ABSTRACT
OBJECTIVES
Shared medical appointments (SMAs) are an increasingly used system-redesign strategy for improving access to and quality of chronic illness care. We conducted a systematic review of the existing literature on SMA interventions for patients with diabetes in order to understand their impact on outcomes.
DATA SOURCES
MEDLINE, EMBASE, CINAHL, PsycINFO, and Web of Science from January 1996 through April 2012. PubMed search updated June 2013.
STUDY SELECTION
English-language peer-reviewed publications of randomized controlled trials (RCTs), nonrandomized cluster controlled trials, controlled before-and-after studies, or interrupted time-series designs conducted among adult patients with diabetes. Two independent reviewers used prespecified criteria to screen titles and abstracts for full text review.
STUDY APPRAISAL AND SYNTHESIS METHODS
Two different reviewers abstracted data and rated study quality and strength of evidence. When possible, we used random-effects models to synthesize the effects quantitatively, reporting by a weighted difference of the means when the same scale was used across studies, and a standardized mean difference when the scales differed. We measured heterogeneity in study effects using Forest Plots, Cochran’s Q, and I2, and explored heterogeneity by using subgroup analyses for categorical variables and meta-regression analyses for continuous or discrete variables. Outcomes not suitable to meta-analysis were summarized qualitatively.
RESULTS
Twenty-five articles representing 17 unique studies compared SMA interventions with usual care. Among patients with diabetes, SMAs improved hemoglobin A1c (∆ = −0.55 percentage points [95 % CI, −0.11 to −0.99]); improved systolic blood pressure (∆ = −5.2 mmHg [95 % CI, −3.0 to −7.4]); and did not improve LDL cholesterol (∆ = −6.6 mg/dl [95 % CI, 2.8 to −16.1]). Nonbiophysical outcomes, including economic outcomes, were reported too infrequently to meta-analyze, or to draw conclusions from. The A1c result had significant heterogeneity among studies, likely secondary to the heterogeneity among included SMA interventions.
LIMITATION
Heterogeneity among the components of diabetes SMAs leads to uncertainty about what makes a particular SMA successful.
CONCLUSION
SMA interventions improve biophysical outcomes among patients with diabetes. There was inadequate literature to determine SMA effects on patient experience, utilization, and costs.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2978-7) contains supplementary material, which is available to authorized users.
KEY WORDS: shared medical appointments, diabetes, chronic illness care
INTRODUCTION
Group medical visits are defined as multiple patients seen together while in the same clinical setting. A subset of group clinics—referred to as shared medical appointments (SMAs)—is defined by groups of patients meeting over time for comprehensive care, usually involving a practitioner with prescribing privileges, for a defining chronic condition or health care state.1 SMAs often use educational or self-management enhancement strategies, paired with medication management, in an effort to improve disease outcomes.1 SMAs usually have more than one health care provider involved; often, the care team will include a person trained or skilled in delivering patient education or facilitating patient interaction (nurse, psychologist) and a prescribing provider empowered to make and initiate a comprehensive care plan.1 The patient group may stay constant in an attempt to facilitate group cohesion, or patients may be allowed to attend sessions chosen from a schedule at their own convenience to promote attendance. Similarly, providers can either be constant with the same patients or vary over time. There has been increased use of and interest in SMAs in recent years, as evidenced by a number of prominent lay press reports.2,3
Shared medical appointments have been studied in an array of primary care settings over the last 10 to 15 years.4–21 They have been researched most in diabetes care,8–18,20,21 but there has been great variability among these studies. In particular, the study settings have been heterogeneous; different chronic health care states have been assessed; and the impact on clinical, cost, and utilization outcomes has been variable. Most important, there has been significant variation in the components of SMA interventions used in diabetes management—in particular, which types of clinical and educational strategies have been tested in the specific SMAs under evaluation.
The Department of Veterans Affairs (VA) commissioned an evidence synthesis,22 from which this paper is derived, to summarize the results of the diverse studies of SMAs for selected chronic illnesses in an effort to understand their impact on clinical outcomes and health care utilization. Because the majority of the evidence review was derived from studies in patients with diabetes mellitus, we focused our manuscript on this population. This paper was informed by an update of the literature search used in the original VA report.
METHODS
We developed and followed a standard protocol for all steps of the review, in addition to following the PRISMA reporting guidelines.23 Methods are summarized here, with detailed methods provided in the VA evidence report available at www.hsrd.research.va.gov/publications/esp.
