 mg]} | Pelletier etal71 (J Hepatol, 1990) | 21 | 24 | NR |
| TACE {Gelfoam particles, cisplatin [70 mg]} | Group d'Etude de Traitment du Carcinome He'patocellulare30 (N Engl J Med, 1995) | 50 | 62 | 38 |
| TACE {Gelfoam, cisplatin [2 mg/kg]} | Pelletier et al75 (J Hepatol, 1998) | 37 | 51 | 24 |
| TACE {Gelfoam, cisplatin [maximum 30 mg]} | Lo et al31 (Hepatology, 2002) | 40 | 57 | 31 |
| TACE {Gelfoam, doxorubicin [25–75 mg/m2] | Llovet et al13 (Lancet, 2002) | 40 | 82 | 63 |
| TACE {Lipiodol, anticancer agent, gelatin sponge} | Takayasu et al19 (Gastroenterology, 2006) | 8510 | 82 | 47 (3 years) |
| TACE {Lipodol, Mitomycin C 10 mg} | Herber et al74 (Rofo, 2007) | 94 | 71.6 | 33.9 |
| TACE {doxorubicin 50 mg, cisplatin 100 mg, 10–20 ml of lipiodol, gelfoam} | Paul et al76 (Indian J Radiol Imaging, 2011) | 71 | 60.8 | 34.4 |
NR = not reported.
It is quite evident that the extent of benefit is based on the technique used and the stringent inclusion criteria. A recently published meta-analysis using the Cochrane of large number of studies, has questioned the beneficial role of TACE in treatment of intermediate HCC. Authors evaluated all the randomized trials comparing TACE or TAE versus placebo, sham or no intervention. They excluded the trials with inadequate randomization. Meta-analysis using inverse variance method and subgroup analysis regarding intervention regime, trial truncation or co-interventions was undertaken. The authors concluded that there was lack of firm evidence to either support or refute the procedure of TACE or TAE in patients with unresectable HCC.77 This generated a lot of controversy. Subsequently, the Society of Interventional Radiology contested this outcome of the Cochrane meta-analysis. They suggested that the conclusion about TACE made by this Cochrane collaboration had lack of validity because two different types of studies were included (TACE and TAE) in the same analysis and secondly there was a selection bias in the studied included. Bases on these facts, they suggested re-analysis of the data.78
TACE is a safe and effective procedure and has become an established palliative therapy for intermediate stage of HCC20–22. TACE also has a role to play as an adjunct to surgical resection, bridging therapy before transplantation and as a combination modality used in conjunction with other ablative therapies or oral chemotherapy for treatment of advanced HCC.
Trans-arterial Chemotherapy (TAC)
Trans-arterial chemotherapy (TAC) pertains to intra-arterial infusion of the chemotherapeutic drugs by selective catheterization of the hepatic artery targeting the tumor(s). The rationale for administering intra-arterial regional chemotherapy is to maximize the drug concentrations within the tumor with minimal systemic toxicity and increased local therapeutic response.79
TAC has been used in patients with unresectable with or without portal vein thrombosis (PVT).80 Role of trans-arterial therapies in the treatment of advanced HCC has been fully established. However, there is still controversy as to which therapy is better. Limited evidence comparing the twin techniques of TACE and TAC are available. No difference in survival has been observed between TACE and TAC,81 however, TAC produces less tumor necrosis than TACE, particularly, in tumors more than 3 cm.82 Additionally, a meta-analysis has suggested lack of clinical benefit following the procedure of TAC.83 Hence TAC is not preferred any more.
The chemotherapeutic drugs can also be periodically administered in an infusion through a catheter port directly through the hepatic artery and this technique is called Hepatic artery infusion chemotherapy (HAIC).84,85 This differs from the procedure of TAC in the sense that no arterial port is left behind and the need for repeating the procedure is assessed on imaging at one month. In HAIC, the arterial port catheter systems are kept for long-term use for delivery of intra-arterial chemotherapy, which historically were placed surgically earlier. However, now, with the advances in the minimally invasive techniques, these ports can be placed percutaneously. This allows easy and repetitive infusion therapy without causing much damage to the vessels and keeps the patient comfortable. HAIC has been primarily used for the treatment of liver metastases86,87 but has also been employed successfully for treating advanced HCC.84,85,88,89 Comparison of trans-arterial chemoembolization and infusion chemotherapy for treating unresectable HCC patients has shown no significant difference in the median overall survival time. Further, treatment intensification by adding embolization has not increased survival over chemoinfusion therapy alone.
