Abstract
Hepatocellular carcinoma (HCC) is different from other malignancies because the prognosis in HCC is not only dependent upon the tumor stage but also on the liver function impairment due to accompanying cirrhosis liver. Various other staging systems used in HCC include the European systems [French staging system, Barcelona Clinic Liver Cancer (BCLC) staging system and the cancer of the liver Italian program (CLIP)] and Asian systems [Okuda staging system, Japan integrated Staging (JIS), Tokyo score and Chinese University Prognostic Index (CUPI)]. Out of all the staging systems used in HCC, Barcelona Clinic Liver Cancer (BCLC) staging system is probably the best because it takes in to account the tumor status (defined by tumor size and number, presence of vascular invasion and extrahepatic spread), liver function (defined either by the Child-Pugh’s class) and general health status of the patient (defined by the ECOG classification and the presence of symptoms). Since most of the extrahepatic spread in HCC occurs to lymph nodes, lungs and bones, the assessment can be done with either PET/CT or a combination of CT (Chest and abdomen) and a bone scan. This article describes the various staging systems used in HCC, guides choosing a staging system particularly in the Indian context and the assessment of extra-hepatic spread in HCC.
Keywords: hepatocellular carcinoma (HCC), BCLC, Okuda
Abbreviations: AJCC, American Joint committee on cancer; BCLC, Barcelona clinic liver cancer; CLIP, cancer of the liver Italian program; CUPI, Chinese University prognostic index; ES, extra-hepatic spread; HCC, hepatocellular carcinoma; ITDV, intra tumor vascular density; LCSGJ, liver cancer study group of Japan; OLT, orthotopic liver transplant; TNM, tumor-node-metastasis; VEGF, vascular endothelial growth factor
Staging of patients with hepatocellular carcinoma (HCC) is important both for the prognostication and deciding about the treatment. Staging in HCC also helps to know the impact of conventional or investigational treatment and to design the prospective trials.1
HCC is different from other malignancies because in this tumor the prognosis not only depends upon the tumor stage (like in other malignancies) but also on the liver function impairment due to underlying cirrhosis liver, which accompanies most of the patients.1 General condition of the patient and the treatment given to the patient also determines the prognosis in a particular patient.
Various parameters have been studied to be of prognostic usefulness in patients with HCC. These include parameters related to the patient demographics like age, gender and general health of the patient. Liver function tests like estimation of bilirubin and albumin are important prognostic variables in HCC as are the presence of ascites or encephalopathy. Tumor characteristics are also important determinants of prognosis which include tumor stage, number (single, multicentric), growth rate and aggressiveness of the tumor, vascular invasion and extrahepatic spread of tumor, presence of tumor markers and receptors and finally the treatment given to the patient.1 Recently various molecular markers (biomarkers) have also been shown to be of prognostic importance in patients with HCC.2 Out of the various biomarkers, alpha feto protein (AFP) has been studied in detail and has a role both in diagnosis and prognosis of HCC.3
Other biomarkers include cellular malignancy phenotype related markers like DNA ploidy, cellular proliferation markers (PCNA, Ki 67 etc), p53 gene, tumor promoter genes (ras, c-myc), apoptosis related markers like Fas and Fas ligand, cell adhesion and extracellular matrix related markers like adhesive molecules (E-adherin, catenins, SICAM etc), angiogenesis related markers like vascular endothelial growth factor (VEGF), platelet derived–ECGF, intra tumor vascular density (ITVD) and genomics and proteomics related markers.2
All above-mentioned prognostic markers can be used either singly or as combination of various markers. Used singly these markers have less prognostic value in comparison to multiple prognostic criteria. The parameter which look at only one aspect of prognosis e.g. Child-Pugh classification for liver function, TNM staging system4 for tumor stage and performance status for general well being of patient5 have limited usefulness because the prognosis in HCC would depend on combination of these factors rather than on one parameter. Treatment given to the patient is an important determinant of prognosis, which in turn depends whether patient presents in early or advanced stage.1
Staging systems in hepatocellular carcinoma
Many staging systems have been used to provide a clinical classification in patients with HCC and as mentioned earlier the best system would be that take in to account the tumor status (defined by tumor size and number, presence of vascular invasion and extrahepatic spread), liver function (defined either by the Child-Pugh’s class or individually by the levels of serum bilirubin and albumin, presence of ascites and portal hypertension) and general health status of the patient (defined by the ECOG classification and the presence of symptoms). Various staging systems used in HCC include the European systems [French staging system,6 Barcelona Clinic Liver Cancer (BCLC) staging system7 and the cancer of the liver Italian program (CLIP)8], Asian systems [Okuda staging system,9 Japan integrated Staging (JIS),10 Tokyo score10 and Chinese University Prognostic Index (CUPI)11].
