Abstract
The current American Association for the Study of Liver Diseases (AASLD) guideline provides strategies for achieving the diagnosis of hepatocellular carcinoma (HCC) based on the size of liver nodules seen on surveillance imaging. For lesions less than 1 cm in size, follow-up surveillance imaging is recommended. Lesions larger than 2 cm require typical radiological hallmark on dynamic imaging. Lesions of 1–2 cm in size require typical imaging features including intense uptake of contrast during arterial phases followed by decreased enhancement during portal venous phases on at least 2 imaging modalities. In cases of atypical radiological features of the suspected lesion, tissue diagnosis either by fine needle aspiration or biopsy should be obtained. Although fine needle aspiration could give a smaller risk of seeding than biopsy, biopsy has been preferred over cytology. Percutaneous biopsy of HCC carries a potential risk of tumor seeding along the needle tract. However the risk is low and there is no clear evidence of post transplant recurrence due to needle tract seeding. Histopathologic assessment can differentiate between premalignant lesions such as dysplastic nodules and early HCC. Atypical variants of HCC can be recognized morphologically which may have associated prognostic value.
Keywords: HCC, pathology, tissue diagnosis
Abbreviations: AASLD, American Association for the Study of Liver Diseases; AFP, alpha-fetoprotein; CK7, cytokeratin 7; CT, computed tomography; DN, dysplastic nodules; EASL, European Association for the Study of the Liver; EMA, epithelial membrane antigen; EpCAM, epithelial cell adhesion molecule; FNA, fine needle aspiration; GPC-3, glypican-3; GS, glutamine synthetase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HSP70, heat shock protein 70; MRI, magnetic resonance imaging; pCEA, polyclonal carcinoembryonic antigen; USG, ultrasonography
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world.1 Its incidence is expected to rise in the future due to anticipated increase in cirrhosis secondary to viral hepatitis. Over the past 2 decades, the incidence of HCC has tripled, and hepatitis C virus (HCV) related HCC is the fastest-rising cause of cancer-related death in the United States.2–4
Hepatocellular carcinoma develops within an established background of chronic liver disease in 70–90% of all patients.5 The most frequent risk factor for HCC is chronic hepatitis B virus (HBV) infection in Asia and Africa. However HCV predominates as a risk factor in Europe and Japan.2 Other well established risk factors are alcoholism, non-alcoholic fatty liver disease and diabetes.6–8
Treatment depends on early diagnosis by screening high-risk patients when HCC is small and remains localized to the liver. Various studies suggest surveillance of HCC in cirrhotic patients irrespective of its etiology. Surveillance of non-cirrhotic patients is also advocated, especially in HBV carriers with serum viral load >10,000 copies/ml9 or HCV infected patients with bridging fibrosis. Patients with HCV infection and advanced fibrosis remain at risk for HCC even after achieving sustained virological response following antiviral treatment.
The preferred imaging method for screening is ultrasonography (USG) which is well tolerated and widely available. However, the sensitivity of USG for HCC detection is low because small nodules can be missed in a cirrhotic liver.10 Use of contrast-enhanced USG improves the diagnostic performance of USG for HCC.
The most used serological test in clinical setting for screening is alpha-fetoprotein (AFP) but it is no longer considered as a surveillance test by most recent guidelines of American Association for the Study of Liver Diseases (AASLD) due to the same reason of low sensitivity.11 Computed tomography (CT) and magnetic resonance imaging (MRI) have a high sensitivity (55%–91%) and specificity (77%–96%) in diagnosing HCC.10
According to the guidelines established by European Association for the Study of the Liver (EASL) and the AASLD, a nodule larger than 2 cm that displays a typical vascular pattern on contrast-enhanced CT or contrast-enhanced MRI can be considered HCC without biopsy.12,13
For lesions measuring between 1 and 2 cm, the diagnosis of HCC is confirmed when typical vascular pattern is seen on both the imaging modalities. Otherwise, these lesions should not be treated as HCC without histological evidence because of a rate of false positives as high as 20%.14,15
Recent prospective studies have reported that up to 67% of new nodules smaller than 2 cm identified during surveillance imaging in patients with cirrhosis are indeed HCC.16 Although the specificity of contrast enhanced MRI has been reported as high as 96% for hepatic nodules of 1–2 cm in size, a significant proportion of small HCC may appear hypovascular or have atypical features, resulting in a false-negative rate of 20%–38%.17
Finally, lesions < 1 cm in diameter may be especially difficult to characterize, even with the best imaging techniques. A lesion less than 1 cm in size should be followed by USG examination repeated at 3 months. These recommendations might be applied to patients with partially developed and fully established cirrhosis and chronic hepatitis B. For all other patients without cirrhosis, the possibility of HCC is much lower; therefore biopsy should be done for definite diagnosis of HCC.12,13
In comparison with EASL and AASLD criteria, the consensus statement from the Asian Oncology Summit from 2009 recommends that for any nodule, regardless of size, the characteristic features on contrast-enhanced CT or contrast-enhanced MRI is sufficient for diagnosis of HCC, and obviates the need for biopsy.18
Is tissue diagnosis required?
