ABSTRACT
BACKGROUND
The US Preventive Services Task Force recommends screening for and treating obesity. However, there are many barriers to successfully treating obesity in primary care (PC). Technology-assisted weight loss interventions offer novel ways of improving treatment, but trials are overwhelmingly conducted outside of PC and may not translate well into this setting. We conducted a systematic review of technology-assisted weight loss interventions specifically tested in PC settings.
METHODS
We searched the literature from January 2000 to March 2014. Inclusion criteria: (1) Randomized controlled trial; (2) trials that utilized the Internet, personal computer, and/or mobile device; and (3) occurred in an ambulatory PC setting. We applied the Cochrane Effective Practice and Organization of Care (EPOC) and Delphi criteria to assess bias and the Pragmatic-Explanatory Continuum Indicator Summary (PRECIS) criteria to assess pragmatism (whether trials occurred in the real world versus under ideal circumstances). Given heterogeneity, results were not pooled quantitatively.
RESULTS
Sixteen trials met inclusion criteria. Twelve (75 %) interventions achieved weight loss (range: 0.08 kg – 5.4 kg) compared to controls, while 5–45 % of patients lost at least 5 % of baseline weight. Trial duration and attrition ranged from 3–36 months and 6–80 %, respectively. Ten (63 %) studies reported results after at least 1 year of follow-up. Interventions used various forms of personnel, technology modalities, and behavior change elements; trials most frequently utilized medical doctors (MDs) (44 %), web-based applications (63 %), and self-monitoring (81 %), respectively. Interventions that included clinician-guiding software or feedback from personnel appeared to promote more weight loss than fully automated interventions. Only two (13 %) studies used publically available technologies. Many studies had fair pragmatism scores (mean: 2.8/4), despite occurring in primary care.
DISCUSSION
Compared to usual care, technology-assisted interventions in the PC setting help patients achieve weight loss, offering evidence-based options to PC providers. However, best practices remain undetermined. Despite occurring in PC, studies often fall short in utilizing pragmatic methodology and rarely provide publically available technology. Longitudinal, pragmatic, interdisciplinary, and open-source interventions are needed.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2987-6) contains supplementary material, which is available to authorized users.
KEY WORDS: weight loss, technology, primary care, obesity, review
INTRODUCTION
The obesity epidemic accounts for unprecedented rates of chronic disease1–3 and influences numerous interactions in primary care (PC).4 The prevalence of overweight and obese adults, while having plateaued over the past decade, remains high, such that among US adults today, 34.9 % are obese,5 up from 10.4 % in 1960 and 19.9 % in 1994.6
Unfortunately, there is a paucity of available weight loss interventions suitable to the real-world PC setting, with most research and guideline formulation conducted inside academic silos,7–10 not in practice settings such as PC offices.11 With 577 million yearly PC visits, effective weight loss interventions within PC could potentially have a large health impact.12
Weight loss, even when modest (≥ 5 %), is associated with significant improvement in cardiovascular disease (CVD) risk factors,13 quality of life,14 and all-cause mortality for those with comorbidities.15–18 Intensive, tailored lifestyle modification programs result in significant weight loss, health benefits,19–21 and perhaps cost savings.22 Those who receive advice from their PC provider (PCP) to lose weight are more likely to do so,23 yet existing programs are challenged by high attrition rates, weight regain, resource-intensive requirements, and poor scalability.24–27 Concurrent provider barriers include limited visit time,28 inadequate reimbursement,29 lack of training,30 and poor competency.31 As a result, PCPs struggle to properly counsel their obese patients,32–34 even as patients are genuinely interested in receiving counseling from them.35,36
Given these large hurdles, there is a strong need to identify novel weight loss interventions that are applicable to the PC office. Technology-assisted interventions have the potential to address barriers to providing care in the PC office through time and cost savings, improved feedback, enhanced self-monitoring, and convenience of use.37 Many studies38,39 and multiple reviews40–42 have assessed technology-assisted weight loss interventions, yet few have placed emphasis on interventions suitable to the real-world PC practice. Many interventions have yet to be integrated into PC, while others are publically unavailable, poorly studied, or expensive.43 Other trials are not pragmatic,44 where pragmatism refers to the extent to which studies operate in real-world circumstances.45
This systematic review examines technology-assisted weight loss interventions specifically provided in PC settings, and aims to highlight their innovation, impact, and pragmatism. To our knowledge, this is the first such systematic review to examine this topic from the lens of the pragmatic PC practice.
METHODS
Our review follows standard PRISMA methodology,46 and the detailed protocol is available on PROPSERO (CRD42013003998)47 and in Appendix 1, available online. We briefly describe our methods below.
Eligibility Criteria
Our aim was to identify and synthesize PC-based weight loss intervention studies that incorporated various technologies. We included peer-reviewed, randomized controlled trials (RCTs) that used the Internet, a personal or in-office computer, and/or mobile device, had weight loss as a primary outcome, and took place in an ambulatory PC (internal medicine, family medicine, OBGYN) setting. We excluded studies that utilized pedometers, postal mail, or telephone calls as the sole technology, non-RCTs, studies conducted in a specialty or non-ambulatory setting, and studies involving pediatric populations. We chose to exclude pedometers, as they have previously been reviewed and demonstrated modest weight loss.48,49 We only considered trials conducted after the year 2000, as few PC-based studies were published before that date and any prior technology-assisted interventions would likely be obsolete.50
Data Sources and Search Strategy
In late 2012, we performed initial scoping searches with Google Scholar. We then finalized our search strategies with the help of a research librarian and searched the following databases without language restrictions to identify citations and trials from 1 January 2000 to 11 March 2014: PubMed/MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, and Cochrane CENTRAL. To limit to RCTs, we used validated search filters.51 We also searched the reference lists of identified studies and reviews.