Drug-eluting Beads
DEBs are microspheres that can be loaded with chemotherapeutic agents and used for chemoembolization (DEB-TACE). There are several commercially available products, the commonest being PVA microspheres (DC Bead; Biocompatibles, Farnham, UK), which imbibe doxorubicin by immersion, and acrylic copolymer microspheres (SAP Quadrophere [Hepasphere in Europe]; BioSphere Medical, Rockland, MA) are beads that can also imbibe doxorubicin or cisplatin. DEBs produce controlled, sustained release of chemotherapy at decreased peak plasma levels within the systemic circulation.90 Due to this the systemic toxicity of the chemotherapeutic drugs is much less and moreover the local response rates (tumor response) is better.
A. Drug Eluting Beads Versus TACE
Studies comparing DEB-TACE with conventional TACE (gelfoam-lipiodol particles) are encouraging.91 In initial reports in patients with preserved liver function (cirrhosis Child Pugh Class A and B) significantly decreased liver toxicity and lower systemic side effects (such as alopecia and marrow suppression) were observed with comparable local response and survival.92 Even in patients with more advanced disease (Child-Pugh B, ECOG 1, and bilobar or recurrent disease), a significantly better local response and survival has been noted.92 Improved local response and fewer treatment sessions have been reported with the use of DEB-TACE in comparison with conventional TACE. Decreased post embolization syndrome with 100–300 μm versus 400–600 μm beads has also been documented.93 DEB-TACE has been associated with fewer side effects and lower hospitalization rates compared to TACE. However, despite these advantages, no cost benefit has been documented.94 Since cost and availability is a major concern in the developing world, this procedure is limited to only few centers in India. Further studies are required to demonstrate the cost-effectiveness of DEB-TACE compared to conventional TACE.95
Trans-arterial radiotherapy (TART)
Trans-arterial radiotherapy (TART) refers to the percutaneous intra-arterial injection of micron-sized embolic particles loaded with a radioisotope. The intra-arterial radioactive compounds have the ability to deliver high doses of radiation to the small target volumes and produce relatively low toxicity profile.
Internal radiation with Iodine 131, Rhenium or Yttrium 90 glass or resin particles have shown antitumoral effects with a safe profile in the registries.96
The therapeutic efficacy of TART essentially depends upon the radiation as opposed to the ischemia associated with chemoembolization or pure embolization. The radio-biological effect results from beta irradiation, which favors destruction of tumor cells surrounding the microvessels containing a high radioactive ligand concentration.
Radioisotopes available for embolization are many. Both 131I-Lipiodol and Yttrium 90 m (90Y) are beta emitters. 90Y has a half-life of 64.2 h and a maximum tissue penetration of 10 mm within the liver. Because of the selective permeation of the tumor vascular plexus, 90Y radioembolization can deliver extremely high levels of radiation (up to 150 Gy), with tolerable exposure to normal parenchyma.97,98 With radio-iodinated lipiodol (131I-Lipiodol), a proportion of the compound migrates towards the tumor microenvironment through an increased vessel permeability. These radioactive compounds are slowly cleared because of the lack of lymphatic vessels, Kupffer cells, and endocytosis in the tumor tissue.131I–Lipiodol has disadvantages mainly due to the radioprotection constraints, logistics of maintaining inventory.
Hence, both Iodine-131 and Rhenium have become obsolete. Current procedures use 90Y referred to as 90Y selective internal radiation therapy (SIRT). Delivery of 90Y is either bound to resin (SIR-Spheres®, Sirtex Medical, Lane Cove, Australia) or embedded in a glass matrix (TheraSphere®, MDS Nordion, Kanata, ON, Canada). In 90Y microsphere therapy, pre-therapy intra-arterial 99mTc-labeled albumin macroaggregate (MAA) scintigraphy is mandatory to quantify potential liver-lung shunting and to exclude blood reflux to bowel, stomach or pancreas.99 The main difference between glass spheres and resin spheres is the activity in each sphere, being much higher in the former (about 2500 Bq) at production time compared to about 50 Bq in one resin sphere. Commercially available vials of glass and resin spheres contain up to 20 GBq and 3 GBq respectively. For the same desired activity, glass spheres probably have less embolic effect on micro vessels, being injected in much limited number. Between the SIR spheres and theraspheres, the later have less embolic load and can be tried even in cases with portal vein thrombosis. While the SIR spheres have a shelf life of 24 h, the theraspheres can be used till 15 days.