Okuda for the first time used the combination of tumor variables (Tumor size < or > 50%) and liver functions (ascites, albumin, bilirubin) and divided the patients into three stages (Table 1).9 Stage I patients have better prognosis in comparison to stage II & III with a median survival of 8.3 months, 2 months and 0.7 month respectively in untreated HCC patients.9 The drawback of Okuda staging is that, it is useful mainly for patients with advanced stage and fails to adequately differentiate early from advanced stage. It does not take into account other tumor variables like the multicentricity of the tumor, vascular invasion, and the extrahepatic spread.12 Japan integrated Staging (JIS),10 which utilizes the assessment of liver damage by the liver cancer study group of Japan (LCSGJ) or Child-Pugh stages and combines it with TNM stage and is considered a useful staging of HCC in Japan. LCSGJ which was originally designed for patients undergoing hepatectomy, takes in to account the same parameters as used in Child-Pugh class except that the hepatic encephalopathy is replaced by the ICG retention at 15 min. JIS has been recently refined as bm-JIS by including biomarkers (AFP, DCP, AFP-L3).13
Table 1.
Okuda Staging of HCC.
| Tumor size |
Ascites | Albumin |
Bilirubin |
|||||
|---|---|---|---|---|---|---|---|---|
| >50% | <50% | <3 mg/dl | >3 mg/dl | >3 mg/dl | 3 mg/dl | |||
| Stage | (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) |
| I | (−) | (−) | (−) | (−) | ||||
| II | 1 or 2 (1) | |||||||
| III | 3 or 4 (+) | |||||||
French staging6 divides the patients into three stages (A, B, C) on the basis of performance status, serum bilirubin, serum alkaline phosphatase, serum alpha feto protein and portal vein obstruction on ultrasound (Table 2). Patients in Stage A (score 0) have higher survival in comparison to stage B (Score 1–5) who have intermediate risks of death, and stage C (Score ≥ 6) who have the worst survival.6 BCLC staging system7 is the treatment based staging system where the patient in early stage (Stage A) are offered the curative treatment of either hepatic resection or orthotopic liver transplant (OLT) (Figure 1). The tumors in this stage are either single <5 cm or 3 nodules of <3 cm with good performance status and have 50–75% 5-year survival. Patients exceeding these limits are in intermediate stage (Stage B) and have 50% 3-year survival. Patients with advanced stage (Stage C) have vascular invasion or extrahepatic spread with poor performance status and their 3-year survival drops down to 10%. Patients in Stage D have a grim prognosis unless they are fit for liver transplantation.7 CLIP staging is the most recent staging system8 that takes into account the Child-Pugh status of the patient with tumor characteristics including the portal vein thrombosis and levels of AFP (Table 3). Patient have scores ranging from 0 to 6, CLIP—0 patient having a better prognosis than those patients with CLIP—6 score.
Table 2.