Study Selection and Data Extraction
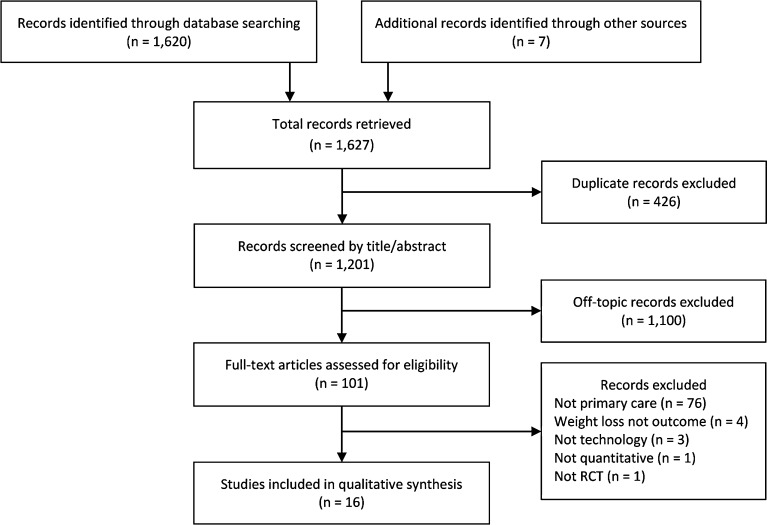
To determine the selection of studies, two authors (DML and SS) independently investigated the titles of all 1,201 initial citations. We excluded 934 articles by title and 166 articles by abstract, leaving 101 for full-text review (Fig. 1). Any disagreements were resolved through consensus, and when needed, a third reviewer (MJ). Two reviewers (DML and SS) independently extracted data for the following variables: baseline demographics, recruitment procedures, setting, intensity,52 mode of customization,53 motivating theory, technology modality, personnel, attrition, weight loss (kg, percent, and percent achieving ≥ 5 % loss), bias, and pragmatism. Because recruitment (all PC offices) and customization (all tailored) did not vary, we do not discuss them further. We similarly do not discuss motivating theory, as studies infrequently reported this.
Figure 1.
PRISMA flow diagram.
Bias Assessment
The Delphi54 and Cochrane Effective Practice and Organization of Care (EPOC)55 criteria were used to assess bias. Delphi and EPOC contain assessment items that pertain to interventions occurring in real-world PC settings, and are commonly used tools for this type of review.56 Two reviewers (DML and SS) independently evaluated the trials (Supplemental Table 1).
Pragmatism Assessment
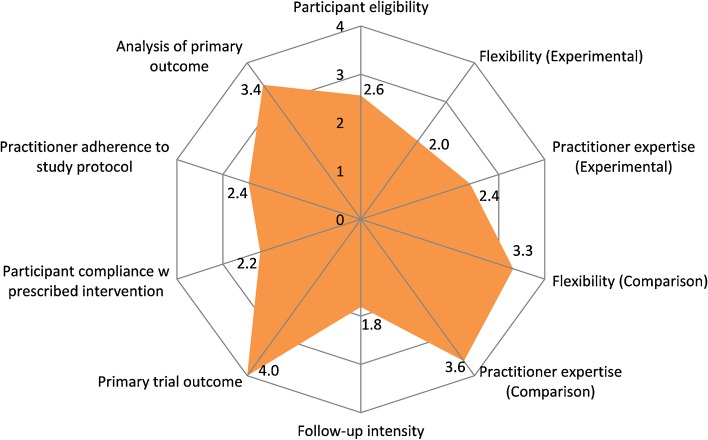
We evaluated the degree of pragmatism of each intervention with the Pragmatic-Explanatory Continuum Indicator Summary (PRECIS).45 This tool helps researchers evaluate the likelihood that a given intervention will work in a real-world setting versus a clinical trial in an ideal setting. An intervention is considered more pragmatic if it is conducted in real-world circumstances; it is considered more explanatory if it occurs in ideal circumstances. PRECIS was initially created by the CONSORT Work Group to assist in trial design and has been adapted to evaluate studies in systematic reviews.57 We modeled our use of PRECIS based on a recent adaptation evaluating the Practice-Based Opportunities for Weight Reduction (POWER) trials (two of which are used in this review), using a 0 (explanatory) to 4 (pragmatic) scale in ten domains.44 A detailed tabular explanation of each of these domains can be found in Fig. 4 and elsewhere.45
Figure 4.
Mean PRagmatic Explanatory Continuum Indicator Summary (PRECIS) Scores. 0-Completely Explanatory (ideal circumstances) → 4-Completely Pragmatic (real-world circumstances). The following descriptions indicate entirely pragmatic trials (see Thorpe et al.45 for detail): Participant eligibility – those with the condition of interest are enrolled without exclusions; Flexibility (experimental arm) – practitioners have considerable leeway in applying the intervention; Practitioner expertise (experimental arm) – no special training or clinical setting is required; Flexibility (comparison arm) – usual care is allowed; Practitioner expertise (comparison arm) – same as experimental arm; Follow-up intensity – no formal follow up; Primary trial outcome – clinically meaningful and testable under usual conditions; Participant compliance – unobtrusive or no measurement of compliance with no rescue strategies; Practitioner compliance – same as participant compliance; Analysis of primary outcome – employs intention to treat analysis.
Statistical Analysis
Study heterogeneity did not allow us to conduct a meta-analysis. We therefore aggregated our results with ranges and narrative summation in most instances.
RESULTS
Sixteen trials58–73 met inclusion criteria and are summarized in Table 1. For readers interested in additional tabular detail and narrative summation of each trial, see Supplemental Table 2 and Appendix 2, available online. Participants were more often female (62 %), white (71 %), and middle-aged. Fifteen (94 %)58–66,68–73 trials were of high intensity and employed tailored interventions. Interventions ranged from 3 – 36 months in duration, with the majority of studies (10/16, 63 %)58,60–64,68,70,71,73 reporting results after at least 1 year of follow-up. Participant attrition rates ranged from 6 to 80 %.
Table 1.