90Y-microspheres are delivered according to the tumor burden. Hepatic arterial catheterization should be performed. In general, the radiopharmaceutical is delivered at the lobar artery level to be distributed to numerous tumors in that lobe. Sometimes, in cases of lower tumor load, a more selective delivery at the segmental artery level is done. To ensure safe and accurate delivery of 90Y-microspheres, the procedure precludes mandatory evaluation of the shunt fraction and mesenteric vascular anatomy to assess arterial variants and to prevent radiation induced pneumonitis, gastric ulcers, pancreatitis etc. This may exclude a significant number of patients and thus add to the cost.
The side effects include transient fever, fatigue, mild transaminitis, thrombocytopenia with 131I lipiodol, and specifically with 90Y spheres; fatal risk of radiation pneumonitis and radiation induced liver fibrosis can occur.100
A. Indications and Contraindications of TART
Many patients of HCC have associated portal vein thrombosis, making them unsuitable for TACE, depending upon the level and severity of thrombosis. Such patients can be offered internal radioisotope therapy to prolong their survival and improve the quality of life.
TART is indicated in patients of unresectable HCC with Child-Pugh A disease, regardless of portal vein thrombosis. In Child-Pugh B and portal vein thrombosis, the outcomes have been poor. However in patients with branch portal vein involvement, it has been considered safe.101,102
Absolute contra-indications to TART include: contraindications to gaining trans-arterial access, and pregnancy/lactation. Relative contraindications are advanced child pugh score [> than 7], renal failure, significant extrahepatic disease and previous treatment with external radiation or oral treatment with the chemotherapeutic drug capebine within 2 months [due to increased risk of radiation induced liver disease]. Specific contraindications for Yttrium spheres are significant artrio-portal shunting, lung shunt fraction >20% [which may cause radiation pneumonitis], significant shunting to other organs like bowel/pancreas [can cause pancreatitis]. Though presence of significant concurrent shunting precludes the use of Yttrium spheres but 131Iodine is not contraindicated though it is not available widely.103
Multiple studies have demonstrated radiological and clinical response with 90Y.104 Patients with Child-Pugh A disease, regardless of portal vein thrombosis, have derived maximum benefit, whereas those with Child-Pugh B and portal vein thrombosis have had poor outcomes.105 Rhennium too has been shown to be effective, safe and well tolerated in patients with unresectable HCC. However, the availability of Rhennium is a constraint here. A multicentre trial from India depicted survival rates at 6, 9, 12, 24 and 36 months as 100%, 95 5%, 90.5%, 58.5% and 30.5% respectively with a median survival of 980 days.106 The overall survival rate of 46% and 23% at 1 and 2 years has also been shown by another multicentric study.107 It has also been tried in patients with post-radiofrequency ablation recurrences as well.108
B. TART Versus TACE
Compared with TACE, TART has shown comparable efficacy in terms of local response and time to progression and superiority in terms of downstaging for transplantation.109 Contradictory outcomes have been reported with TART recently. Retrospective studies comparing TART and TACE have depicted similar efficacy and toxicity110–112 while certain prospective studies have shown better local response and comparable survival with TART.96,105
In conclusion, transcatheter intra-arterial therapies are established effective techniques for the treatment of unresectable HCC. TACE is the established primary palliative therapeutic option for BCLC B, C (normal main portal vein) and in some select cases of BCLC A stage of HCC. The procedures of TAE and TAC have not documented any additional clinical benefit and are no longer practiced these days. DEB-TACE achieves good local response rates and less systemic side effects. Further evidence for long term survival and cost benefit are still emerging. Use of Iodine-131 and Rhenium for the procedure of TART have become obsolete. TART with Yttrium 90 is the current standard and may be preferred in select patients of advanced HCC with portal vein thrombosis having good liver function. These newer techniques of DEB-TACE and TART with Yttrium 90 have no doubt added on to the existing options but have limitations of cost and availability for wider use in the developing world. Choice of the type of intra-arterial therapy should be based on the careful assessment on patient to patient basis and taking into cognizance the important factors like indications, side effects, efficacy, availability, and cost-effectiveness.
Conflicts of interest
All authors have none to declare.