Data Sources and Searches
In consultation with a master librarian, we searched MEDLINE (via PubMed), EMBASE, CINAHL, PsycINFO, and Web of Science for peer-reviewed publications from January 1996 through April 2012 that compared SMAs with usual care; the article generally held to be the seminal article on SMAs was published in 1997. Because all of the eligible studies in the evidence report were identified via PubMed, we updated the PubMed search for the current manuscript through June 2013. We used medical subject headings and text words for terms relevant to populations and interventions (e.g., shared medical appointments, group visits, cluster visits), and validated search strategies for both randomized controlled trials (RCTs) and relevant observational studies (Appendix Table 1, available online). We limited the search to articles published in English involving human subjects 18 years of age and older. We supplemented electronic searches with a review of the bibliographies of key articles. As a mechanism to assess risk of publication bias, we searched www.clinicaltrials.gov/ for completed but unpublished studies.
Study Selection, Data Extraction, and Quality Assessment
Two reviewers used prespecified eligibility criteria to screen titles and abstracts. Eligible studies were RCTs, nonrandomized cluster controlled trials, controlled before-and-after studies, or interrupted time-series designs conducted among adult patients with diabetes that assessed SMA interventions. (Appendix Table 2, available online, presents full eligibility criteria.) Titles and abstracts included by either reviewer for full text review underwent further evaluation by a different pair of reviewers. For included studies, we abstracted data on study populations, interventions, outcomes, quality, and applicability. Interventions were categorized using the following domains: (1) whether the team was continuous, (2) whether the group was closed, (3) whether individual breakout sessions were conducted, (4) whether medication changes were made, (5) how long each session was, and (6) whether there was contact outside the session.
Based on our understanding of the theoretical underpinnings of SMA interventions,14 we constructed an SMA “robustness score” that ranged from 0 to 9, based on seven intervention elements chosen a priori. These included: being at or above median in visit frequency; individual contact time with a provider; the presence of a theoretical framework guiding the intervention; groups facilitated by a licensed or trained behaviorist; continuity between patients and clinical team, and medication changes. The last characteristics were scored 0 (absent) or 2 (present); the behaviorist category was scored 2 for a licensed person, and 1 for a formally trained person, leading groups; all other items were scored as 0 or 1. Disagreements at any step were reconciled through discussion or by a third reviewer.
We assessed quality as described in the Agency for Healthcare Research and Quality’s (AHRQ’s) “Methods Guide for Effectiveness and Comparative Effectiveness Reviews.”24 Briefly, this approach: specifically addresses methodological quality as a key source of potential bias; assesses specific categories of bias, including selection bias, detection bias, and selective outcome reporting bias; includes validity and reliability of outcome measurement as a source of detection bias; and allows for different bias ratings for different outcomes within the same study. Again, assessments of bias were performed by two reviewers, and disagreements were reconciled through discussion or by a third reviewer.
Data Synthesis and Analysis
When meta-analysis was appropriate, we used random-effects models to synthesize the effects quantitatively, reporting by a weighted difference of the means when the same scale was used (e.g., mmHg for blood pressure) and a standardized mean difference when the scales differed across studies (e.g., for health-related quality of life). Heterogeneity was examined among the studies using Forrest plots and test statistics (Cochran’s Q and I2); p < 0.10 or I2 > 50 % were interpreted as showing significant heterogeneity.25 We explored identified heterogeneity in study effects by using subgroup analyses for categorical variables (e.g., study quality) and meta-regression analyses for continuous or discrete variables (e.g., baseline HbA1c, intervention robustness). We used Comprehensive Meta-Analysis (version 2.2.064) to conduct these analyses. Outcomes not suitable to meta-analysis were summarized qualitatively. We evaluated the strength of evidence for each outcome using the approach described by AHRQ,24 which assesses the following domains for each outcome: risk of bias, consistency, directness, precision, strength of association (magnitude of effect), and publication bias. These domains were considered qualitatively, and a summary rating of high, moderate, low, or insufficient strength of evidence was assigned for each outcome after discussion by two reviewers.
Role of the Funding Source
The VA’s Quality Enhancement Research Initiative funded the research, but did not participate in the literature search, data analysis, interpretation of the results, or decision to submit a manuscript for publication. VA QUERI coordinated peer review for the related VA Evidence Report prior to publication, and approved that final document for publication.