Technology-Assisted Weight Loss Interventions in Primary Care
| Participants | Intervention | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | n | Age ( ) | % F | Ethnicity (%) | BMI () | Intervention (n) | Duration (mos) | Attrition (%) | Weight Δ (kg) | ≥ 5 % weight loss (%) |
| Appel58 | 415 | 54 | 64 | 56W, 41B | 37 | All: PCP visit at 6/12/24 mos for encouragement IG1 (138): in-person support, website: self-monitoring, auto feedback, & email reminder to login IG2 (139): remote support via telephone, same website & emails as IG1 CG (138): 1 weight loss coach meeting, brochures, weight loss websites |
24 | 16 | IG1: −5.1* IG2: −4.6* CG: −0.8 |
IG1: 41* IG2: 38* CG: 19 |
| Bennett (2010)59 | 101 | 54 | 47 | 50W, 31B, 5H | 35 | IG (51): self-monitoring website, 4 RD visits via phone and in-person CG (50): usual care & basic materials |
3 | 16 | IG: −2.3* CG: +0.3 |
IG: 26NR
CG: 0 |
| Bennett (2012)60 |
365 | 55 | 69 | 71B, 13H | 37 | IG (180): web-based tailored behavior change goals, skills training via website or interactive voice response, telephone counseling w trained community health educator, primary care provider endorsement, 12 optional in-person group support sessions, walking kit w pedometer CG (185): usual care & self-help booklet |
24 | 14 | IG: −1.5* CG: −0.5 |
IG: 20 CG: 20 |
| Christian (2008)61 | 310 | 53 | 66 | 100H | 35 | IG (155): goal-setting computer program, then regular MD clinic visits w 3/6/9 mos goals reassessment CG (155): usual care & info packet |
12 | 12 | IG: −0.08 CG: +0.6 |
IG: 21* CG: 11 |
| Christian (2011)62 | 279 | 50 | 68 | 51W, 44H | 34 | IG (140): goal-setting computer program, then MD clinic visits to reinforce goals, 6 mos goals reassessment w computer CG (139): usual care & info packet |
12 | 6 | IG: −1.5* CG: +0.1 |
IG: 26* CG: 8 |
| Ma63 / Xiao†79 | 171 | 53 | 46 | 79W, 4H | 32 | All: Heart360 website, standardized monthly emails, ability to submit questions online IG1 (79): 3-mo intensive in-person weekly DPP w physical activity & food tastings, personalized monthly emails on Heart360 progress IG2 (81): 3-mo intensive at-home DPP DVD, standardized weekly emails CG (81): usual care |
24 | 29 | IG1: −5.4* IG2: −4.5* CG: −2.4 |
IG1: 45* IG2: 30 CG: 17 |
| McConnon64 | 221 | 46 | 77 | 95W | 34 | IG (111): website: tailored advice, tools & information to support behavior change in terms of dietary & physical activity patterns, reminder emails CG (110): usual care, small info booklet |
12 | 41 | IG: −1.3 CG: −1.9 |
IG: 22NR
CG: 18 |
| McDoniel65 | 111 | 46 | 61 | 78W, 20H | 37 | All: MI counseling at wk 4 & wk 8; core topic email newsletters weekly IG (55): MedGem Analyzer for nutrition program & Balance-Log for SM CG (56): Usual care: 3-day food menu, paper journal) |
3 | 28 | IG: −3.5 CG: −3.7 |
IG: 31 CG: 42 |
| Mehring66 | 186 | 48 | 69 | NR | 34 | IG (109): HausMed eHealth web-based coaching program w MD input and 3 phone calls from MD or MA at wks 1,5,12 for motivation CG (77): usual care |
3 | 20 | IG: −2.9* CG: −1.6 |
IG: 26 CG: 16 |
| Nanchahal (2009)67 | 123 | 47 | 80 | 96W | 36 | IG (61): ProHealthClinical structured lifestyle support w tailored diet, self-monitoring w diary, coping skills, & RN feedback CG (62): usual care |
3 | 15 | IG: −4.0* CG: +1.2 |
IG: 17NR
CG: 10 |
| Nanchahal (2012)68 | 381 | 49 | 72 | 73W | 33 | IG (191): structured one-on-one in-person sessions (6 in 1st 12 wks, 4 in 2nd 12 wks, 3 in 3rd 12 wks), perfect-diet-tracker.com for SM; adamsportionpot.com, pedometers CG (190): usual care & asked to seek weight loss from PCP |
12 | 43 | IG: −2.4 CG: −1.3 |
IG: 34* CG: 19 |
| Rothert‡69 | 2862 | 45 | 83 | 56W, 36B | 32 | All: 20min computer assessment IG (1475): Web-tailored weight management program x 6 wks w 1/3/6 wk email asking participants to enter weight CG (1387): Web-info-only materials on Kaiser’s website |
6 | 80 | IG: −2.8* CG: −1.1 |
NR |
| Spring70 | 70 | 58 | 14 | 75W, 25B, 6H | 36 | All: 2 wk run-in baseline IG (35): Weight Loss Phase (mos 0–6): twice weekly MOVE sessions, PDA for self-monitoring w automated feedback, coach-derived feedback; Maintenance Phase (mos 7–12): monthly MOVE sessions, telephone coach conversation if no data transmitted CG (34): All MOVE sessions as IG, but no PDA, no coach calls |
12 | 23 | IG: −2.9* CG: +0.02 |
IG: 30* CG: 15 |
| ter Bogt71 | 457 | 56 | 52 | NR | 30 | All: baseline online or paper questionnaire IG (225): 4 visits w NP using software, 1 telephone f/u in yr 1, then 1 visit & 2 telephone f/u’s in yr 2 & yr 3 CG (232): MD usual care |
36 | 22 | IG: −1.1 CG: −0.5 |
IG: 5 CG: 5 |
| Verheijden72 | 146 | 63 | 45 | NR | 29 | IG (73): Heartweb online program: monthly stage-of-change questionnaire w subsequent tailored nutrition suggestions, bulletin board, dietary fat tracker, low fat recipes CG (73): usual care |
8 | 9 | NR | NR |
| Wylie-Rosett‡73 | 588 | 52 | 82 | 83W | 36 | All: computer assessment IG1 (236): 6 group workshops, RD/MSW consult (telephone or face-to-face) up to 18times + IG2 + CG IG2 (236): kiosks weekly (20–30 mins) x 3 mos, then monthly + CG CG (116): workbook |
12 | 21 | IG1: −3.4* IG2: −2.1* CG: −1.0 |
IG1: 31* IG2: 23* CG: 15 |
B Black; BMI Body Mass Index (kg/m2); CG Control Group; Δ Change; DPP Diabetes Prevention Program; H Hispanic; IG Intervention Group; NR Not Reported; PCP Primary Care Provider; PDA Personal Digital Assistant; W White; Mean
Technology Modality: App Mobile App; CS Clinician Software; HC Home Computer (no internet); Int Internet; K Kiosk; EP Exercise Physiologist; MSW Master of Social Work; MD Medical Doctor; None No Personnel; NP Nurse Practitioner; O Other Personnel; RD Registered Dietician; RN Registered Nurse
Elements: AF Automated Feedback; Con Contests; IPF In-Person Feedback; LC Lifestyle Coaching; MDF MD Feedback; O Other; P2P Peer-to-Peer Support; Rem Reminders; SM Self Monitoring
* p < 0.05
† Xiao et al. re-consented the patients from Ma et al. and followed them for nine additional months; we report their data with this extension period. Also, their data is for ≥ 7% weight loss, not ≥ 5%.
‡ Completers-only analysis (i.e., not intention to treat)
Compared to the control group, most (12/16, 75 %)58–63,66–70,73 technology-assisted interventions achieved weight loss at the end of the study period. Weight loss in active treatment arms ranged from 0.08 kg to 5.4 kg (0.8 % – 5.8 % of initial body weight). The percentage of patients losing at least 5 % of baseline weight ranged from 5 % to 45 %.