References
- 1.Yumoto Y., Jinno K., Tokuyama K. Hepatocellular carcinoma detected by iodized oil. Radiology. 1985;154:19–24. doi: 10.1148/radiology.154.1.2981112. [DOI] [PubMed] [Google Scholar]
- 2.Konno T., Maeda H., Iwai K. Effect of arterial administration of high-moleculan- weight anticancer agent SMANCS with lipid lymphographic agent on hepatoma. Eur J Cancer Clin Oncol. 1983;19:1053–1065. doi: 10.1016/0277-5379(83)90028-7. [DOI] [PubMed] [Google Scholar]
- 3.Ohishi H., Uchida H., Yoshimura H. Hepatocellular carcinoma detected by iodized oil: use of anticancer agents. Radiology. 1985;154:25–29. doi: 10.1148/radiology.154.1.2981114. [DOI] [PubMed] [Google Scholar]
- 4.Takayasu K., Shima Y., Muramatsu Y. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987;163:345–351. doi: 10.1148/radiology.163.2.3031724. [DOI] [PubMed] [Google Scholar]
- 5.Paul S.B., Manjunatha Y.C., Acharya S.K. Palliative treatment in advanced hepatocellular carcinoma: has it made any difference? Trop Gastroenterol. 2009;30:125–134. [PubMed] [Google Scholar]
- 6.Lewandowski R.J., Geschwind J.F., Liapi E., Salem R. Transcatheter intraarterial. therapies: rationale and overview. Radiology. 2011;259:641–657. doi: 10.1148/radiol.11081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markowitz J. The hepatic artery. Surg Gynecol Obstet. 1952;95:644–646. [PubMed] [Google Scholar]
- 8.Llovet J.M., Burroughs A., Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 9.Marelli L., Stigliano R., Triantos C. Trans-arterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Interv Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 10.Coldwell D.M., Stokes K.R., Yakes W.F. Embolotherapy: agents, clinical applications, and techniques. Radiographics. 1994;14:623–643. doi: 10.1148/radiographics.14.3.8066276. [DOI] [PubMed] [Google Scholar]
- 11.Chung J.W. Transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatogastroenterology. 1998;45:1236–1241. [PubMed] [Google Scholar]
- 12.Maluccio M.A., Covey A.M., Porat L.B. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2008;19:862–869. doi: 10.1016/j.jvir.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Llovet J.M., Real M.I., Montana X. Arterial embolisation or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J., Llovet J.M., Castells A. Trans-arterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–1583. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 15.Llovet J.M., Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 16.Morse M.A., Hanks B.A., Suhocki P. Improved time to progression for trans-arterial chemoembolization compared with trans-arterial embolization for patients with unresectable hepatocellular carcinoma. Clin Colorectal Cancer. 2012 Sep;11(3):185–190. doi: 10.1016/j.clcc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Yamada R., Sato M., Kawabata M., Nakatsuka H., Nakamura K., Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]
- 18.Yamada R., Nakatsuka H., Nakamura K. Hepatic artery embolization in 32 patients with unresectable hepatoma. Osaka City Med J. 1980;26:81–96. [PubMed] [Google Scholar]
- 19.Takayasu K., Arii S., Ikai I. Prospective cohort study of trans-arterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Kudo M., Izumi N., Kokudo N., HCC Expert Panel of Japan Society of Hepatology Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 21.European Association for The Study of The Liver, European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012 Apr;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J., Sherman M. Management of hepatocellular carcinoma. An update AASLD practice guidelines. Hepatology. 2010;0:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruix J., Sala M., Llovet J.M. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Cillo U., Vitale A., Grigoletto F. Prospective validation of the barcelona clinic liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Omata M., Laurentius L.A., Ryosuke T. Asian Pacific Association for the Study of the liver consensus recommendations on hepatocellular carcinoma. Hepatlogy Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chua T.C., Liauw W., Saxena A. Systematic review of neoadjuvant trans-arterial chemoembolization for resectable hepatocellular carcinoma. Liver Int. 2010;30:166–174. doi: 10.1111/j.1478-3231.2009.02166.x. [DOI] [PubMed] [Google Scholar]