Histologic diagnosis is not necessary when the diagnosis of HCC is determined by diagnostic imaging (Level of evidence 1a, grade of recommendation A). Histologic diagnosis by biopsy is indicated when imaging findings are atypical (Level of evidence 3b, grade of recommendation C).
Fine needle aspiration (FNA) biopsy is not without its complications, though rare. The role and efficacy of FNA of small liver lesions (less than and equal to 2 cm) is actively debated.
Percutaneous FNA biopsy performed under image guidance has been adopted as a safe, effective and minimally invasive procedure for the diagnosis of liver lesions. This technique is especially advantageous in patients with advanced malignancies. However controversies were raised over the role of FNA in the detection of HCC.19 These include 1) high accuracy, sensitivity and specificity of dynamic imaging modalities,20 2) the risk of needle tract seeding21 3) intraprocedural hematogenous dissemination21 4) need of accurate cytohistological characterization in small well-differentiated hepatocellular lesions.22 All these reasons preclude use of pre-operative FNA diagnosis of HCC. However false-positive results from imaging techniques have also occurred.14,15 Now there is a need to decide the strategy accordingly in an individual patient. It has to be weighed whether the risk of futile transplantation is more or the risk of seeding? The risk of seeding is overall lower than that of a futile transplantation.23 There is no clear evidence of post transplantation recurrence due to biopsy-induced hematogenous dissemination.
The percutaneous transabdominal technique under CT or US guidance is the most popular method for performing liver FNA. The sensitivity and specificity of FNA for detection of liver malignancy are around 90% and 100%, respectively. False positives are rare.24
Although liver biopsy is not used as frequently for a definitive histopathological diagnosis of HCC, it has an important role in lesions with atypical features on imaging studies. The ability to discriminate between dysplastic nodules and early HCC has become increasingly important, as the efficacy of treatments for HCC, depends on recognition at an early phase. Hence, guided liver biopsy is now mostly used for lesions with equivocal imaging features measuring over 1 cm. The differential diagnosis includes large regenerative nodule, focal nodular hyperplasia-like nodule, dysplastic nodule, early HCC and classic HCC. The first 2 lesions lack cytologic and structural atypia in contrast to dysplastic nodule, early HCC, and classic HCC.
With the new AASLD guidelines, approximately 52%–56% of patients with nodules 10–20 mm in size will need to undergo biopsy.25
Hence, biopsy has been strongly recommended before transplantation in patients with small nodules whose nature is uncertain on imaging and in patients with compensated cirrhosis whose only indication for a costly transplantation is the presence of malignancy.
Overall, the specificity and positive predictive value of tumor biopsy is 100% based on the studies available in literature. However the sensitivity varies from 66 to 93% which depends upon the size of the needle and nodule.23,26,27 Biopsy results obtained by 21- to 22 gage needle and of nodules ≤1 cm show less sensitivity. Tumor biopsy is excellent for ruling in the diagnosis of HCC. However, negative predictive value of biopsy is relatively low. For ruling out the diagnosis, tumor biopsy is less reliable, especially if the nodule is ≤1 cm. Therefore, patients with negative biopsy findings should continue to undergo careful surveillance with repeated imaging.23,26–28
Biopsy is not indicated in following situations: A. if there is a focal lesion in a cirrhotic liver and the patient is not a candidate for any form of therapy B. in decompensated cirrhosis and the patient is on the waiting list for liver transplantation C. if the patient is a candidate for resection.
FNAC or biopsy?