RESULTS
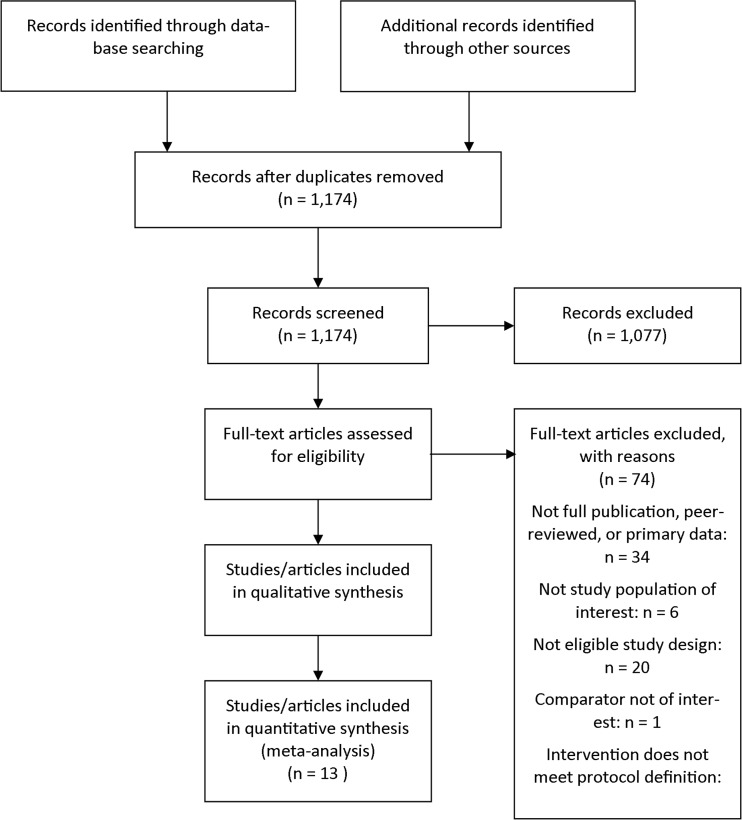
We identified 1,172 unique citations from a combined search of MEDLINE (n = 397), CINAHL (n = 290), Embase (n = 145), PsycINFO (n = 157), and Web of Science (n = 186). Manual searching of bibliographies of included studies and review articles identified two more citations, for a total of 1,174 citations. After applying inclusion and exclusion criteria at the title-and-abstract level, 97 full-text articles were retrieved and screened. Of these, 74 were excluded at the full-text screening stage, leaving 17 unique studies (represented by 23 articles) for data abstraction (Fig. 1). Our search of www.clinicaltrials.gov/ did not suggest publication bias. We found three ongoing trials, but there were no completed studies that were unpublished.
Figure 1.
Literature flow diagram.
All 17 studies compared diabetes SMAs with usual care or enhanced usual care; there were no direct comparisons with other types of quality-improvement strategies. Among the 17 studies, 13 were RCTs9–11,13,15–20,26–28 and four were observational studies8,12,14,29 with concurrent controls. Most studies were conducted in primary care settings that are part of integrated health systems in the United States. Of the 17 studies, 12 reported outcomes at 1 year or later (Table 1). Study quality was rated as good for six RCTs, fair for six RCTs and two observational studies, and poor for the three remaining studies. For RCTs, methodological problems included (a) failure to describe allocation concealment (n = 9), (b) outcomes assessed without blinding to intervention (n = 6), and (c) an inadequate approach to addressing incomplete data (n = 6). Detailed study characteristics are given in Appendix Table 3 (available online).
Table 1.
Study Details for SMAs Enrolling Adults with Diabetes
| Characteristic | Randomized controlled trials | Observational studies |
|---|---|---|
| N studies (participants) | 13 (2,921)* | 4 (326) |
| Median age of sample (range)† | 60.8 (29 to 69.8) | 58.1 (56 to 61.0) |
| Sex: N (%) | ||
| Male | 1,585 (54.3 %) | 93 (28.5 %) |
| Female | 1,137 (38.9 %) | 128 (39.3 %) |
| Not reported (4 studies) | 190 (6.8 %) | 105 (32.2 %) |
| Race: N (%)‡ | ||
| African American | 425 (16.4 %) | – |
| White | 952 (36.7 %) | – |
| Other | 127 (4.9 %) | – |
| Not reported | 1,088 (42.0 %) | 326 (100 %) |
| Study quality: N (%) | ||
| Good | 6 (46 %) | 0 |
| Fair | 6 (46 %) | 2 (50 %) |
| Poor | 1 (8 %) | 2 (50 %) |
| Setting: N studies (participants) | ||
| Primary care | 10 (1932) | 4 (326) |
| Medical Subspecialty | 3 (989) | 0 (0) |
| Health care system: N studies (participants) | ||
| Government (VA, FQC) | 5 (631) | 2 (140) |
| Private clinic/integrated system (HMO) | 2 (892) | 1 (26) |
| University-affiliated clinic | 6 (1,398) | 1 (160) |
| Country: N studies (participants) | ||
| United States | 10 (1,932) | 4 (326) |
| Europe | 3 (989) | 0 (0) |
| Sites: N studies (participants) | ||
| Single | 11 (1,806) | 4 (326) |
| Multisite | 2 (1,115) | 0 (0) |
| Study duration§: N studies (participants) | ||
| < 6 months | 0 (0) | 2 (105) |
| 6 to 12 months | 3 (331) | 0 (0) |
| > 12 months | 10 (2,590) | 2 (221) |
*Participant number is based on the number included in description of population characteristics, which is a smaller sample than those randomized
†Mean age was not reported in one study
‡Of studies reporting race, 329 participants were not accounted for; therefore, percentage is of n = 2,592
§Study duration is measured from time of randomization to most distal follow-up
Abbreviations: FQC federally qualified center; VA Department of Veterans Affairs; HMO health maintenance organization
Characteristics of Shared Medical Appointments
In the studies we assessed, SMAs were led by teams of one to three clinicians that included a physician (n = 13), clinical pharmacist (n = 9; the prescribing clinician in three studies), and registered nurse (n = 10) (Appendix Table 4, available online). The clinical team was multidisciplinary in 16 studies; eight studies employed pharmacists and four employed licensed mental health professionals. Sessions were designed so that the same patients stayed together in the same groups through the length of the intervention in all but three studies; these latter studies used drop-in models. Group size was six to ten members for ten studies, with group size ranging between ten and 20 in five studies, and being as large as 25 in one study. The planned visit frequency ranged from approximately every 3 weeks to every 3 months. SMA visits were a median of 2 h (range, 1 to 4 h).