- 27.Gerundi G.E., Neri D., Merenda R. Role of trans-arterial chemoembolization before liver resection for hepatocarcinoma. Liver Transpl. 2000;6:619–626. doi: 10.1053/jlts.2000.8312. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J.P., Zhou W.P., Fu S.Y., Shan Y.F., Yao X.P., Wu M.C. Influence of preoperative transcatheter arterial chemoembolization on liver function in patients with resectable large hepatocellular carcinoma. Gandan Waike Zazhi. 2003;11:256–258. [Google Scholar]
- 29.Stockland A.H., Walser E.M., Paz-Fumagalli R., McKinney J.M., May G.R. Preoperative chemoembolization in patients with hepatocellular carcinoma undergoing liver transplantation: influence of emergent versus elective procedures on patient survival and tumor recurrence rate. Cardiovasc Interv Radiol. 2007;30(5):888–893. doi: 10.1007/s00270-007-9111-9. [DOI] [PubMed] [Google Scholar]
- 30.Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332(11):1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 31.Lo C.M., Ngan H., Tso W.K. Randomized controlled trial of trans-arterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 32.Kim K.M., Kim J.H., Park I.S. Reappraisal of repeated trans-arterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806–814. doi: 10.1111/j.1440-1746.2008.05728.x. [DOI] [PubMed] [Google Scholar]
- 33.Luo J., Guo R.P., Lai E.C.H. Trans-arterial chemoembolization for unresectable hepatocellular carcinoma with Portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 34.De Lope C.R., Tremosini S., Forner A., Reig M., Bruix J. Management of HCC. J Hepatol. 2012;56(suppl 1):S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 35.Shi M., Chen J.A., Lin X.J. Trans-arterial chemoembolization as initial treatment for unresectable hepatocellular carcinoma in southern China. World J Gastroenterol. 2010;16:264–269. doi: 10.3748/wjg.v16.i2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Association for Study of Liver European Organisation for Research and treatment of Cancer. Eur J Cancer. 2012;48:599–641. doi: 10.1016/j.ejca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Raoul J.L., Heresbach D., Bretagne J.F. Chemoembolization of hepatocellular carcinomas: a study of the biodistribution and pharmacokinetics of doxorubicin. Cancer. 1992;70:585–590. doi: 10.1002/1097-0142(19920801)70:3<585::aid-cncr2820700308>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura H., Hashimoto T., Oi H., Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783–786. doi: 10.1148/radiology.170.3.2536946. [DOI] [PubMed] [Google Scholar]
- 39.Lee J., Park J.O., Kim W.S. Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004;54:385–390. doi: 10.1007/s00280-004-0837-7. [DOI] [PubMed] [Google Scholar]
- 40.Bobbio-Pallavicini E., Porta C., Moroni M. Epirubicin and etoposide combination chemotherapy to treat hepatocellular carcinoma patients: a phase II study. Eur J Cancer. 1997;33:1784–1788. doi: 10.1016/s0959-8049(97)00163-9. [DOI] [PubMed] [Google Scholar]
- 41.Petruzzi N.J., Frangos A.J., Fenkel J.M. Single-center comparison of three chemoembolization Regimens for hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24:266–273. doi: 10.1016/j.jvir.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 42.Georgiades C.S., Hong K., D'Angelo M., Geschwind J.F. Safety and efficacy of trans-arterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–1659. doi: 10.1097/01.RVI.0000182185.47500.7A. [DOI] [PubMed] [Google Scholar]
- 43.Yau T., Chan P., Epstein R., Poon R.T. Management of advanced hepatocellular carcinoma in the era of targeted therapy. Liver Int. 2009;29:10–17. doi: 10.1111/j.1478-3231.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 44.Bartolozzi C., Lencioni R., Caramella D. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812–818. doi: 10.1148/radiology.197.3.7480761. [DOI] [PubMed] [Google Scholar]
- 45.Narvaez-Lugo J., Cáceres W.W., Toro D.H. Transcatheter arterial chemoembolization and percutaneous ethanol injection for hepatocellular carcinoma: a retrospective review of the veterans affairs Caribbean healthcare system. Cancer Control. 2008;15:80–85. doi: 10.1177/107327480801500110. [DOI] [PubMed] [Google Scholar]
- 46.Xu Z.M., Wang J.H., Zhen Z.J., Chen H.W., Cui W.Z. Percutaneous radiofrequency ablation combined with transcatheter arterial chemoembolization and percutaneous ethanol injection for recurrent small hepatocellular carcinoma. Nan Fang Yi Ke Da Xue XueBao. 2006;26:1626–1628. [PubMed] [Google Scholar]
- 47.Seror O., N’Kontchou G., Haddar D. Large infiltrative hepatocellular carcinomas: treatment with percutaneous intraarterial ethanol injection alone or in combination with conventional percutaneous ethanol injection. Radiology. 2005;234:299–309. doi: 10.1148/radiol.2341031008. [DOI] [PubMed] [Google Scholar]