Fine needle aspiration could give a smaller risk of seeding than biopsy. Although the specificity and the positive predictive value of FNAC for focal liver lesions is very high, the sensitivity ranges between 67% and 93% and thus diagnostic accuracy is less than for histology.29 In addition to distinguish malignant from non-malignant lesions is difficult on cytology especially when the nodule is 2 cm or less.30 Image guided biopsy is often advocated for small suspicious nodules and is the preferred method to FNAC for diagnosing HCC histologically (Level of evidence 5, grade of recommendation D). Therefore biopsy with an 18-gage needle is preferred to cytology. Tumor biopsy is a safe procedure with excellent sensitivity and specificity for lesions > 10 mm.
Risk of needle tract seeding
A level 3a systematic review showed that the risk of tumor seeding was 2.7% (0–11%) and the median time between biopsy and seeding was 17 months.28,31
In the single studies, the seeding risk varied from 0% to 5.1%, and the seeding occurred 3 months to 4 years after biopsy. The currently available evidence is grade B.27,32 In studies looking at the risk of seeding, a long follow-up period (up to 4 years) is essential.28,33,34
In a large series, the incidence of needle tract seeding in more than 1000 patients with HCC was 0.76%.34 Other studies with more than 100 patients have reported the incidence of tumor seeding following biopsy was in the range of 1.6%–3.4%.35
Percutaneous biopsy of HCC carries a potential risk of tumor seeding along the needle tract. Needle tract seeding can occur in the post transplantation period. Risk factors for needle tract seeding have not been clearly known. It has been suggested that the risk of seeding can be reduced by the use of a coaxial cutting needle technique.36 On the contrary the risk is increased after radiofrequency ablation, possibly because of the use of larger diameter needle.37 The frequency of track seeding will also vary with the diameter of the needle used, the number of passes, and the amount of normal parenchyma traversed by the needle.31
Pathological characteristics of hepatocellular carcinoma
Gross Examination
Hepatocellular carcinoma may form a large solitary mass with or without adjacent smaller satellite nodules. It may consist of multiple nodules scattered throughout the liver, or it may infiltrate the liver diffusely without forming nodules. Hepatocellular carcinoma is usually soft, tan to yellow in color, sometimes bile stained and show areas of necrosis and hemorrhage. Invasion of small and/or large portal vein or hepatic vein branches may be seen.38,39
Microscopic Examination (Fine Needle Aspiration and/or Biopsy)
Hepatocellular carcinoma cells resemble hepatocytes in function, cytologic features, and growth patterns. In routine diagnostic practice, HCC is graded as well, moderate, or poorly differentiated types. Both architectural and cytologic features are helpful in establishing diagnosis of HCC. The pattern of growth may be trabecular, pseudoacinar, or diffuse. The individual tumor cells are polygonal, have granular and eosinophilic cytoplasm with nuclear pleomorphism and high nuclear to cytoplasmic ratio. The cells may secrete bile and contain fat, glycogen, Mallory-Denk bodies, hyaline globules, or fibrinogen (Figure 1A–C).
Figure 1.
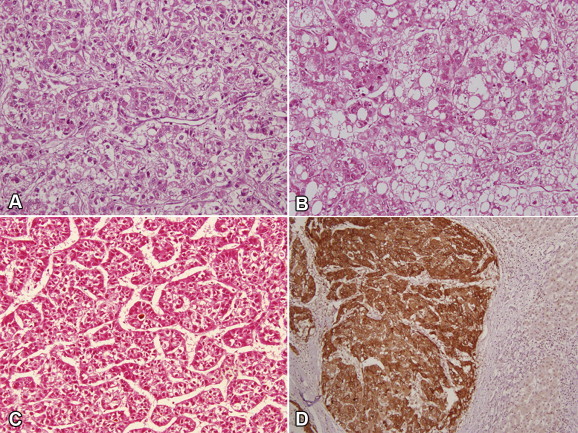
A: Photomicrograph shows trabecular pattern of HCC; B: with intracytoplasmic fat and Mallory hyaline; C: pseudoglandular pattern is seen; (A, B, C × 200 H&E). D: Glypican-3 shows intracytoplasmic positivity in tumor cells whereas nonneoplastic hepatocytes are negative.
Characteristically, there is no intercellular stroma except for the desmoplastic stroma in rare scirrhous type and fibrolamellar type, and the malignant cells are lined directly by endothelial cells. Unpaired arteries are identified amid tumor cells. Portal tracts are not present however, at the tumor margin, entrapped portal tracts may be seen among the invading neoplastic cells. Vascular invasion is commonly seen.