At least 14 studies offered individual breakouts with a physician or clinical pharmacist, since part of the SMA design specified that medication changes could be made at group visits. Three studies did not report this information. Seven studies invited participation by family members or friends; six did not specify and four did not allow this participation. Three studies described the educational approach as “patient-centered adult learning,”18–20 and two studies used the stages-of-change model to design the intervention;17,26 no other study described a theoretical model. In six studies, patients participated in selecting or prioritizing educational topics, and printed materials were tailored to the individual patient. Few studies used telephone contact as a part of the SMA intervention. (For details of each SMA intervention, refer to the appendix of the full report.22)
Effects of SMAs on Diabetes Outcomes
Patient Selection
Of the 13 RCTs, ten examined type 2 diabetes only, one examined type 1 only, and two examined a mixed patient population. Studies enrolled patients with poor glucose control (thresholds varied from A1c 6.5 to > 9 %); a few studies required elevated blood pressure or lipids. Of the 17 total studies, 16 compared SMAs with usual care and one15 compared SMAs with a traditional, two-session diabetes education intervention.
Hemoglobin A1c
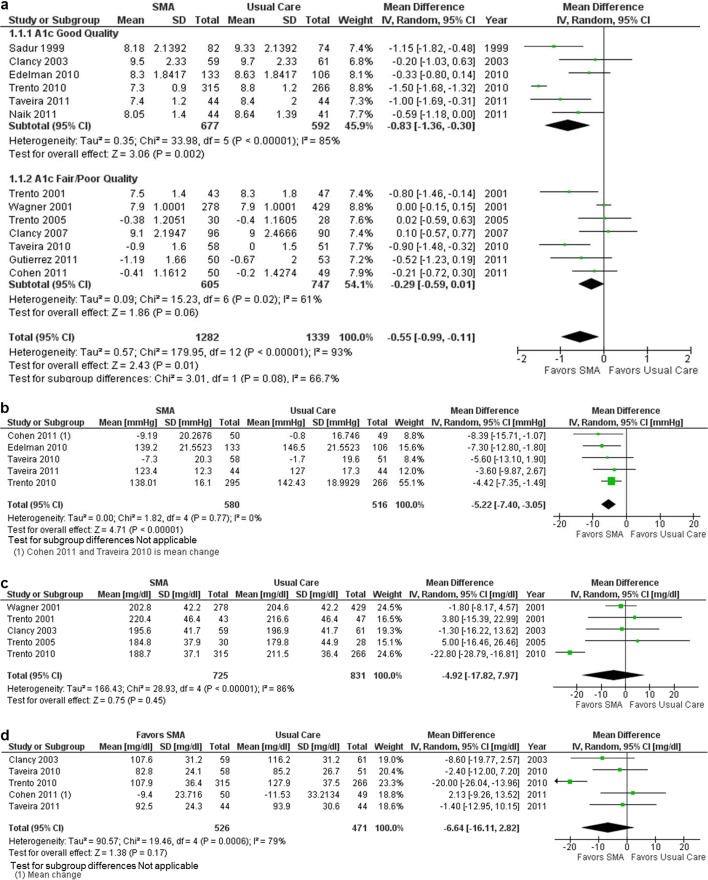
Figure 2a shows the forest plots for the random-effects meta-analyses of SMA interventions on glucose control (A1c). All studies reported effects on average glucose at the end of the intervention, assessed at 6 months to 4 years. SMAs were associated with lower A1c compared with usual care (mean difference −0.55 [95 % CI, −0.99 to −0.11]). However, effects varied significantly across studies (Q 179.9; degrees of freedom 12; p < 0.001; I2 93 %), and study quality was not predictive of effect size. A sensitivity analysis that excluded the study in patients with type 1 diabetes19 did not decrease heterogeneity (I2 94 %). Using meta-regression, we did not find an association between baseline A1c (ß = 0.10, p = 0.58) or intervention robustness (ß = 0.02, p = 0.88) and treatment effects. Thus, SMAs were associated with a mean decrease in A1c, but effects varied markedly and were not explained by factors we hypothesized a priori to be associated with variation in treatment effects.