- 48.Decaens T., Roudot-Thoraval F., Bresson-Hadni S. Impact of pretransplantation trans-arterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11(7):767–775. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 49.Graziadei I.W., Sandmueller H., Waldenberger P. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9(6):557–563. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 50.Yamakado K., Nakatsuka A., Ohmori S. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225–1232. doi: 10.1016/s1051-0443(07)61969-1. [DOI] [PubMed] [Google Scholar]
- 51.Pacella C.M., Bizzarri G., Cecconi P. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219(3):669–678. doi: 10.1148/radiology.219.3.r01ma02669. [DOI] [PubMed] [Google Scholar]
- 52.Graf H., Jüngst C., Straub G. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion. 2008;78:34–38. doi: 10.1159/000156702. [DOI] [PubMed] [Google Scholar]
- 53.Zangos S., Eichler K., Balzer J.O. Large-sized hepatocellular carcinoma (HCC): a neoadjuvant treatment protocol with repetitive trans-arterial chemoembolization (TACE) before percutaneous MR-guided laser-induced thermotherapy (LITT) Eur Radiol. 2007;17(2):553–563. doi: 10.1007/s00330-006-0343-x. [DOI] [PubMed] [Google Scholar]
- 54.Vogl T.J., Mack M.G., Balzer J.O. Liver metastases: neoadjuvant downsizing with trans-arterial chemoembolization before laser-induced thermotherapy. Radiology. 2003;229:457–464. doi: 10.1148/radiol.2292021329. [DOI] [PubMed] [Google Scholar]
- 55.Kim S.K., Lim H.K., Kim Y.H. Hepatocellular carcinoma treated with radiofrequency ablation: spectrum of imaging findings. RadioGraphics. 2003;23:107–121. doi: 10.1148/rg.231025055. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y.B., Chen M.H., Yan K., Yang W., Dai Y., Yin S.S. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Qual Life Res. 2007;16:389–397. doi: 10.1007/s11136-006-9133-9. [DOI] [PubMed] [Google Scholar]
- 57.Bai W., Wang Y.J., Zhao Y. Sorafenib in combination with trans-arterial chemoembolization improves survival of unresectable hepatocellular carcinoma: a propensity-score matching study. J Dig Dis. 2013;14:181–190. doi: 10.1111/1751-2980.12038. [DOI] [PubMed] [Google Scholar]
- 58.Iwazawa J., Ohue S., Mitani T. Identifying feeding arteries during tace of hepatic tumors: comparison of c-arm ct and digital subtraction angiography. AJR. 2009 apr;192(4):1057–1063. doi: 10.2214/AJR.08.1285. [DOI] [PubMed] [Google Scholar]
- 59.Kim H.C., Chung J.W., Lee W., Jae H.J., Park J.H. Recognizing extrahepatic collateral vessels that Supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. 2005 Oct;25(suppl 1):S25–S39. doi: 10.1148/rg.25si055508. [DOI] [PubMed] [Google Scholar]
- 60.Chung J.W., Kim H.C., Yoon J.H. Transcatheter arterial chemoembolization of hepatocellular carcinoma: prevalence and causative factors of extrahepatic collateral arteries in 479 patients. Korean J Radiol. 2006 Oct-Dec;7(4):257–266. doi: 10.3348/kjr.2006.7.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng L.F., Ma K.F., Fan W.C., Yung A.W., Li T.M., Wong C.S. Hepatocellular carcinoma with extrahepatic collateral arterial supply. J Med Imaging Radiat Oncol. 2010 Feb;54(1):26–34. doi: 10.1111/j.1754-9485.2010.02134.x. [DOI] [PubMed] [Google Scholar]
- 62.Kim H.C., Chung J.W., Choi S.H. Hepatocellular carcinoma with internal mammary artery supply: feasibility and efficacy of trans-arterial chemoembolization and factors affecting patient prognosis. J Vasc Interv Radiol. 2007 May;18(5):611–619. doi: 10.1016/j.jvir.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 63.Miyayama S., Yamashiro M., Okuda M. Chemoembolization for the treatment of large hepatocellular carcinoma. J Vasc Interv Radiol. 2010 Aug;21(8):1226–1234. doi: 10.1016/j.jvir.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 64.Nakai M., Sato M., Kawai N. Hepatocellular carcinoma: involvement of the internal mammary artery. Radiology. 2001;219:147–152. doi: 10.1148/radiology.219.1.r01mr28147. [DOI] [PubMed] [Google Scholar]
- 65.Miller A.B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 67.Bruix J., Sherman M., Llovet J.M. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 68.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 69.Shim J.H., Lee H.C., Kim S.O. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012 Feb;262(2):708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]