French Staging of HCC.
| Parameter | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Karnofsky index | ≥80 | <80 | ||
| Serum bilirubin (μmol/l) | <50 | ≥50 | ||
| Serum alkaline phosphatase (ULN2) | <2 | ≥2 | ||
| Serum alfa fetoprotein (μg/l) | <35 | ≥35 | ||
| Portal obstruction (ultrasonography) | No | Yes |
Figure 1.
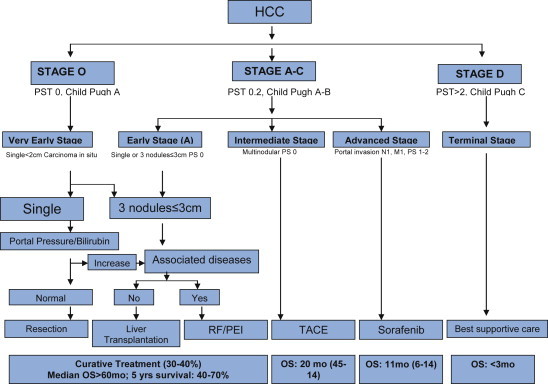
BCLC staging system of HCC.
Table 3.
CLIP Staging of HCC.
| Variable | Score |
|---|---|
| Child-Pugh score | |
| A | 0 |
| B | 1 |
| C | 2 |
| Tumor morphology | |
| Uninodular and extension ≤50 | 0 |
| Multinodular and extension ≤50 | 1 |
| Massive or extension > 50% | 2 |
| AFP | |
| <400 | 0 |
| ≥400 | 1 |
| Portal vein thrombosis | |
| No | 0 |
| Yes | 1 |
Recommendation
Staging system for HCC should take into account tumor stage, liver function and physical status and the impact of treatment (Level of evidence—2a).
Choosing the staging system
There have been many studies comparing various staging systems in HCC and have found variable results. The difference in results are predominantly dependent on the difference in the tumor characteristics, whether the disease was early or advanced, geographical and racial differences in patients and on the fact whether the stage was used only for the purpose of prognosis or for both prognosis and treatment allocation. A study from Thailand evaluated American Joint Committee on Cancer (AJCC), Tumor-Node-Metastasis (TNM), Okuda staging, Cancer of the Liver Italian Program (CLIP), Barcelona Clinic Liver Cancer (BCLC), Chinese University Prognostic Index (CUPI), and Japan Integrated Staging (JIS), Child-Pugh classification in 99 patients with HCC for the overall and disease-free survival.14 All staging systems except Okuda were significant in determining overall survival in univariate analyses. In multivariate analyses, TNM and Child-Pugh demonstrated better predictive power for overall survival. In terms of disease-free survival, univariate analyses revealed that TNM, CLIP, BCLC, CUPI, and JIS were significant, and TNM was the best predictive staging system in multivariate analyses. A study from Hong Kong, China, including 595 patients (80.2% with chronic HBV infection) found that both CUPI and CLIP had the most favorable performance in terms of discriminatory ability, homogeneity and monotonicity. CUPI performed the best in predicting 3-month survival while CLIP performed better in predicting the outcome of 6- and 12-month survival rate.11 A study from Japan involving 1173 HCC patients found that the bm-JIS score showed good stratification ability and was demonstrated to be a better predictor of the prognosis than the c-JIS score and the BALAD score, especially for the patients with a good prognosis.13 In a recent study15 comparing CLIP staging with Okuda staging system and Child-Pugh scoring system, CLIP staging was found to give more accurate prognostic information in comparison to Okuda and Child-Pugh classification. Five-year survival rate in CLIP—0 was found to be 65% in comparison to 37% with Okuda stage I.