Well Differentiated Hepatocellular Carcinoma
These include thin cell plates of 1–3 cells in thickness, presence of abundant pseudoglandular structures, cytologic atypia and paucity of reticulin fibers. The most important differential diagnosis is adenoma/macroregenerative nodule/dysplastic nodule. It is difficult to diagnose correctly in small samples. Helpful features to diagnose adenoma include clinical history, absence of cirrhosis and thick fibrous pseudocapsule, non-trabecular and insignificant pseudoglandular growth pattern, maintained reticulin framework and minimal atypia. Immunohistochemical stains for GPC-3 or alpha-fetoprotein can be of help in distinguishing well differentiated HCC from hepatocellular adenoma (Figure 1D).
Moderately Differentiated Hepatocellular Carcinoma
Tumors show trabecular pattern with more than 4 cells in thickness. The cells are larger than well differentiated HCC with more eosinophilic cytoplasm and distinct nucleoli. This is the most common pattern seen in established HCC.
Poorly Differentiated Hepatocellular Carcinoma
Poorly differentiated HCCs display a great variety of histologic features including trabecular and diffuse patterns with or without areas of necrosis. The tumor cell nuclei are hyperchromatic with prominent nucleoli. Occasionally, the tumor cells are highly pleomorphic (pleomorphic cell variant) or spindly (sarcomatoid HCC). The differential diagnoses include variety of metastatic poorly differentiated adenocarcinomas, renal cell carcinoma, neuroendocrine carcinomas, and melanomas. A battery of immunohistochemical stains will be required to confirm the diagnosis of poorly differentiated HCC and to rule out metastatic neoplasms. These include Hep-Par1, glypican-3 (GPC-3), polyclonal carcinoembryonic antigen (pCEA) and CD10.40,41
Small HCCs are defined as tumors measuring up to 2 cm in diameter and these are further classified into distinctly nodular type and vaguely nodular type.42
Distinctly nodular type or progressed HCC is a type with gross and histologic features similar to those of larger classic HCC. On histology these are mostly moderately differentiated, lacks portal tracts, and show evidence of microvascular invasion. These tumors contain well-developed unpaired tumor arteries, which facilitate their detection by contrast enhanced imaging methods.
Vaguely nodular type or early HCC is a well differentiated type with indistinct margins. On histology it lacks fibrous capsule, and contains portal tracts. Most of these HCCs are clinically hypovascular due to insufficient development of unpaired tumor arteries and incomplete sinusoidal capillarization.43
Dysplastic nodules (DN). These are mostly less than 2 cm in diameter. Grossly these nodules differ from the surrounding liver parenchyma with regard to size, color, texture and degree of bulging of the cut surface. Histologically the presence of portal tracts and ductular reaction is diagnostic of non-malignant process (Level 3 of diagnostic strength). Low grade DN features a nodule showing mild increase in cell density with a monotonous pattern and/or clonal changes. High grade DN shows cytological and architectural atypia. Few unpaired non-triadal arteries can be seen. Stromal and vascular invasion is absent. The most important differential diagnosis is well differentiated HCC. In the appropriate clinico-morphological context, unequivocal positivity for 2 immunostains out of 3 (GPC-3, heat shock protein 70, glutamine synthetase) can detect early and well differentiated HCC (Level 3 of diagnostic strength).
Immunohistochemistry
One established approach is to use the 4 stains of cytokeratin 7 (CK7), cytokeratin 20 (CK20), Hep-Par 1 and pCEA. CK7 and CK 20 will be negative in HCC whereas the latter 2 will be positive.40,41 The other approach will be to use the trio of GPC-3, glutamine synthetase (GS) and heat shock protein 70 (HSP70) immunostains (sensitivity and specificity of 72% and 100%, respectively). This combination proves to be very good for the diagnosis of HCC, particularly when any two of the three are positive.44 Recent study by Timek et al suggest role of arginase-1, Hep-Par1 and GPC-3 in diagnosis of HCC and distinguishing it from metastatic tumors especially in small biopsies and FNA material.45 Arginase-1 is considered a more sensitive marker of hepatic differentiation than either HepPar-1 or GPC-3.46 CD34 shows diffuse strong staining of the endothelial lining of a large number of sinusoid-like tumor vessels in the majority of HCC whereas it is limited to sinusoidal endothelium confined to the vicinity of portal tracts in normal liver.47 Although a germ cell marker, SALL-4, an oncofetal gene, has also been seen in HCC and considered as a marker of aggressiveness by virtue of its stem cell properties.48–50
Atypical hepatocellular carcinoma variants
Variants which have no clinical significance but important for distinguishing from other cancer mimics on either morphology or imaging:
Pseudoglandular Hepatocellular Carcinoma
Pure pseudoglandular HCC is quite uncommon (<5%). It has to be differentiated from metastatic adenocarcinoma and cholangiocarcinoma.39
Clear Cell Hepatocellular Carcinoma
This variant has to be distinguished from more common metastatic clear cell renal cell carcinoma, adrenocortical carcinomas and angiomyolipomas.