- 70.Kim B.K., Kim K.A., Park J.Y. Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolization. Eur J Cancer. 2013 Mar;49(4):826–834. doi: 10.1016/j.ejca.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 71.Pelletier G., Roche A., Ink O. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990;11:181–184. doi: 10.1016/0168-8278(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 72.Jansen M.C., van Hillegersberg R., Chamuleau R.A., van Delden O.M., Gouma D.J., van Gulik T.M. Outcome of regional and local ablative therapies for hepatocellular carcinoma: a collective review. Eur J Surg Oncol. 2005;31:331–347. doi: 10.1016/j.ejso.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Liad’o L., Virgilli J., Figueras J. A prognostic index of survival of patients with unrestable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 74.Herber S.C., Otto G., Woerns M. Single center experience over a 5-year period with sequential transarterial chemoembolization (TACE) in the treatment of hepatocellular carcinoma. Rofo. 2007 Mar;179(3):289–299. doi: 10.1055/s-2006-927355. [DOI] [PubMed] [Google Scholar]
- 75.Pelletier G., Ducreux M., Gay F. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129–134. doi: 10.1016/s0168-8278(98)80187-6. [DOI] [PubMed] [Google Scholar]
- 76.Paul S.B., Gamanagatti S., Sreenivas V. Trans-arterial chemoembolization (TACE) in patients with unresectable hepatocellular carcinoma: experience from a tertiary care centre in India. Indian J Radiol. Imaging. 2011;21:113–120. doi: 10.4103/0971-3026.82294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oliveri R.S., Wetterslev J., Gluud C. Trans-arterial (chemo) embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011 Mar 16;(3):CD004787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PubMed] [Google Scholar]
- 78.Rose S.C., Kikolski S.G., Gish R.G., Kono Y., Loomba R., Hemming A.W. Society of interventional radiology critique and commentary on the Cochrane Report on trans-arterial (chemo)embolisation. Hepatology. 2013;57:1675–1676. doi: 10.1002/hep.26000. [DOI] [PubMed] [Google Scholar]
- 79.Collins J.M. Pharmacologic rationale for regional drug delivery. J Clin Oncol. 1984;2(5):498–504. doi: 10.1200/JCO.1984.2.5.498. [DOI] [PubMed] [Google Scholar]
- 80.Tsochatzis E.A., Germani G., Burroughs A.K. Trans-arterial chemoembolization, trans-arterial chemotherapy, and intra-arterial chemotherapy for hepatocellular carcinoma treatment. Semin Oncol. 2010;37:89–93. doi: 10.1053/j.seminoncol.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Okusaka T., Kasugai H., Shioyama Y. Trans-arterial chemotherapy alone versus trans-arterial chemoembolization for hepatocellular carcinoma: a randomized phase III trial. J Hepatol. 2009;51:1030–1036. doi: 10.1016/j.jhep.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Maeda S., Fujiyama S., Tanaka M., Ashihara H., Hirata R., Tomita K. Survival and local recurrence rates of hepatocellular carcinoma patients treated by trans-arterial chemolipiodolization with and without embolization. Hepatol Res. 2002;23:202–210. doi: 10.1016/s1386-6346(01)00174-7. [DOI] [PubMed] [Google Scholar]
- 83.Leung D.A., Goin J.E., Sickles C., Raskay B.J., Soulen M.C. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321–326. doi: 10.1016/s1051-0443(07)61911-3. [DOI] [PubMed] [Google Scholar]
- 84.Tajima T., Yoshimitsu K., Kuroiwa T. Percutaneous femoral catheter placement for long-term chemotherapy infusions: preliminary technical results. AJR. 2005;184:906–914. doi: 10.2214/ajr.184.3.01840906. [DOI] [PubMed] [Google Scholar]
- 85.Herrmann K.A., Waggershauser T., Sittek H., Reiser M.F. Liver intraarterial chemotherapy: use of the femoral artery for percutaneous implantation of catheter-port systems. Radiology. 2000;215:294–299. doi: 10.1148/radiology.215.1.r00ap14294. [DOI] [PubMed] [Google Scholar]
- 86.Kemeny N., Daly J., Reichman B., Geller N., Botet J., Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma: a randomized trial. Ann Intern Med. 1987;107(4):459–465. doi: 10.7326/0003-4819-107-4-459. [DOI] [PubMed] [Google Scholar]
- 87.Martin J.K., Jr., O’Connell M.J., Wieand H.S. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg. 1990;125(8):1022–1027. doi: 10.1001/archsurg.1990.01410200086013. [DOI] [PubMed] [Google Scholar]
- 88.Ando E., Tanaka M., Yamashita F. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 89.Seno H., Ito K., Kojima K., Nakajima N., Chiba T. Efficacy of an implanted drug delivery system for advanced hepatocellular carcinoma using 5-fluorouracil, epirubicin and mitomycin C. J Gastroenterol Hepatol. 1999;14:811–816. doi: 10.1046/j.1440-1746.1999.01956.x. [DOI] [PubMed] [Google Scholar]