Personnel
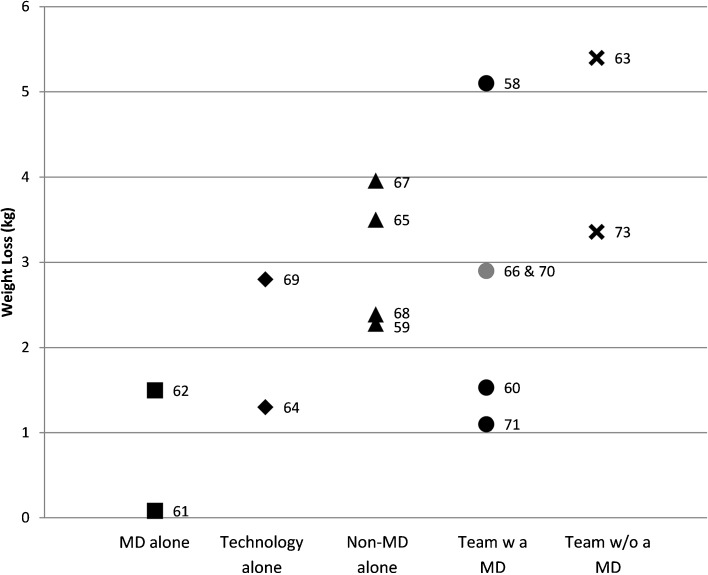
Figure 2 illustrates the association between weight loss and mix of personnel delivering the intervention. Seven (44 %)58,60,63,66,70,71,73 trials used more than one type of personnel. Trials most frequently utilized medical doctors (MDs) (7/16, 44 %)58,60–62,66,70,71. When MDs participated, they were solely in a supportive role (no active counseling) in 3/7 (43 %)58,60,71 studies, whereas they actively counseled patients in 3/7 (43 %)61,62,66 studies. In 1/7 (14 %)70 studies, they co-taught group lifestyle sessions with registered dieticians (RDs) and psychologists. Four studies utilized RDs who most often counseled participants one-on-one (3/4, 75 %),59,63,73 using a combination of in-person, telephone, and email-based exchanges. In one study, RDs co-taught group sessions with MDs and psychologists.70 Several studies used “other” personnel (5/16, 31 %).58,60,63,68,70 These ranged from “fitness instructors,”63 to “weight loss coaches,”58 to “community health educators.”60 These personnel had varied levels of training and were most often involved in lifestyle coaching delivered via various modalities, including in-person, telephone, and email-based exchanges similar to the RD studies.
Figure 2.
Weight loss by personnel type. Square: MD alone (e.g., no additional personnel assisted MD). Diamond: Technology alone (e.g., no personnel, intervention automated). Triangle: Non-MD alone (e.g., RN, weight loss coach, etc.). Circle: Team with an MD. X: Team without an MD. Gray: Two overlapping data points. Numbers beside each shape indicate citation.
Only one study included nurse practitioners (NPs), where their counseling was guided by software.71 Although this did not demonstrate significant weight loss compared with usual PC by MDs at the study’s 3 year conclusion, significant weight loss was demonstrated at 1 year.74
Three (19 %)64,69,72 studies utilized no personnel, instead relying entirely on automated advice based on stage of change72 or based on dietary and physical activity patterns.64,69 All employed web-based self-monitoring. Importantly, 2/3 (67 %)64,72 of these studies did not demonstrate significant weight loss, while 1/3 (33 %)69 showed 2.8 kg weight loss, but had 80 % attrition, did not use intention to treat analysis, and used self-reported data.
Technology Modality
Technology modalities included web-based applications (63 %),58–60,63–66,68,69,72 clinician-guiding software (44 %),58,60–62,66,67,71 kiosks (19 %),61,62,73 home PCs (13 %),65,68 mobile applications (6 %),70 and short message services (SMS, “texting”) (6 %).66 Half of the studies59,64,67,69–73 employed a single technology modality (weight loss: 1.1 – 3.96 kg), while others58,60–63,65,66,68 used multiple modalities (weight loss: 0.082 – 5.4 kg). Two out of 16 (13 %)63,73 used publically available open-source technology.
Ten (63 %)58–60,63–66,68,69,72 trials employed web-based applications. All utilized proprietary technology for self-monitoring with the exception of Heart360.org.63 Spring et al.70 used a proprietary mobile app, while Mehring et al. employed texting through a commercial website.66
Clinician-guiding software, used in 7/16 (44 %)58,60–62,66,67,71 studies, assisted various practitioners (MDs, RNs, and NPs) in guiding participants toward achieving their weight loss goals. In Christian et al.,61,62 for example, participants took a kiosk-based survey that provided the MD with a one-page assessment of the patient and assisted in goal-setting and goal-resetting over 9 months. Participants in all seven studies had significant weight loss at 3 months,66,67 1 year,61,62,74 and 2 years,58,60 although in ter Bogt et al., weight loss in the intervention group was no longer significantly different from that in the control group at 3 years.71
Three (19 %)61,62,73 studies used kiosks to obtain baseline health and behavior-related patient information61,62 or to facilitate a computer-based weight loss program while in the waiting room.73 In Wylie-Rosett et al.,73 only about 50 % of participants enjoyed working on the kiosks, 75 % found them a poor substitute for human contact, yet 75 % found the kiosk easy to use. In contrast, about 75 % and 67 % of participants in Nanchahal (2009) et al.67 found automated and RN web-based feedback and motivation very/extremely helpful, respectively.
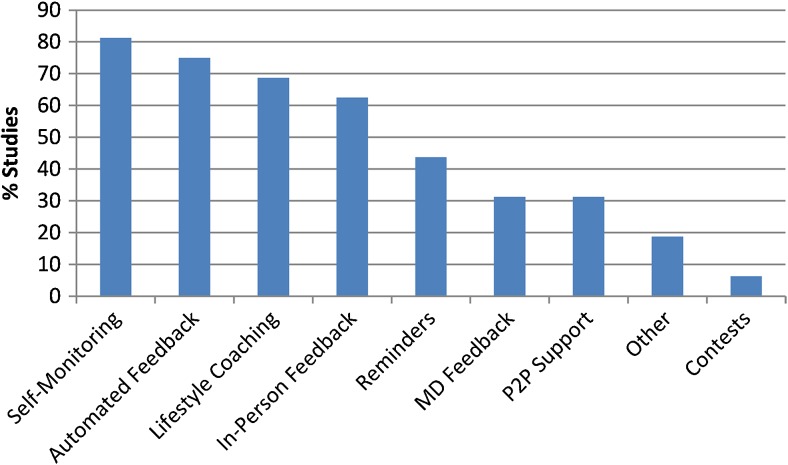
Behavior Change Elements
All studies used evidence-based elements to promote behavior change (Fig. 3). The most common was self-monitoring (13/16, 81 %),58–60,63–72 where participants recorded daily dietary and physical activity behaviors, usually on web-based software. This technology-supported self-monitoring occurred in 11/13 (85 %) studies (weight loss: 1.3 – 5.4 kg), compared to paper and pencil methods in 2/13 (15 %) (weight loss: 1.1 – 3.96 kg).67,71 Of the automated response feedback studies,64,69,72 only one (33 %) was a positive trial, while of the feedback delivered by personnel (phone, in-person, or email),58–63,65–68,70,71,73 11/13 (85 %) were positive trials.