Scirrhous Hepatocellular Carcinoma
Due to extensive fibrosis this tumor is commonly mistaken for cholangiocarcinoma on imaging.51
Diffuse Cirrhosis like Hepatocellular Carcinoma
This is clinically and radiographically undetected variant of HCC which mimics cirrhosis. It evades radiographic detection even on dynamic imaging due to the small size of tumor nodules.52
Variants which have Prognostic Importance
Giant Cell Variant
Consists of multinucleated tumor cells and it is considered as a bad prognostic sign.38,39,51
Combined Hepatocellular and Cholangiocarcinoma
It may represent collision of 2 different tumors or may result from malignant transformation of stem/progenitor cells which are identified by Keratin 19 and epithelial cell adhesion molecule (EpCAM). Both primary intrahepatic cholangiocarcinoma and HCC may arise secondary to chronic liver disease and cirrhosis. It is important to recognize this variant as prognosis is poorer than HCC alone. This variant has got a tendency for multifocal disease, frequent vascular invasion and lymph nodal metastasis.
The hepatocellular component is positive for hepatocellular markers whereas the cholangiocellular component is positive for cytokeratins 7 and 19, epithelial membrane antigen (EMA) and monoclonal CEA. Keratin 19, a progenitor cell/biliary marker, at a cut-off of 5% of positive tumor cells on immunohistochemistry, has been shown to correlate with poor clinical outcome.53–55
Fibrolamellar Hepatocellular Carcinoma
This type usually develops in non-cirrhotic liver in older children and adults and carries a better prognosis due to better resectability and absence of cirrhosis.38,39
Pedunculated Hepatocellular Carcinoma
It is a rare variant and usually located on the posterior and inferior surfaces of the right lobe. It is associated with good prognosis due to easy resectability.56
Ablated Hepatocellular Carcinoma
Hepatocellular carcinoma subsequent to presurgical ablation therapy is characterized by large areas of necrosis with or without viable tumor cells. Pathological evaluation of resected or explanted liver should include comment on degree of ablation as feedback for therapeutic success.57
Recently described variants
Glycogenotic Hepatocellular Carcinoma
It is typified by ground-glass hepatocytes due to accumulation of glycogen in neoplastic cells.58
Steatohepatitic Hepatocellular Carcinoma
This newly described morphological variant is recognized in association with underlying metabolic syndrome-related liver disease in which features of steatohepatitis are seen in the tumor cells.59–62
Conclusion
The diagnosis of HCC is based on either a tissue specimen or on very specific CT/MRI findings [1a, A]. Pathological diagnosis of HCC requires a biopsy of the tumor or a resection specimen [3b, C]. Stromal invasion or tumor cell invasion into the portal tracts or fibrous septa, defines HCC and is not present in dysplastic lesions [3a, A]. Immunostaining for GPC-3, HSP70, and GS is recommended to differentiate high grade dysplastic nodules from early HCC [2d, B]. Non-invasive diagnosis is based on imaging techniques and characterized by identification of the typical radiological hallmark of HCC.
Conflicts of interest
The author has none to declare.