As mentioned earlier the best staging system should not only look at the prognosis without treatment but should also provide guidelines about treatment and the prognosis after treatment. The system should take in to account the tumor status, liver function and general health status of the patient. BCLC is one such classification which takes in to account most of these aspects. Most of the American literature supports BCLC and it has also been endorsed by the EASL and AASLD.16,17 In one of the recent studies done over 5 years (2003 and 2008), 1717 treatment-naïve HCC patients were enrolled prospectively. 167 (9.8%) patients were classified as BCLC stage 0, 526 (30.6%) as A, 333 (19.4%) as B, 608 (35.4%) as C and 83 (4.8%) as D. Median overall survival was 22.5 months, and 1-, 2-, 3-, 4-, and 5-year survival rates were 62.6, 48.3, 39.9, 34.7, and 29.3% respectively.18 Of six staging systems, BCLC had the highest area under ROC (AUROC; 0.821) for overall survival, followed by JIS (0.809), Tokyo score (0.771), CLIP (0.746), CUPI (0.701) and GRETCH (0.685) system. In both subgroups stratified according to treatment strategy (curative vs. palliative), BCLC also showed the best AUROCs (curative, 0.708/palliative, 0.807) for overall survival.18
Recommendation
The BCLC staging system is recommended for prognostic prediction and treatment allocation (Level of evidence—1a).
Assessment of extra-hepatic spread
In recent years extra-hepatic spread (ES) of HCC seems to have been observed more frequently than in the past and the probability of finding ES is higher in patients with advanced intrahepatic HCC. The more frequent ES sites are lungs, abdominal lymph nodes and bones, but head and neck can also be affected. In a study looking at the CT findings in 403 consecutive patients with HCC, 148 patients with extrahepatic metastatic HCC were identified.19 A majority (128 [86%] of 148) of patients with extrahepatic HCC had either intrahepatic stage IVA tumor (112 [76%] patients) or an intrahepatic stage III tumor (16 [11%] patients). Most common metastatic sites of extrahepatic metastasis were the lung in 81 (55%) patients, the abdominal lymph nodes in 60 (41%) patients, and the bone in 41 (28%) patients. Lungs were the most frequent site of the first detectable metastasis [58 (39%) patients].19 In another study, all 995 consecutive HCC patients were followed up at regular intervals and 151 (15.2%) patients were found to have extrahepatic metastases at the initial diagnosis of primary HCC or developed such tumors during the follow-up period.20 The most frequent site of extrahepatic metastases was the lungs (47%), followed by lymph nodes (45%), bones (37%), and adrenal glands (12%). The cumulative survival rates after the initial diagnosis of extrahepatic metastases at 6, 12, 24, and 36 mo were 44.1%, 21.7%, 14.2%, 7.1%, respectively. The median survival time was 4.9 mo (range, 0–37 mo). Fourteen patients (11%) died of extrahepatic HCC, others died of primary HCC or liver failure.20
Extrahepatic metastases can be diagnosed by CT, MRI, bone scintigraphy, X-ray, and/or positron emission tomography (PET) with18 F-fluorodeoxyglucose (FDG), or by the histopathological examination of surgically resected specimen or biopsy. Though PET–CT is not a good modality per se for HCC but is emerging as a useful modality in patients with HCC with extrahepatic spread. In one of the studies involving 121 patients, all patients had undergone a “dual-tracer” PET/CT same-day protocol with (11)C-ACT PET/CT followed by (18)F-FDG PET/CT.21 On patient basis, dual-tracer PET/CT had a sensitivity of 98%, a specificity of 86%, a positive predictive value of 97%, a negative predictive value of 90%, and an accuracy of 96% in the detection of HCC metastasis. On a lesion basis, 273 metastatic HCC lesions considered as true-positive were detected and categorized according to the organ or site of metastasis: lymph node (abdominal and thoracic, 49%), lung (32%), bone (8%), and others (10%). The lesion-based and patient-based detection sensitivities were 60% and 64%, respectively, by (11) C-ACT and 77% and 79%, respectively, by (18) F-FDG, and they were complementary. Authors confirmed that (18) F-FDG PET/CT is useful in the evaluation of HCC metastasis, although its role in the diagnosis of primary HCC is more limited. In addition they suggested that dual-tracer PET/CT had an incremental value and complementary advantage when compared with single-tracer imaging in the evaluation of HCC metastasis.21 In another study 45 patients were found to have focal intrahepatic HCC recurrence after surgical interventions, and 9 patients were free of HCC recurrence.22 Twenty-three patients developed extrahepatic metastasis, among whom 19 also had intrahepatic tumor recurrence and 4 had extrahepatic metastasis only. The sensitivity, specificity, and accuracy of (18)F-FDG PET/CT in the detection of HCC recurrence were 88.9% (40/45), 77.8% (7/9), and 87.0% (47/54), respectively, as compared with those of 57.8% (26/45), 100% (9/9), and 64.8% (35/54) by CECT detection. Authors suggested that in comparison to CECT, (18)F-PET/CT shows a high sensitivity and accuracy in detecting postoperative tumor residual or recurrence in the liver, and can also be an effective modality for detecting extrahepatic lesions in HCC patients.22
Recommendation
A CT scan of abdomen plus chest and a bone scan is recommended for the assessment of extrahepatic spread in patients with HCC (Level of evidence—4).