Figure 2.
Effects of shared medical appointments. a On Hemoglobin A1c; b On systolic blood pressure; c On total cholesterol; d On LDL cholesterol. Abbreviations: CI confidence interval; LDL low-density lipoprotein; SD standard deviation; SMA shared medical appointment.
Effects of SMAs on glucose from the observational studies were generally consistent with the RCT data. Two of the four observational studies8,14 found statistically significant reductions in A1c from baseline to follow-up among patients participating in SMAs. Only one study14 compared this change with a control group, finding a statistically significant benefit from SMA participation (p = 0.002).
Blood Pressure
Figure 2b shows the forest plot for the random-effects meta-analysis of SMA interventions on systolic blood pressure. Five studies reported effects on systolic blood pressure.11,17,20,25,27 SMAs were associated with improved blood pressure control (mean difference −5.22 [95 % CI, −7.40 to −3.05]). Results were consistent across studies (Q 1.82; degrees of freedom 4; p = 0.77; I2 0 %).
Of the four observational studies, only one14 found a statistically significant pre–post change in systolic blood pressure for the SMA participants. In this study, the blood pressure effects were also greater for the SMA group (−14.93 mmHg) than for the control group (−2.54 mmHg, P = 0.04).
Cholesterol
Figures 2c and d show the forest plots for the random-effects meta-analyses of the effect of SMAs on total cholesterol (five studies) and LDL cholesterol (five studies). For both outcomes, SMAs were associated with a decrease in cholesterol that was not statistically significant. For each outcome, treatment effects varied significantly across studies. Because of the small number of studies, we did not perform meta-regression analyses to examine variability in treatment effects. One additional study13 reported a statistically nonsignificant increase in the proportion of patients achieving an LDL of less than 100—findings that are consistent with the analysis of mean change in LDL.
Only two of the observational studies reported effects on cholesterol. Both found reductions in LDL cholesterol, but only one14 compared the SMA with the control group, and the differences were not statistically significant.
Patient Experience Outcomes
We had insufficient studies to perform meta-analysis of patient-experience outcomes. No more than two studies reported effects on any one of a wide range of experiential outcomes. For example, two RCTs reported no change in patients’ satisfaction with SMAs compared with control.19,27 One study11 reported no effects on medication adherence, and another18 reported no effects on blood glucose self-monitoring.
Utilization and Economic Outcomes
Hospital Admissions and Emergency Department Visits
The effect of SMAs on hospital admissions was reported in five studies.9,11,16,26,27 In four of these, admission rates were lower with SMAs, but the result was statistically significant in only one study.16 Effects on emergency department visits were reported in the same five studies. Two studies reported significantly lower emergency department use.11,27 Rates were not significantly different in the other three studies. Observational studies did not report comparative effects on admission rates or emergency department visits.
Costs
Four studies reported effects on total costs: one in a large health maintenance organization,27 two in a university-affiliated general medical clinic serving low-income patients,9,30 and another in an Italian diabetes clinic.18 For all studies, the purpose was to assess the relative costs of the intervention, the cost analysis was a secondary aim, and the perspective of the cost analysis was that of the health care system under study. Findings were mixed. Both university-based studies showed significant cost differences, but in different directions; the earlier study found significantly higher total costs (inpatient, outpatient, and emergency department costs) for SMAs compared with usual care ($2,886 versus $1,490 per patient over 6 months; p = 0.0003). Higher total costs were driven by both outpatient and inpatient costs. In the later study, 1-year charges were significantly lower for the SMA group ($5,869 versus $8,412 per patient, p < 0.05). Lower total charges were heavily influenced by lower inpatient costs for the SMA group. Two other studies18,27 showed no significant difference in costs between SMAs and usual care arms.
DISCUSSION
SMAs have the potential to offer chronic disease care that is more efficient while improving staff satisfaction and patient outcomes. We identified 13 RCTs and four observational studies of varying quality comparing diabetes SMAs with usual care or enhanced usual care. The most robust finding of this evidence synthesis is that SMAs for patients with diabetes appear to have a significant impact on biophysical outcomes. Hemoglobin A1c improved by approximately 0.6 percentage points, and systolic blood pressure by about 5 mmHg; both these findings were statistically significant. LDL-C improved by approximately 7 mg/dl, but this was not statistically significant. These findings are similar to those seen in another recent meta-analysis.31 While each individual finding is only moderately robust given the limitations in study quality and unexplained variability in intervention effects, the constellation of findings taken together indicates that SMAs help intermediate clinical outcomes for type 2 diabetes.