Figure 3.
Behavior change elements: Elements used to promote behavior change. P2P: Person-To-Person.
Bias
Trials scored between 4 and 8 of nine points for both the Delphi and EPOC bias criteria (Supplemental Table 1). Of studies meeting seven or more EPOC criteria,58,60,62,63,65,67,68,72 weight loss ranged from 1.5 kg to 5.4 kg, whereas those meeting less than seven EPOC criteria59,61,64,66,69–71,73 reported 0.08 to 3.36 kg weight loss. Blinding of the provider, patient, and outcome assessor were the most common sources of bias.
Pragmatism
PRECIS scores generally, leaned more toward pragmatic (mean: 2.8 [SD 0.46], range: 2 – 3.6) (Fig. 4 and Supplemental Table 3). On average, studies scored lower (more explanatory) on follow-up intensity (1.8/4 [SD 1.2]) (i.e., subjects had more frequent visits and additional data collection than in routine practice), experimental intervention flexibility (2.0/4 [SD 1.1]) (i.e., strict intervention protocols), participant compliance (2.2/4 [SD 0.66]) (i.e., compliance was closely monitored and included measures to maintain and regain high compliance), and practitioner expertise in the experimental groups (2.4/4 [SD 1.3]) (i.e., used only highly trained practitioners). Studies scored highest (more pragmatic) in analysis of the primary outcome (3.4/4 [SD 1.2]) (e.g., intention-to-treat analysis), flexibility in the control group (3.3/4 [SD 1.4]) (i.e., usual practice control), and practitioner expertise in the control group (3.6/4 [SD 0.81]) (i.e., additional expertise and training were not required). Five (31 %) trials were highly pragmatic, scoring on average > 3/4 on the PRECIS scale.62–64,67,72 Of note, our scores remained within one standard deviation of previously published data.44
DISCUSSION
Results of this review demonstrate that compared to usual PC, most (12/16, 75 %)58–63,66–70,73 technology-assisted interventions in the PC setting help patients to achieve significant weight loss, indicating that technology can supplement and enhance the work of the PCP for weight loss outcomes. These technology-assisted interventions employ elements already demonstrated to be effective in facilitating weight loss: self-monitoring, in-person feedback, and targeted, structured lifestyle coaching.75
The degree of weight loss in this review compares favorably to other PC-based weight loss interventions without technology. Weight loss results in Tsai and Wadden’s review50 of PC obesity treatment via counseling and pharmacotherapy (0.1 kg–7.7 kg) and in McCombie and colleagues’ appraisal76 of PC options in the UK (1.1 kg–6.6 kg) were overall similar to our findings (0.08 kg–5.4 kg). Attrition rates were also comparable to those in our review. At the very least, this portrays technology-assisted weight loss in the PC setting as having similar outcomes to traditional methods and offers further options for PCPs to consider.
Moreover, technology may give PCPs and patients the option to undergo weight loss intervention semi-remotely. Appel et al.58 demonstrated that remote treatment produced weight loss comparable to an in-person intervention. Ma et al.63 showed that in-person and self-directed Diabetes Prevention Program (DPP) arms produced comparable weight loss, while Spring et al.70 similarly confirmed the additive benefit of remote support. This is promising, given that many practices are moving toward the patient-centered medical home model with office visits less central to PC practice.
Given the variation among the 12 studies with significant weight loss, surmising the most effective practices and/or their amalgamation is difficult. Our results suggest that interventions employing clinician-guiding software and feedback from personnel may be more likely to promote weight loss, as 86 % and 85 % of studies using these tools showed significant weight loss, respectively. In contrast, interventions without personnel (fully automated) were less likely to do so, with only 33 % demonstrating weight loss, suggesting that technology cannot fully replace human interactions with the healthcare team. Furthermore, whether employing multiple interventions (e.g., Bennett [2012] et al.60) is more effective than utilizing fewer technology modalities (e.g., Christian et al.61) remains unclear, as in this example both studies led to 1.5 kg weight loss. Similarly, we cannot determine the ideal combination of practitioners. While teams without MDs showed the highest weight loss (Fig. 2), the n is too small to justify any robust conclusions. Further trials could study the effect of specific team groupings on weight outcomes.
Of the four studies that did not achieve significant weight loss, each had specific deficiencies. ter Bogt et al.71 randomized at the level of the patient, not practice, potentially introducing contamination bias as the study progressed. They also transitioned to three yearly NP visits after the first year, an intensity less likely to promote weight loss.52 Verheijden et al.72 suffered from poor website uptake (33 %) and no change in online social support. McConnon et al.64 had similar problems of poor website utilization (29 %) and high attrition (41 %). Finally, McDoniel et al.65 demonstrated an age discrepancy between those who completed the study (older) and those who did not (younger).
For web-based interventions, poor web utilization was common. For example, in Bennett (2012) et al.,60 only 25 % of participants used a self-monitoring platform for at least 75 % of trial weeks for unclear reasons. We speculate this was due to outdated design unable to keep pace with rapidly changing web standards77,78 or an age-related “digital divide” as seen in Verheijden et al.,72 where users were significantly younger than nonusers.
Only 4/16 (25 %)58,60,63,71 interventions lasted more than 1 year, with most lasting for 12 months (6/16, 38 %)61,62,64,68,70,73 or less (6/16, 38 %).59,65–67,69,72 All reported outcomes at the end of the intervention, except for Spring et al.,70 Nanchahal (2012) et al.,68 and Ma et al.63 (extension Xiao et al.79), who had maintenance phases of 6, 4, and 9 months, respectively. Whether after 1 year shorter trials would have continued to show significant weight loss is unclear. Judging from other weight loss literature and ter Bogt et al., where encouraging 1 year data74 unfortunately resulted in insignificant 3-year weight loss,71 the need for long-term interventions and/or follow-up post intervention greater than 1 year is imperative to provide patients with long-term outcomes.
Overall, the studies were of moderate to excellent quality. Our findings on Delphi and EPOC are similar to previously published results.80 Due to the nature of this research, blinding was difficult. Studies of higher quality (≥ 7 EPOC criteria)58,60,62,63,65,67,68,72 reported greater weight loss, an encouraging finding. Interestingly, and not part of our study’s initial intent, Delphi and EPOC provide comparable quality rankings.