Staging of hepatocellular carcinoma—Indian scenario
As mentioned earlier, geographical differences do play a role in determining the prognosis in patients with HCC. Overall, Asian trials almost always reported poorer survival than non-Asian trials in patients with advanced HCC. A systematic review of randomized trials [fourteen trials (6 Asians, 8 non-Asians)] for unresectable HCC used systemic therapy as an experimental arm and placebo or supportive care as control.23 The median survival of patients in the control arm, which indicated natural history of advanced HCC patients, was 3.57 ± 1.88 months in Asian trials and 5.96 ± 1.46 months in non-Asian trials (P = 0.02). Independent predictors of better survival included non-Asian trials (P = 0.0007), higher percentage of Child A cirrhosis (P = 0.01) and hepatitis B (HBV)-related HCC (P = 0.02). Subgroup analysis suggested that Asian trials tended to enroll patients with more advanced diseases. Independent predictors of better treatment effect included non-Asian trials, higher percentage of extra-hepatic metastasis, HBV-related HCC, and poorer trial quality.23
In a study from AIIMS, New Delhi involving 324 patients with HCC, patients were staged according to Child-Pugh class, Okuda staging, CLIP staging and BCLC staging. Out of all staging systems, Okuda staging was observed as the independent predictor of survival.24 A study from GB Pant hospital involving 191 patients predominantly used the TNM staging and Okuda staging system.25 In a prospective study from our center 101 HCC patients were diagnosed and stratified according to 7 different staging systems; CLIP, Tokyo score and BCLC staging system showed a significant difference in the probability of survival. All other staging systems failed to show a significant difference in survival.26 Most of the patients in India report at an advanced stage, hence the staging systems like JIS which predominantly are helpful in patients in early stage may not be applicable to Indian patients. Staging systems like BCLC are helpful in even advanced stage and not only help in prognostication but also in deciding the treatment modalities; hence may be more applicable to Indian patients with HCC.
Recommendation
The BCLC staging system is recommended for prognostic prediction and treatment allocation in Indian patients with HCC (Level of evidence—5).
Conclusions
The prognosis in HCC is not only dependent upon the tumor stage but also on the liver function impairment due to accompanying cirrhosis liver in most cases. Of the various staging systems used in HCC, BCLC staging system is probably the best because it takes in to account the tumor status, liver function and general health status of the patient. The evaluation of extra-hepatic spread lungs, abdominal lymph nodes, and bones can be done with either PET/CT or a combination of CT (Chest and abdomen) and a bone scan.
Conflicts of interest
The author has none to declare.