When assessing the impact of SMAs on utilization and cost, four of five studies suggested hospitalization was lower in the intervention arm, but only one found a statistically significant effect. Effects on other economic outcomes were even more preliminary and/or mixed. We also looked for effects on patient and staff experience and satisfaction with SMAs as well as treatment adherence, but found too little evidence from which to draw conclusions. The impact of SMAs on clinical and economic outcomes is summarized in Table 2. Our judgments about the strength of evidence prioritized data from RCTs.
Table 2.
Summary of Effects of Diabetes SMAs on Clinical and Economic Outcomes
| Number of studies (subjects)* | Domains pertaining to strength of evidence (SOE) | |||||
|---|---|---|---|---|---|---|
| Risk of bias: study design/quality | Consistency | Directness | Precision | Effect estimate (95 % CI) SOE |
||
| Clinical outcomes | ||||||
| A1c | 13 (2,921) | RCT/Good | Inconsistent | Direct | Some imprecision | MD −0.55 (−0.99 to −0.11) Moderate SOE |
| Blood pressure | 5 (1,125) | RCT/Good | Consistent | Direct | Some imprecision | MD −5.2 (−7.4 to −3.1) Moderate SOE |
| Total cholesterol | 5 (1,556) | RCT/Fair | Inconsistent | Direct | Imprecise | MD −4.9 (−17.8 to 7.9) Low SOE |
| LDL cholesterol | 5 (997) | RCT/Fair | Inconsistent | Direct | Imprecise | MD −6.6 (−16.1 to 2.8) Low SOE |
| Economic outcomes | ||||||
| Emergency department visits | 5 (1,339) | RCT/Good | Inconsistent | Direct | Imprecise | Lower rates in 2 of 5 studies Insufficient SOE |
| Hospitalizations | 5 (1,339) | RCT/Good | Consistent | Direct | Imprecise | Insignificantly lower rates in 4 of 5 studies Low SOE |
| Total costs | 4 (1,125) | RCT/Fair | Inconsistent | Direct | Imprecise | Total costs range from lower to higher Insufficient SOE |
*Studies (subjects) given are for randomized trials; observational studies were also considered in strength of evidence ratings, but are not listed separately in the table
Abbreviations: CI confidence interval; MD mean difference; NA not applicable; RCT randomized controlled trial; SOE strength of evidence
It is challenging to place into context these improvements seen in biophysical parameters with diabetes SMAs. However, we can discuss the clinical importance of these findings in at least two ways. First, we can compare the meta-analytic outcome improvements to clinical trial data relative to starting a new medication for these conditions. The improvement seen in 1 year on systolic blood pressure across all arms of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) trial after adding the chosen first medication was approximately 6.6 mmHg; patients in SMAs achieved approximately 75 % of that level of improvement.31 Similarly, adding a first-line oral hypoglycemic agent at a maximally tolerated dose usually lowers A1c by 1 to 1.5 percentage points;32 patients in SMAs achieved 33 to 50 % of that goal. The change in LDL-C of 7 mg/dl is much smaller compared with a drug effect, approximately 15 % of what would be expected with clinical trial doses of an HMG-CoA reductase inhibitor (i.e., statin). However, each drug comparison is made relative to placebo controls. For SMA interventions, the comparator is usual care, which typically includes medication treatment, and thus one would expect the effects to be smaller. It is also important to note that, since SMAs utilize medication management, it may be that addition of new medications is part of the reason for improvements demonstrated by SMAs.
Another way to evaluate the improvements observed with SMA interventions is against the known standard deviations for outcomes in the population of patients with disease, and then calculate effect sizes. While many different values for standard deviations for the relevant parameters are reported in the literature, Cohen’s effect sizes of SMA interventions for systolic blood pressure, A1c, and LDL-C are approximately 0.5, 0.33, and 0.25 standard deviations, respectively. These are considered moderate to small effect sizes, but all would be considered clinically important.33
The improvements in A1c and blood pressure, and the more modest improvement in LDL-C, are possibly synergistic, or at least additive, in prevention of the macrovascular and microvascular complications of diabetes.34 Thus, as a whole, SMA interventions may have an impact on the risk of complications among patients with diabetes. Even if half the effect were lost in translation due to lower treatment fidelity when implemented outside of clinical trials, there would still likely be an important improvement in complication risk for patients enrolled in a diabetes SMA intervention. However, it is important to remember that the degree of synergy in the context of improvements in multiple outcomes is guesswork at best; SMAs—and indeed multicomponent health services in general—have not been studied with enough patients to directly determine their actual effects on major cardiovascular or microvascular complications.