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag H.B. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Nayak N.C., Jain D. Liver Cirrhosis: Causes Diagnosis and Treatment. Nova Publishers, Inc; New York, USA: 2011. End stage chronic Liver Disease – Yesterday, Today and Tomorrow. [Google Scholar]
- 4.Nayak N.C., Jain D., Vasdev N., Gulwani H., Saigal S., Soin A. Etiologic types of end-stage chronic liver disease in adults: analysis of prevalence and their temporal changes from a study on native liver explants. Eur J Gastroenterol Hepatol. 2012 Oct;24(10):1199–1208. doi: 10.1097/MEG.0b013e32835643f1. [DOI] [PubMed] [Google Scholar]
- 5.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]
- 6.Marrero J.A., Fontana R.J., Fu S., Conjeevaram H.S., Su G.L., Lok A.S. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag H.B., Tran T., Everhart J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 8.Jain D., Nayak N.C., Saigal S. Hepatocellular carcinoma arising in association with von-Meyenburg's complexes: an incidental finding or precursor lesions? A clinicopatholigic study of 4 cases. Ann Diagn Pathol. 2010 Oct;14(5):317–320. doi: 10.1016/j.anndiagpath.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Chen C.J., Yang H.I., Su J., REVEAL-HBV Study Group Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Yu N.C., Chaudhari V., Raman S.S. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161–167. doi: 10.1016/j.cgh.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Chen J.G., Parkin D.M., Chen Q.G. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J., Sherman M. Practice guidelines committee, American Association for the Study of Liver Diseases: management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J., Sherman M., Llovet J.M. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference – European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 14.Levy I., Greig P.D., Gallinger S., Langer B., Sherman M. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg. 2001;234:206–209. doi: 10.1097/00000658-200108000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong Y.Y., Mitchell D.G., Kamishima T. Small (<20 mm) enhancing hepatic nodules seen on arterial phase MR imaging of the cirrhotic liver: clinical implications. AJR Am J Roentgenol. 2002;178:1327–1334. doi: 10.2214/ajr.178.6.1781327. [DOI] [PubMed] [Google Scholar]
- 16.Sangiovanni A., Manini M.A., Iavarone M. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–644. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- 17.Leoni S., Piscaglia F., Golfieri R. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol. 2010;105:599–609. doi: 10.1038/ajg.2009.654. [DOI] [PubMed] [Google Scholar]
- 18.Poon D., Anderson B.O., Chen L.T. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 19.Wang P., Meng Z.Q., Chen Z. Diagnostic value and complications of fine needle aspiration for primary liver cancer and its influence on the treatment outcome. A study based on 3011 patients in China. Eur J Surg Oncol. 2008;34:541–546. doi: 10.1016/j.ejso.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Torzilli G., Minagawa M., Takayama T. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 21.Stigliano R., Marelli L., Yu D. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 22.de Boer W.B., Segal A., Frost F.A., Sterrett G.F. Cytodiagnosis of well differentiated hepatocellular carcinoma: can indeterminate diagnoses be reduced? Cancer. 1999;87:270–277. doi: 10.1002/(sici)1097-0142(19991025)87:5<270::aid-cncr6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Wee A. Fine needle aspiration biopsy of hepatocellular carcinoma and hepatocellular nodular lesions: role, controversies and approach to diagnosis. Cytopathology. 2011 Oct;22(5):287–305. doi: 10.1111/j.1365-2303.2011.00882.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuo F.Y., Chen W.J., Lu S.N., Wang J.H., Eng H.L. Fine needle aspiration cytodiagnosis of liver tumors. Acta Cytol. 2004;48:142–148. doi: 10.1159/000326307. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2010;000:1–35. doi: 10.1002/hep.24199. http://publish.aasld.org/practiceguidelines/Documents/Bookmarked%20Practice%20Guidelines/HCCUpdate2010.pdf Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caturelli E., Bisceglia M., Fusilli S., Squillante M.M., Castelvetere M., Siena D.A. Cytological vs microhistological diagnosis of hepatocellular carcinoma: comparative accuracies in the same fine-needle biopsy specimen. Dig Dis Sci. 1996;41:2326–2331. doi: 10.1007/BF02100122. [DOI] [PubMed] [Google Scholar]