PRECIS scores suggest that existing PC-based technology-assisted weight loss studies have many pragmatic elements. The PRECIS tool is helpful in pinpointing areas for improving pragmatism. For example, Ma et al.63 had uniformly excellent scores with the exception of participant compliance (2/4) to intervention, suggesting that measurement of participant compliance could be less obtrusive. In general, interventions frequently scored poorly with respect to follow-up intensity, participant compliance, flexibility, and practitioner expertise, all key areas for successful real-world translation and implementation. This is concerning, as trials with low pragmatism are likely difficult to replicate and disseminate. Thus, future studies should strive for pragmatic design, particularly with attention to the aforementioned areas. On the other hand, the five trials that averaged > 3/4 on the PRECIS scale can serve as models for future protocol design.
Areas for Future Research
Unfortunately, most studies are still performed outside of the PC setting—we excluded 76/101 studies during full text review for this reason. Given the paucity of PC-based trials, we suggest adapting technology-assisted interventions from non-PC settings into the office. Multiple innovative technologies tested only outside of PC have been summarized elsewhere and are awaiting possible implementation.40,42,81,82 For instance, Ma et al.63 adapted the DPP to the PC office, while Appel et al.58 and Bennett (2012) et al.60 similarly demonstrated an important step toward tailoring interventions for PC. Also encouraging is Morgan et al.’s Self-Help, Exercise, and Diet using Information Technology (SHED-IT) series. Translation of SHED-IT from a university83 to a community84 sample is encouraging, as further adaptation into PC appears a straightforward step.
Some technologies are currently under-represented in the literature, despite their widespread use. Interestingly, only one intervention in this review utilized texting, a consistently popular tool despite continued technology advances. A focus on texting could prove fruitful, as seen in smoking cessation85,86 and treatment adherence research.87,88 Of note, Heart360.org added texting capability after initiation of Ma et al.’s63 study. Similarly, only one study employed a mobile application, even though internet traffic is increasingly occurring on mobile platforms.89 Another untapped area similarly lies in social media, for its peer support outlets.90
We found it remarkable that few (2/16, 13 %) studies presented open-source or non-proprietary interventions.63,73 Most websites and tools studied in trials are entirely unavailable to the practicing PCP, even though many patients could conceivably benefit from access. Instead, a poorly or unstudied commercial alternative is often the only remaining choice.43 Notable exceptions to this include the AHA’s Heart360, the MOVE!23 questionnaire and its mobile app, and the Healthy Highways website.
Currently missing from the literature is a cost-effectiveness analysis of technology-assisted weight loss interventions in PC. Only four trials64,73,91,92 provide a critical appraisal of their costs. Also presently unclear is the usability of many of the trials’ technology. Formal usability studies may help to improve reach and uptake of these weight loss interventions. Furthermore, the role of different clinicians in providing weight loss interventions in PC remains undetermined. Non-MD providers as a group were utilized most often, but large differences in training and role existed among them. As we continue to transition into the era of accountable care organizations and PC medical homes, multiple providers other than MDs will be expected to execute technology-assisted interventions.
Limitations
The search terms may not have identified all pertinent studies. We find this unlikely, given the large number of articles extracted and the use of a research librarian to conduct the search. The participants in the included trials also may not represent the ethnic, gender, or age breakdown of typical PC practices. Some studies focused solely on disadvantaged populations,60–62 while others occurred in ethnically homogenous countries,72 perhaps precluding generalization. Furthermore, a “digital divide” may exist both with respect to socioeconomic status (SES) and age, whereby those with lower SES and older age may have difficulty interacting with technology-assisted interventions.93 Moreover, while there are only a small number of RCTs on this topic, there are many prospective cohort studies, yet these may not provide us with sufficient rigor to guide clinical decision-making.
CONCLUSIONS
At the present time, there is no “first-line” therapy for technology-assisted weight loss in PC, but several meaningful choices exist that compare favorably to traditional weight loss interventions. Results of this review reveal that little is known about the optimal use of technology for weight loss in the PC setting. From this review, we can conclude the following: (1) technology-assisted weight loss is a valid tool for PCPs as they counsel patients, although a best iteration has yet to be determined; (2) technology-assisted weight loss interventions compare favorably to other modalities; and (3) longer, pragmatic, interdisciplinary, open-source interventions are needed.
Electronic supplementary material
(DOCX 66 kb)
Acknowledgements
Contributors
We would like to thank Adina Kalet, MD, MPH for her feedback and editing of the manuscript.
Funders
Veteran Affairs Career Development Award
Prior presentations
Levine D, Savarimuthu S, Nicholson J, Jay M. Technology-assisted weight loss interventions in primary care: A systematic review. Poster Presentation, Society of General Internal Medicine; Denver, CO 2013.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Footnotes
Registration
PROPSERO CRD42013003998
REFERENCES
- 1.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 2.Oster G, Edelsberg J, O’Sullivan AK, Thompson D. The clinical and economic burden of obesity in a managed care setting. Am J Manage Care. 2000;6(6):681–689. [PubMed] [Google Scholar]
- 3.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–25. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 4.Williams BC, Philbrick JT, Becker DM, McDermott A, Davis RC, Buncher PC. A patient-based system for describing ambulatory medicine practices using diagnosis clusters. J Gen Intern Med. 1991;6(1):57–63. doi: 10.1007/BF02599394. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8:200. doi: 10.1186/1471-2458-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushner RF. Roadmaps for clinical practice: case studies in disease prevention and health promotion—assessment and management of adult obesity: a primer for physicians. Chicago: American Medical Association; 2003. [Google Scholar]
- 8.Bray GA, Wilson JF. In the clinic. Obesity. Ann Intern Med. 2008;149(7):ITC4–1–15. [DOI] [PubMed]
- 9.National Institute for Health and Clinical Excellence. Obesity: guidance on the prevention, identification, assessment and management of overweight and obesity in adults and childen. NICE clinical guideline no. 43. 2006. Available at http://www.nice.org.uk/CG43. Accessed 7/17/14. [PubMed]