Limitations of the Literature
Our evidence synthesis uncovered far more gaps in the literature than it found definitive results. The most important of these gaps is likely the heterogeneity of what comprises “diabetes SMAs.” Complex health services interventions are often a black box; that is, they contain many components that are hard to capture and tease out, even in a well-conducted analysis. The intervention processes used in the diabetes SMAs we reviewed had enough differences such that we could not identify what makes a particular SMA intervention successful. There were also precious few data on satisfaction, patient access, or other key patient-centered outcomes. No conclusions could be drawn regarding the most appropriate patient for a diabetes SMA referral, nor were there enough data to support a consistent mechanism of action for SMAs (e.g., improved peer support or improved self-management).
Conclusions
Our review suggests that SMAs, typically using closed panels with individual breakouts and opportunity for medication management, can help intermediate clinical outcomes for type 2 diabetes. Clinical leaders interested in implementation of SMAs for diabetes patients should feel comfortable that it is reasonably likely patients will be helped by SMAs as an add-on to routine clinical care. The next generation of diabetes SMA research should work to close the gaps we identified in the literature. In particular, mechanistic studies that measure behaviors more closely and in a standard fashion, simple large-scale trials that measure clinical events as well as costs, and quasi-experimental implementation studies that measure real-world impacts on patient-centered and staff-centered outcomes would be important in defining the future role of this new model of care.
Electronic supplementary material
(DOCX 33 kb)
Acknowledgements
The authors thank Connie Schardt, MLS, for help with the literature search and retrieval, Avishek Nagi, MS, for organizational support, and Liz Wing, MA, for editorial assistance.
Conflict of Interest
No investigators have any affiliations or financial involvement (e.g., employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties) that conflict with material presented in the report.
Financial Support
U.S. Veterans Affairs Office of Research and Development, Quality Enhancement Research Initiative (VA-ESP Project 09-010;2012). The sponsor chose the topic and guided the key questions for the review, and provided access to peer review and edits for the original VA Evidence Report, but had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of this manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Noffsinger EB. Operational challenges to implementing a successful physicals shared Medical Appointment Program. Part 1: Choosing the right type of shared medical appointment. Group Pract J. 2002;51:24–34. [Google Scholar]
- 2.Park A. Need to see the doctor? You may have company on your next visit. Time Magazine. August 7, 2013. Available at: http://healthland.time.com/2013/08/07/need-to-see-the-doctor-you-may-have-company-on-your-next-visit. Accessed July 14 2014.
- 3.Gorman A. Group meetings turn doctor visits inside out. Los Angeles Times. September 16, 2013. Available at: http://axis.cdrewu.edu/axis_doc/functions/AXIS/group_meetings_turn_doctor_visits_inside_out-latimes.com.pdf. Accessed July 14 2014.
- 4.Beck A, Scott J, Williams P, et al. A randomized trial of group outpatient visits for chronically ill older HMO members: the Cooperative Health Care Clinic. J Am Geriatr Soc. 1997;45:543–549. doi: 10.1111/j.1532-5415.1997.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 5.Levine MD, Ross TR, Balderson BH, Phelan EA. Implementing group medical visits for older adults at group health cooperative. J Am Geriatr Soc. 2010;58:168–172. doi: 10.1111/j.1532-5415.2009.02628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JC, Conner DA, Venohr I, et al. Effectiveness of a group outpatient visit model for chronically ill older health maintenance organization members: a 2-year randomized trial of the cooperative health care clinic. J Am Geriatr Soc. 2004;52:1463–1470. doi: 10.1111/j.1532-5415.2004.52408.x. [DOI] [PubMed] [Google Scholar]
- 7.Coleman EA, Eilertsen TB, Kramer AM, Magid DJ, Beck A, Conner D. Reducing emergency visits in older adults with chronic illness. A randomized, controlled trial of group visits. Eff Clin Pract. 2001;4:49–57. [PubMed] [Google Scholar]
- 8.Bray P, Thompson D, Wynn JD, Cummings DM, Whetstone L. Confronting disparities in diabetes care: the clinical effectiveness of redesigning care management for minority patients in rural primary care practices. J Rural Health. 2005;21:317–321. doi: 10.1111/j.1748-0361.2005.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 9.Clancy DE, Cope DW, Magruder KM, Huang P, Wolfman TE. Evaluating concordance to American Diabetes Association standards of care for type 2 diabetes through group visits in an uninsured or inadequately insured patient population. Diabetes Care. 2003;26:2032–2036. doi: 10.2337/diacare.26.7.2032. [DOI] [PubMed] [Google Scholar]