- 27.Caturelli E., Solmi L., Anti M. Ultrasound guided fine needle biopsy of early hepatocellular carcinoma complicating liver cirrhosis: a multicentre study. Gut. 2004;53:1356–1362. doi: 10.1136/gut.2003.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müllhaupt B., Durand F., Roskams T., Dutkowski P., Heim M. Is tumor biopsy necessary? Liver Transpl. 2011 Oct;17(suppl 2):S14–S25. doi: 10.1002/lt.22374. [DOI] [PubMed] [Google Scholar]
- 29.Cochand-Priollet B., Chagnon S., Ferrand J., Blery M., Hoang C., Galian A. Comparison of cytologic examination of smears and histologic examination of tissue cores obtained by fine needle aspiration biopsy of the liver. Acta Cytol. 1987;31(4):476–480. [PubMed] [Google Scholar]
- 30.Tsai Y.Y., Lu S.N., Changchien C.S. Combined cytologic and histologic diagnosis of liver tumors via one-shot aspiration. Hepatogastroenterology. 2002;49(45):644–647. [PubMed] [Google Scholar]
- 31.Silva M.A., Hegab B., Hyde C., Guo B., Buckels J.A., Mirza D.F. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 32.Durand F., Regimbeau J.M., Belghiti J. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001;35:254–258. doi: 10.1016/s0168-8278(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 33.Takamori R., Wong L.L., Dang C., Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000;6:67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- 34.Chang S., Kim S.H., Lim H.K. Needle tract implantation after sonographically guided percutaneous biopsy of hepatocellular carcinoma: evaluation of doubling time, frequency, and features on CT. AJR Am J Roentgenol. 2005;185:400–405. doi: 10.2214/ajr.185.2.01850400. [DOI] [PubMed] [Google Scholar]
- 35.Durand F., Belghiti J., Paradis V. Liver transplantation for hepatocellular carcinoma:role of biopsy. Liver Transpl. 2007;13:S17–S23. doi: 10.1002/lt.21326. [DOI] [PubMed] [Google Scholar]
- 36.Maturen K.E., Nghiem H.V., Marrero J.A. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006;187:1184–1187. doi: 10.2214/AJR.05.1347. [DOI] [PubMed] [Google Scholar]
- 37.Llovet J.M., Vilana R., Bru C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 38.Goodman Z.D., Terraciano L.M. Tumours and tumour-like lesions of the liver. In: Burt A.D., Portmann B.C., Ferrell L.D., editors. MacSween's Pathology of the Liver. 5th ed. Churchill Livingstone; Philadelphia, PA: 2007. pp. 761–814. [Google Scholar]
- 39.Ishak K.G., Goodman Z.D., Stocker J.T. Armed Forces Institute of Pathology; Washington, DC: 2001. Tumors of the Liver and Intrahepatic Bile Ducts. (Atlas of Tumor Pathology. 3rd Series. Fascicle 31). [Google Scholar]
- 40.Minervini M.I., Demetris A.J., Lee R.G., Carr B.I., Madariaga J., Nalesnik M.A. Utilization of hepatocyte-specifi c antibody in the immunocytochemical evaluation of liver tumors. Mod Pathol. 1997;10(7):686–692. [PubMed] [Google Scholar]
- 41.Kakar S., Gown A.M., Goodman Z.D., Ferrell L.D. Best practices in diagnostic immunohistochemistry: hepatocellular carcinoma versus metastatic neoplasms. Arch Pathol Lab Med. 2007;131(11):1648–1654. doi: 10.5858/2007-131-1648-BPIDIH. [DOI] [PubMed] [Google Scholar]
- 42.Nakashima O., Sugihara S., Kage M., Kojiro M. Pathomorphologic characteristics of small hepatocellular carcinoma: a special reference to small hepatocellular carcinoma with indistinct margins. Hepatology. 1995;22(1):101–105. [PubMed] [Google Scholar]
- 43.International Consensus Group for Hepatocellular Neoplasia Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49(2):658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 44.Di Tommaso L., Franchi G., Park Y.N. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology. 2007;45:725–734. doi: 10.1002/hep.21531. [DOI] [PubMed] [Google Scholar]