- 10.McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;139(11):933–49. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 11.Levy RL, Finch EA, Crowell MD, Talley NJ, Jeffery RW. Behavioral intervention for the treatment of obesity: strategies and effectiveness data. Am J Gastroenterol. 2007;102(10):2314–21. doi: 10.1111/j.1572-0241.2007.01342.x. [DOI] [PubMed] [Google Scholar]
- 12.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 2011;13(169):1–38. [PubMed] [Google Scholar]
- 13.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–6. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pi-Sunyer FX. Short-term medical benefits and adverse effects of weight loss. Ann Intern Med. 1993;119:722–6. doi: 10.7326/0003-4819-119-7_Part_2-199310011-00019. [DOI] [PubMed] [Google Scholar]
- 15.Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000;23(10):1499–504. doi: 10.2337/diacare.23.10.1499. [DOI] [PubMed] [Google Scholar]
- 16.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009;22(1):93–108. doi: 10.1017/S0954422409990035. [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415. [PubMed] [Google Scholar]
- 19.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–93. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 20.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17(4):713–22. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cecchini M, Sassi F, Lauer JA, YY L, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. 2010;376(9754):1775–84. doi: 10.1016/S0140-6736(10)61514-0. [DOI] [PubMed] [Google Scholar]
- 23.Sciamanna CN, Tate DF, Lang W, Wing RR. Who reports receiving advice to lose weight? Results from a multistate survey. Arch Intern Med. 2010;160(15):2334–9. doi: 10.1001/archinte.160.15.2334. [DOI] [PubMed] [Google Scholar]
- 24.McTigue KM, Conroy MB. Use of the internet in the treatment of obesity and prevention of type 2 diabetes in primary care. Proc Nutr Soc. 2013;72(1):98–108. doi: 10.1017/S0029665112002777. [DOI] [PubMed] [Google Scholar]
- 25.Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med (Baltimore) 1995;24(6):546–52. doi: 10.1006/pmed.1995.1087. [DOI] [PubMed] [Google Scholar]
- 26.Forman-Hoffman V, Little A, Wahls T. Barriers to obesity management: a pilot study of primary care clinicians. BMC Fam Pract. 2006;7:35. doi: 10.1186/1471-2296-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster GD, Wadden TA, Makris AP, et al. Primary care physicians’ attitudes about obesity and its treatment. Obes Res. 2003;11(10):1168–77. doi: 10.1038/oby.2003.161. [DOI] [PubMed] [Google Scholar]
- 28.Yarnall KSH, Pollak KI, Østbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–41. doi: 10.2105/AJPH.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iacobucci G. Pay GPs to tackle obesity, doctors urge UK government. BMJ. 2014;348:g232. doi: 10.1136/bmj.g232. [DOI] [PubMed] [Google Scholar]
- 30.Block JP, DeSalvo KB, Fisher WP. Are physicians equipped to address the obesity epidemic? Knowledge and attitudes of internal medicine residents. Prev Med. 2003;36(6):669–75. doi: 10.1016/S0091-7435(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 31.Jay M, Gillespie C, Ark T, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care?: using a needs assessment to drive curriculum design. J Gen Intern Med. 2008;23(7):1066–70. doi: 10.1007/s11606-008-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafford RS, Farhat JH, Misra B, Schoenfeld DA. National patterns of physician activities related to obesity management. Arch Fam Med. 2000;9(7):631–8. doi: 10.1001/archfami.9.7.631. [DOI] [PubMed] [Google Scholar]
- 33.Schauffler HH, Rodriguez T, Milstein A. Health education and patient satisfaction. J Fam Pract. 1996;42(1):62–8. [PubMed] [Google Scholar]
- 34.Bleich SN, Pickett-Blakely O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns. 2011;82(1):123–9. doi: 10.1016/j.pec.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy BT, Williamson PS. Patient perceptions and weight loss of obese adults. J Fam Pract. 1988;27(3):285–90. [PubMed] [Google Scholar]
- 36.Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract. 2001;50(6):513–8. [PubMed] [Google Scholar]
- 37.Budman SH, Portnoy D, Villapiano AJ. How to get technological innovation used in behavioral health care: Build it and they still might not come. Psychother Theory Res Prac Train. 2013;40:45–54. doi: 10.1037/0033-3204.40.1-2.45. [DOI] [Google Scholar]
- 38.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA. 2001;285(9):1172–7. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 39.Harvey-Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev Med. 2010;51(2):123–8. doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wieland LS, Falzon L, Sciamanna CN, et al. Interactive computer-based interventions for weight loss or weight maintenance in overweight or obese people. Cochrane Database Syst Rev. 2012;8:CD007675. doi: 10.1002/14651858.CD007675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neve M, Morgan PJ, Jones PR, Collins CE. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: a systematic review with meta-analysis. Obes Rev. 2010;11(4):306–21. doi: 10.1111/j.1467-789X.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- 42.Arem H, Irwin M. A review of web-based weight loss interventions in adults. Obes Rev. 2011;12(5):e236–43. doi: 10.1111/j.1467-789X.2010.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142(1):56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- 44.Glasgow RE, Gaglio B, Bennett G, et al. Applying the PRECIS criteria to describe three effectiveness trials of weight loss in obese patients with comorbid conditions. Health Serv Res. 2012;47:1051–67. doi: 10.1111/j.1475-6773.2011.01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62(5):464–75. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013003998. Accessed 7/17/14.
- 48.Bravata D, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 49.Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. A Meta-Analysis of Pedometer-Based Walking Interventions and Weight Loss. Ann Fam Med. 2008;6(1):69–77. doi: 10.1370/afm.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24(9):1073–9. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lefebvre C, Manheimer E, Glanville J. Chapter 6: searching for studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009.
- 52.US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139(11):930–2. [DOI] [PubMed]
- 53.Bensley RJ, Brusk JJ, Rivas J. Key principles in internet-based weight management systems. Am J Health Behav. 2010;34(2):206–13. doi: 10.5993/AJHB.34.2.8. [DOI] [PubMed] [Google Scholar]
- 54.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–41. doi: 10.1016/S0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 55.Cochrane Effective Practice and Organisation of Care Review Group. Risk of bias. 2009. Available at: http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/Suggested%20risk%20of%20bias%20criteria%20for%20EPOC%20reviews.pdf. Accessed 7/17/14.