References
- 1.Bruix J., Llovet J.M. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 2.Qin Lx, Tang Z.Y. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nomura F., Ohnishi K., Tanabe Y. Clinical features, and prognosis of hepatocellular carcinoma with reference to serum alfa fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64:1700–1701. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Marsh J.W., Dvorchik Igor, Bonham C.A., Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–543. doi: 10.1002/(sici)1097-0142(20000201)88:3<538::aid-cncr7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen J.B., Klee M., Palshof T., Hansen H.H. Performance status assessment in cancer patients. An inter observer variability study. Br J Cancer. 1993;67:773–775. doi: 10.1038/bjc.1993.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevret S., Trinchet J.C., Mathieu D., Rached A.A., Beaugrand M., Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. J Hepatol. 1999;3:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 7.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 8.Llovet J.M., Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32:679–680. doi: 10.1053/jhep.2000.16475. [DOI] [PubMed] [Google Scholar]
- 9.Okuda K., Ohtsuki T., Obata H. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Chung H., Kudo M., Takahashi S. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol. 2008;23:445–452. doi: 10.1111/j.1440-1746.2007.05075.x. [DOI] [PubMed] [Google Scholar]
- 11.Chan S.L., Mo F.K., Johnson P.J. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 2011;26:340–347. doi: 10.1111/j.1440-1746.2010.06329.x. [DOI] [PubMed] [Google Scholar]
- 12.Shouval D. HCC: what's the score? Gut. 2002;50:749–750. doi: 10.1136/gut.50.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitai S., Kudo M., Minami Y. Validation of a new prognostic staging system for hepatocellular carcinoma: a comparison of the biomarker-combined Japan Integrated Staging Score, the conventional Japan Integrated Staging Score and the BALAD Score. Oncology. 2008;75(suppl 1):83–90. doi: 10.1159/000173428. [DOI] [PubMed] [Google Scholar]
- 14.Sirivatanauksorn Y., Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. HPB Surg. 2011;2011:818217. doi: 10.1155/2011/818217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy I., Sherman M., The Liver Cancer Study Group of the University of Toronto Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:365–369. doi: 10.1136/gut.50.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J., Sherman M., American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim B.K., Kim S.U., Park J.Y. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naïve patients with hepatocellular carcinoma. Liver Int. 2012;32:1120–1127. doi: 10.1111/j.1478-3231.2012.02811.x. [DOI] [PubMed] [Google Scholar]
- 19.Katyal S., Oliver J.H., 3rd, Peterson M.S., Ferris J.V., Carr B.S., Baron R.L. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 20.Uka K., Aikata H., Takaki S. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho C.L., Chen S., Yeung D.W., Cheng T.K. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med. 2007;48:902–909. doi: 10.2967/jnumed.106.036673. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z.F., Liang H., Zhang X.S. Value of (18) F-FDG PET/CT and CECT in detecting postoperative recurrence and extrahepatic metastasis of hepatocellular carcinoma in patients with elevated serum alpha-fetoprotein. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1615–1619. [PubMed] [Google Scholar]
- 23.Hsu C., Shen Y.C., Cheng C.C., Hu F.C., Cheng A.L. Geographic difference in survival outcome for advanced hepatocellular carcinoma: implications on future clinical trial design. Contemp Clin Trials. 2010;31:55–61. doi: 10.1016/j.cct.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Paul S.B., Chalamalasetty S.B., Vishnubhatla S. Clinical profile, etiology and therapeutic outcome in 324 hepatocellular carcinoma patients at a tertiary care center in India. Oncology. 2009;77:162–171. doi: 10.1159/000231886. [DOI] [PubMed] [Google Scholar]
- 25.Kumar R., Saraswat M.K., Sharma B.C., Sakhuja P., Sarin S.K. Characteristics of hepatocellular carcinoma in India: a retrospective analysis of 191 cases. QJM. 2008;101:479–485. doi: 10.1093/qjmed/hcn033. [DOI] [PubMed] [Google Scholar]
- 26.Sarma S., Sharma B., Chawla Y.K. Comparison of 7 staging systems in north Indian cohort of hepatocellular carcinoma. Trop Gastroenterol. 2010;31:271–278. [PubMed] [Google Scholar]