- 45.Timek D.T., Shi J., Liu H., Lin F. Arginase-1, HepPar-1, and Glypican-3 are the most effective panel of markers in distinguishing hepatocellular carcinoma from metastatic tumor on fine-needle aspiration specimens. Am J Clin Pathol. 2012 Aug;138(2):203–210. doi: 10.1309/AJCPK1ZC9WNHCCMU. [DOI] [PubMed] [Google Scholar]
- 46.Fujiwara M., Kwok S., Yano H., Pai R.K. Arginase-1 is a more sensitive marker of hepatic differentiation than HepPar-1 and glypican-3 in fine-needle aspiration biopsies. Cancer Cytopathol. 2012 Aug 25;120(4):230–237. doi: 10.1002/cncy.21190. [DOI] [PubMed] [Google Scholar]
- 47.Kong C., Appenzeller M., Ferrell L. Utility of CD34 reactivity in evaluating focal nodular hepatocellular lesions sampled by fine needle aspiration biopsy. Acta Cytol. 2000;44:218–222. doi: 10.1159/000326363. [DOI] [PubMed] [Google Scholar]
- 48.Jain D. Sal-like protein 4: an epithelial and germ cell marker. Hum Pathol. 2013 Jul;44(7):1453. doi: 10.1016/j.humpath.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Oikawa T., Kamiya A., Zeniya M. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology. 2013 Apr;57(4):1469–1483. doi: 10.1002/hep.26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yong K.J., Gao C., Lim J.S. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med. 2013 Jun 13;368(24):2266–2276. doi: 10.1056/NEJMoa1300297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirohashi S., Ishak K.G., Kojiro M. Hepatocellular carcinoma. In: Hamilton S.R., Aaltonen L.A., editors. Tumors of the Digestive System. IARC Press; Lyon: 2000. pp. 159–172. World Health Organization Classification of Tumors. [Google Scholar]
- 52.Jakate S., Yabes A., Giusto D. Diffuse cirrhosis-like hepatocellular carcinoma: a clinically and radiographically undetected variant mimicking cirrhosis. Am J Surg Pathol. 2010;34:935–941. doi: 10.1097/PAS.0b013e3181ddf52f. [DOI] [PubMed] [Google Scholar]
- 53.Jarnagin W.R., Weber S., Tickoo S.K. Combined hepatocellular and cholangiocarcinoma. Demographic, clinical, and prognostic factors. Cancer. 2002;94:2040–2046. doi: 10.1002/cncr.10392. [DOI] [PubMed] [Google Scholar]
- 54.Kim H., Choi G.H., Na D.C. Human hepatocellular carcinomas with “Stemness”–related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54:1707–1717. doi: 10.1002/hep.24559. [DOI] [PubMed] [Google Scholar]
- 55.Durnez A., Verslype C., Nevens F. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49(2):138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 56.Yeh C.N., Lee W.C., Jeng L.B., Chen M.F. Pedunculated hepatocellular carcinoma: clinicopathologic study of 18 surgically resected cases. World J Surg. 2002;26:1133–1138. doi: 10.1007/s00268-002-6401-x. [DOI] [PubMed] [Google Scholar]
- 57.Shiina S. Image-guided percutaneous ablation therapies for hepatocellular carcinoma. J Gastroenterol. 2009;44(suppl 19):122–131. doi: 10.1007/s00535-008-2263-9. [DOI] [PubMed] [Google Scholar]
- 58.Callea F., Giovannoni I., Stefanelli M., Villanacci V., Lorini G., Francalanci P. Glycogenotic hepatocellular carcinoma with glycogen-ground-glass hepatocytes: histological, histochemical and microbiochemical characterization of the novel variant. Histopathology. 2012 May;60(6):1010–1012. doi: 10.1111/j.1365-2559.2011.04168.x. [DOI] [PubMed] [Google Scholar]
- 59.Salomao M., Yu W.M., Brown R.S., Jr., Emond J.C., Lefkowitch J.H. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus—related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010;34:1630–1636. doi: 10.1097/PAS.0b013e3181f31caa. [DOI] [PubMed] [Google Scholar]
- 60.Jain D., Nayak N.C., Saigal S. Hepatocellular carcinoma in nonalcoholic fatty liver cirrhosis and alcoholic cirrhosis: risk factor analysis in liver transplant recipients. Eur J Gastroenterol Hepatol. 2012 Jul;24(7):840–848. doi: 10.1097/MEG.0b013e3283534b40. [DOI] [PubMed] [Google Scholar]
- 61.Jain D., Nayak N.C., Kumaran V., Saigal S. Steatohepatitic hepatocellular carcinoma, a morphologic indicator of associated metabolic risk factors. A Study from India. Arch Pathol Lab Med. 2013 Jul;137(7):961–966. doi: 10.5858/arpa.2012-0048-OA. [DOI] [PubMed] [Google Scholar]
- 62.Jain D. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012 May;43(5):769–770. doi: 10.1016/j.humpath.2011.12.016. [DOI] [PubMed] [Google Scholar]