- 56.Verhagen AP, de Vet HC, de Bie RA, Boers M, van den Brandt PA. The art of quality assessment of RCTs included in systematic reviews. J Clin Epidemiol. 2001;54(7):651–4. doi: 10.1016/S0895-4356(00)00360-7. [DOI] [PubMed] [Google Scholar]
- 57.Koppenaal T, Linmans J, Knottnerus JA, Spigt M. Pragmatic vs explanatory: an adaptation of the PRECIS tool helps to judge the applicability of systematic reviews for daily practice. J Clin Epidemiol. 2011;64(10):1095–101. doi: 10.1016/j.jclinepi.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 58.Appel LJ, Clark MJ, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–68. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based weight loss in primary care: a randomized controlled trial. Obesity (Silver Spring) 2010;18(2):308–13. doi: 10.1038/oby.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett GG, Warner ET, Glasgow RE, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172(7):565–74. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christian JG, Bessesen DH, Byers TE, Christian KK, Goldstein MG, Bock BC. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med. 2008;168(2):141–6. doi: 10.1001/archinternmed.2007.13. [DOI] [PubMed] [Google Scholar]
- 62.Christian JG, Byers TE, Christian KK, et al. A computer support program that helps clinicians provide patients with metabolic syndrome tailored counseling to promote weight loss. J Am Diet Assoc. 2011;111(1):75–83. doi: 10.1016/j.jada.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–21. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McConnon A, Kirk SF, Cockroft JE, et al. The Internet for weight control in an obese sample: results of a randomised controlled trial. BMC Health Serv Res. 2007;7:206. doi: 10.1186/1472-6963-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDoniel SO, Wolskee P, Shen J. Treating obesity with a novel hand-held device, computer software program, and Internet technology in primary care: the SMART motivational trial. Patient Educ Couns. 2010;79(2):185–91. doi: 10.1016/j.pec.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 66.Mehring M, Haag M, Linde K, et al. Effects of a general practice guided web-based weight reduction program–results of a cluster-randomized controlled trial. BMC Fam Pract. 2013;14(1):76. doi: 10.1186/1471-2296-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanchahal K, Townsend J, Letley L, Haslam D, Wellings K, Haines A. Weight-management interventions in primary care: a pilot randomised controlled trial. Br J Gen Pract. 2009;59(562):e157–66. doi: 10.3399/bjgp09X420617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nanchahal K, Power T, Holdsworth E, et al. A pragmatic randomised controlled trial in primary care of the Camden Weight Loss (CAMWEL) programme. BMJ Open. 2012;2(3). [DOI] [PMC free article] [PubMed]
- 69.Rothert K, Strecher V, Doyle L. Web based Weight Management Programs in an Integrated Health Care Setting: A Randomized. Controlled Trial. Obesity. 2006;14(2):266–272. doi: 10.1038/oby.2006.34. [DOI] [PubMed] [Google Scholar]
- 70.Spring B, Duncan JM, Janke EA, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med. 2013;173(2):105–11. doi: 10.1001/jamainternmed.2013.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ter Bogt NCW, Bemelmans WJE, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing Weight Gain by Lifestyle Intervention in a General Practice Setting. Arch Intern Med. 2011;171(4):306–313. doi: 10.1001/archinternmed.2011.22. [DOI] [PubMed] [Google Scholar]
- 72.Verheijden M, Bakx JC, Akkermans R, et al. Web-based targeted nutrition counselling and social support for patients at increased cardiovascular risk in general practice: randomized controlled trial. J Med Internet Res. 2004;6(4):e44. doi: 10.2196/jmir.6.4.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wylie-Rosett J, Swencionis C, Ginsberg M, et al. Computerized Weight Loss Intervention Optimizes Staff Time. J Am Diet Assoc. 2001;101(10):1155–1162. doi: 10.1016/S0002-8223(01)00284-X. [DOI] [PubMed] [Google Scholar]
- 74.ter Bogt NCW, Bemelmans WJE, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain: one-year results of a randomized lifestyle intervention. Am J Prev Med. 2009;37(4):270–7. doi: 10.1016/j.amepre.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010;16(9):931–8. doi: 10.1089/tmj.2010.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCombie L, Lean MEJ, Haslam D. Effective UK weight management services for adults. Clin Obes. 2012;2(3–4):96–102. doi: 10.1111/j.1758-8111.2012.00049.x. [DOI] [PubMed] [Google Scholar]
- 77.Bennett GG, Glasgow RE. The delivery of public health interventions via the Internet: actualizing their potential. Annu Rev Public Health. 2009;30:273–92. doi: 10.1146/annurev.publhealth.031308.100235. [DOI] [PubMed] [Google Scholar]
- 78.Krukowski RA, Harvey-Berino J, Ashikaga T, Thomas CS, Micco N. Internet-based weight control: the relationship between web features and weight loss. Telemed J E Health. 2008;14(8):775–82. doi: 10.1089/tmj.2007.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao L, Yank V, Wilson SR, Lavori PW, Ma J. Two-year weight-loss maintenance in primary care-based Diabetes Prevention Program lifestyle interventions. Nutr Diabetes. 2013;3:e76. doi: 10.1038/nutd.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoong SL, Carey M, Sanson-Fisher R, Grady A. A systematic review of behavioural weight-loss interventions involving primary-care physicians in overweight and obese primary-care patients (1999–2011) Public Health Nutr. 2013;16(11):2083–99. doi: 10.1017/S1368980012004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kodama S, Saito K, Tanaka S, et al. Effect of Web-based lifestyle modification on weight control: a meta-analysis. Int J Obes. 2012;36(5):675–85. doi: 10.1038/ijo.2011.121. [DOI] [PubMed] [Google Scholar]
- 82.Reed VA, Schifferdecker KE, Rezaee ME, O’Connor S, Larson RJ. The effect of computers for weight loss: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2012;27(1):99–108. doi: 10.1007/s11606-011-1803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12-month outcomes and process evaluation of the SHED-IT RCT: an internet-based weight loss program targeting men. Obesity (Silver Spring) 2011;19(1):142–51. doi: 10.1038/oby.2010.119. [DOI] [PubMed] [Google Scholar]
- 84.Morgan PJ, Callister R, Collins CE, et al. The SHED-IT community trial: a randomized controlled trial of internet- and paper-based weight loss programs tailored for overweight and obese men. Ann Behav Med. 2013;45(2):139–52. doi: 10.1007/s12160-012-9424-z. [DOI] [PubMed] [Google Scholar]
- 85.Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD006611. doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed] [Google Scholar]
- 86.Shahab L, McEwen A. Online support for smoking cessation: a systematic review of the literature. Addiction. 2009;104(11):1792–804. doi: 10.1111/j.1360-0443.2009.02710.x. [DOI] [PubMed] [Google Scholar]
- 87.Vervloet M, van Dijk L, Santen-Reestman J, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform. 2012;81(9):594–604. doi: 10.1016/j.ijmedinf.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 89.Bacigalupo R, Cudd P, Littlewood C, Bissell P, Hawley MS, Buckley WH. Interventions employing mobile technology for overweight and obesity: an early systematic review of randomized controlled trials. Obes Rev. 2013;14(4):279–91. doi: 10.1111/obr.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang T, Chopra V, Zhang C, Woolford SJ. The role of social media in online weight management: systematic review. J Med Internet Res. 2013;15(11):e262. doi: 10.2196/jmir.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ritzwoller DP, Glasgow RE, Sukhanova AY, et al. Economic analyses of the Be Fit Be Well program: a weight loss program for community health centers. J Gen Intern Med. 2013;28(12):1581–8. doi: 10.1007/s11606-013-2492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsai AG, Wadden TA, Volger S, et al. Cost-effectiveness of a primary care intervention to treat obesity. Int J Obes. 2013;37(S1):S31–7. doi: 10.1038/ijo.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loges WE, Jung J-Y. Exploring the Digital Divide: Internet Connectedness and Age. Commun Res. 2001;28(4):536–562. doi: 10.1177/009365001028004007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 66 kb)