- 90.Varela M., Real M.I., Burrel M. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 91.Van Malenstein H., Maleux G., Vandecaveye V. A randomized phase II study of drug-eluting beads versus trans-arterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34:368–376. doi: 10.1159/000329602. [DOI] [PubMed] [Google Scholar]
- 92.Lammer J., Malagari K., Vogl T. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Interv Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Padia S.A., Shivaram G., Bastawrous S. Safety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particles. J Vasc Interv Radiol. 2013;24:301–306. doi: 10.1016/j.jvir.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 94.Prajapati H.J., Dhanasekaran R., El-Rayes B.F. Safety and efficacy of doxorubicin drug-eluting bead trans-arterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24:307–315. doi: 10.1016/j.jvir.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 95.Vadot L., Boulin M., Malbranche C. Result and cost of hepatic chemoembolization with drug eluting beads in 21 patients. Diagn Interv Imaging. 2013 Jan;94(1):53–59. doi: 10.1016/j.diii.2012.05.001. Epub 2012 Nov 10. [DOI] [PubMed] [Google Scholar]
- 96.Sangro B., Carpanese L., Cianni R. Survival after Yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 97.Kennedy A.S., Nutting C., Coldwell D., Gaiser J., Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 98.Gulec S.A., Mesoloras G., Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med. 2006;47:1209–1211. [PubMed] [Google Scholar]
- 99.Lambert B., Mertens J., Sturm E.J., Stienaers S., Defreyne L., D'Asseler Y. 99mTc-labelled macroaggregated albumin (MAA) scintigraphy for planning treatment with 90Y microspheres. Eur J Nucl Med Mol Imaging. 2010;37:2328–2333. doi: 10.1007/s00259-010-1566-2. [DOI] [PubMed] [Google Scholar]
- 100.Strigari L., Sciuto R., Rea S. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: radiobiologic considerations. J Nucl Med. 2010;51:1377–1385. doi: 10.2967/jnumed.110.075861. [DOI] [PubMed] [Google Scholar]
- 101.Kulik L.M., Carr B.I., Mulcahy M.F. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(1):71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 102.Inarrairaegui M., Thurston K.G., Bilbao J.I. Radioembolization with use of Yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2010;21:1205–1212. doi: 10.1016/j.jvir.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 103.Raoul J.L., Guyader D., Bretagne J.F. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26(5):1156–1161. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 104.Geschwind J.F., Salem R., Carr B.I. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 105.Salem R., Lewandowski R.J., Mulcahy M.F. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 106.Kumar A., Srivastava D.N., Chau T.T. Inoperable hepatocellular carcinoma; trans-arterial rhenium-188 HDD-labelled iodised oil for treatment-prospective multicentre clinical TRIAL. Radiology. 2007;243:509–519. doi: 10.1148/radiol.2432051246. [DOI] [PubMed] [Google Scholar]
- 107.Bernal P., Raoul J.L., stare J. International Atomic Energy Agency sponsored multination study of intra-arterial Rhenium 188 labeled lipiodol in the treatment of inoperable hepatocellular carcinoma: results with special emphasis on prognostic value dosimetric study. Semin Nucl Med. 2008;38:s40–s45. doi: 10.1053/j.semnuclmed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 108.Kumar A., Bal C., Srivastava D.N. Management of multiple intrahepatic recurrences after radiofrequency ablation of hepatocellular carcinoma with rhenium-188-HDD-lipiodol. Eur J Gastroenterol Hepatol. 2006;18:219–223. doi: 10.1097/00042737-200602000-00016. [DOI] [PubMed] [Google Scholar]
- 109.Lewandowski R.J., Kulik L.M., Riaz A. A comparative analysis of trans-arterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transpl. 2009 Aug;9(8):1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 110.Carr B.I., Kondragunta V., Buch S.C., Branch R.A. Therapeutic equivalence in survival for hepatic arterial chemoembolization and Yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305–1314. doi: 10.1002/cncr.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kooby D.A., Egnatashvili V., Srinivasan S. Comparison of Yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 112.Salem R., Lewandowski R.J., Kulik L. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140 doi: 10.1053/j.gastro.2010.10.049. 497.e2–507.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]