- 10.Clancy DE, Huang P, Okonofua E, Yeager D, Magruder KM. Group visits: promoting adherence to diabetes guidelines. J Gen Intern Med. 2007;22:620–624. doi: 10.1007/s11606-007-0150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelman D, Fredrickson SK, Melnyk SD, et al. Medical clinics versus usual care for patients with both diabetes and hypertension: a randomized trial. Ann Intern Med. 2010;152:689–696. doi: 10.7326/0003-4819-152-11-201006010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Culhane-Pera K, Peterson KA, Crain AL, et al. Group visits for Hmong adults with type 2 diabetes mellitus: a pre-post analysis. J Health Care Poor Underserved. 2005;16:315–327. doi: 10.1353/hpu.2005.0030. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez N, Gimple NE, Dallo FJ, Foster BM, Ohagi EJ. Shared medical appointments in a residency clinic: an exploratory study among Hispanics with diabetes. Am J Manag Care. 2011;17:e212–e214. [PubMed] [Google Scholar]
- 14.Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349–353. doi: 10.1136/qshc.2006.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171:453–459. doi: 10.1001/archinternmed.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadur CN, Moline N, Costa M, et al. Diabetes management in a health maintenance organization. Efficacy of care management using cluster visits. Diabetes Care. 1999;22:2011–2017. doi: 10.2337/diacare.22.12.2011. [DOI] [PubMed] [Google Scholar]
- 17.Taveira TH, Friedmann PD, Cohen LB, et al. Pharmacist-led group medical appointment model in type 2 diabetes. Diabetes Educ. 2010;36:109–117. doi: 10.1177/0145721709352383. [DOI] [PubMed] [Google Scholar]
- 18.Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995–1000. doi: 10.2337/diacare.24.6.995. [DOI] [PubMed] [Google Scholar]
- 19.Trento M, Passera P, Borgo E, et al. A 3-year prospective randomized controlled clinical trial of group care in type 1 diabetes. Nutr Metab Cardiovasc Dis. 2005;15:293–301. doi: 10.1016/j.numecd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Trento M, Gamba S, Gentile L, et al. Rethink Organization to iMprove Education and Outcomes (ROMEO): a multicenter randomized trial of lifestyle intervention by group care to manage type 2 diabetes. Diabetes Care. 2010;33:745–747. doi: 10.2337/dc09-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krzywkowski-Mohn SM. Diabetic control and patient perception of the scheduled in group medical appointment at the Cincinnati Veterans Administration Medical Center. ProQuest Information & Learning; 2009.
- 22.Edelman D, McDuffie JR, Oddone E, Gierisch JM, Nagi A, Williams JW. Shared medical appointments for chronic medical conditions: a systematic review. U.S. Veterans Affairs Evidence-based Synthesis Program, July 2012. Available at: www.hsrd.research.va.gov//publications/esp. [PubMed]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research and Quality. Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=318. Accessed July 14, 2014.
- 25.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;1539–1558 [DOI] [PubMed]
- 26.Taveira TH, Dooley AG, Cohen LB, Khatana SA, Wu WC. Pharmacist-led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults. Ann Pharmacother. 2011;45:1346–1355. doi: 10.1345/aph.1Q212. [DOI] [PubMed] [Google Scholar]
- 27.Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695–700. doi: 10.2337/diacare.24.4.695. [DOI] [PubMed] [Google Scholar]
- 28.Cohen LB, Taveira TH, Khatana SA, Dooley AG, Pirraglia PA, Wu WC. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Educ. 2011;37:801–812. doi: 10.1177/0145721711423980. [DOI] [PubMed] [Google Scholar]
- 29.Jessee BT, Rutledge CM. Effectiveness of nurse practitioner coordinated team group visits for type 2 diabetes in medically underserved Appalachia. J Am Acad Nurse Pract. 2012;24:735–743. doi: 10.1111/j.1745-7599.2012.00764.x. [DOI] [PubMed] [Google Scholar]
- 30.Clancy DE, Dismuke CE, Magruder KM, Simpson KN, Bradford D. Do diabetes group visits lead to lower medical care charges? Am J Manag Care. 2008;14(1):39–44. [PubMed] [Google Scholar]
- 31.Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185(13):E635–E644. doi: 10.1503/cmaj.130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fowler MJ. Diabetes treatment, part 2: Oral agents for glycemic management. Clin Diabetes. 2007;25(4):131–134. doi: 10.2337/diaclin.25.4.131. [DOI] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 34.Sehestedt T, Hansen TW, Li Y, et al. Are blood pressure and diabetes additive or synergistic risk factors? Outcome in 8494 subjects randomly recruited from 10 populations. Hypertens Res. 2011;34:714–721. doi: 10.1038/hr.2011.6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33 kb)