Abstract
Background
Generation of genetically stable and non-tumoric immortalization cell line from primary cells would be enormously useful for research and therapeutic purposes, but progress towards this goal has so far been limited. It is now universal acceptance that immortalization of human fetal hepatocytes based on recent advances of telomerase biology and oncogene, lead to unlimited population doubling could be the possible source for bioartificial liver device.
Methods
Immortalization of human fetal hepatocytes cell line by ectopic expression of human telomerase reverse transcriptase (hTERT), human papilloma virus gene (E7) and simian virus 40 large T (SV40 T) antigens is main goal of present study. We used an inducible system containing human telomerase and E7, both of which are cloned into responder constructs controlled by doxycycline transactivator. We characterized the immortalized human fetal hepatocyte cells by analysis of green fluorescent cells (GFP) positive cells using flow cytometry (FACs) cell sorting and morphology, proliferative rate and antigen expression by immunohistochemical analysis. In addition to we analysized lactate formation, glucose consumption, albumin secretion and urea production of immortalized human fetal hepatocyte cells.
Results
After 25 attempts for transfection of adult primary hepatocytes by human telomerase and E7 to immortalize them, none of the transfection systems resulted in the production of a stable, proliferating cell line. Although the transfection efficiency was more than 70% on the first day, the vast majority of the transfected hepatocytes lost their signal within the first 5–7 days. The remaining transfected hepatocytes persisted for 2–4 weeks and divided one or two times without forming a clone. After 10 attempts of transfection human fetal hepatocytes using the same transfection system, we obtained one stable human fetal hepatocytes cell line which was able albumin secretion urea production and glucose consumption.
Conclusion
We established a conditional human fetal hepatocytes cell line with mesenchymal characteristics. Thus immortalization of human fetal hepatocytes cell line by telomerase biology offers a great challenge to examine basic biological mechanisms which are directly related to human and best cell source having unlimited population doubling for bioartificial support without any risk of replicative senescence and pathogenic risks.
Keywords: bioartificial liver device, human fetal hepatocytes, hTERT, E7, SV T 40 antigen
Abbreviations: AFP, alpha-fetoprotein; BLD, bioartificail liver device; E7, human papilloma virus; EBV, epstein barr virus; EGFP, enhanced green fluorescent protein; FACs, flow cytometry; FH, fetal hepatocytes; GFP, green fluorescent cells positive cells; HPV, human papilloma virus; hTERT, human telomerase reverse transcriptase; iPS, pluripotent stem cell; SV40 T, simian virus 40 large T
Bioartificail liver device (BLD) has been used for bridge for acute and chronic liver patients, however the clinical success of BAD relies on the cell source. Primary adult hepatocytes are sensitive and have very limited proliferation potential. Therefore, these primary human hepatocytes are not suitable for large scale application such as bioartificial liver systems. In contrast, human fetal hepatocyte could be reliable option to solve the existing limitation of proliferation of mature primary hepatocytes. The present study was taken to address some existing limitations. Infinite proliferation is possible by transfer of an immortalizing oncogene by immortalization process.1,2 Simian virus 40 (SV40) T antigen, Epstein Barr virus (EBV), gene E1A and E1B from Adenovirus, and gene E6 and E7 from human papiloma virus (HPV) has been used to induce immortalization in different cell types. Although these genes allow normal cells to overcome senescence signals and continue proliferating upto 20 population doubling.3 But in the case of SV40 T antigen which is most used immortalizing agent which gives additional life span, still restricted by another barrier represented entrance into crisis during there is a slowing of cellular growth and widespread apotosis.4,5 The crisis can be getting out by ectotopic expression of the catalytic subunit of telomerase reverse transcriptase enzyme (hTERT).6–8 Actually telomerase consists of two essential components: one is the functional RNA component (in humans called hTR or hTERC)9 which serves as a template for telomeric DNA synthesis; the other is a catalytic protein (hTERT) with reverse transcriptase activity.10–13 The expression of hTERT of cancer cells is fivefold-higher expression than normal cells.14 In contrast, the expression (mRNA) of the human catalytic component hTERT is estimated at less than 1–5 copies per cell14 and is closely associated with telomerase activity in cells. Further, over expression of hTERT Increase stem cell properties.15 Recently it has been demonstrated that expression of human telomerase alone is sufficient for the immortalization of various cell types.16–19 But cell population without hTERT expression in even in SV 40 expressing will enter into crisis, hence immortalization is imcomplete.20 It has been demonstrated that immortalization of primary rat hepatocytes by early region of T-Ag comprising large T and small t gene have led to tumorigenic phenotype.21 It is important to use role of hTERT for complete functional immortalization. Hence it has paid much attention on cell immortalization technology by either human telomere reverse transcriptase (hTERT) or combination of other immortalization gene like SV40 T antigen, human papilloma virus gene (E7). Induction of telomerase activity alone in some cell types is sufficient for cell immortalization.22 So it is urgent need to develop immortalized hepatocytes cell line to overcome above mentioned limitations and which suits for BLD. Immortalized cell lines have great advantages than primary mature cell such as better uniform cultures, easier availability, easy maintenance, easier genetic manipulation, suitable for drug biotransformation. Taken together, these research reports indicate that telomerase plays an important role in cellular aging for infinite proliferation and give greatest challenge towards regenerative medicine and tissue engineering.
Herein, we successfully established an immortalized human fetal hepatocytes cell line with mesenchymal characteristics by inducible system comprising human telomerase, human papilloma virus gene (E7) and SV 40 large T.
Materials and methods
Choice of Vectors
We used an inducible system containing human telomerase and E7 (human papilloma virus gene), both of which are cloned into responder constructs controlled by doxycyclin transactivator construct. The inducible system used the transactivators: pCMV-KRAB-rtTA: 6131 bp Tet On, pUHT 61-1: 4194 bp Tet Off, pUHrT 62-1: 4194 bp Tet On, conditional immortalization vector; pBI-EGFP/E: 5450 bb, pBI DTAloxP hTert: 9500 bp, not conditional SV40 large T: pRNS-1, 10979 bp.
Plasmid Amplification
Each vector was single-introduced into the E. coli-strain XL1-Blue-strain as described by the manufacturer (Stratagene, Heidelberg). After introduction, bacteria were plated onto agar-coated plates containing 100 μg/ml ampicillin and incubated for 16 h at 37 °C. Single colonies were placed in 3 ml of culture medium (2x YT-Medium + 100 μg/ml ampicillin) and incubated overnight at 37 °C. 1 ml of the culture was then expanded in 250 ml of the same medium for another 14 h at 37 °C with 300 rpm shaking.
Plasmid Preparation
The bacterial culture was centrifuged at 3000 ×g for 15 min at 4 °C, and the supernatant was discarded. Plasmids were obtained from bacteria by using the QIAGEN-filter Plasmid Maxi Kit (Qiagen, Hilden). The concentration and purity of the isolated plasmid was determined by reading the absorbance at 260 and 280 nm.
Digestion of the Plasmids
Plasmids were analyzed by digestion with restriction enzymes to verify their accurate sequences and purity. 1.5 μg of each plasmid was digested by BamHI und EcoRI for 1 h at 37 °C, and fragments were then separated on a 0.8% agarose gel.
Plasmid Linearization
To allow an optimal transfection and DNA incorporation, it is necessary to liberalize the plasmids before transfection. Each plasmid was incubated with a plasmid-specific enzyme (see below) in a restriction enzyme-specific buffer containing BSA (100 μg/ml) for 3 h at 37 °C.
| Plasmid | Restriction enzyme |
|---|---|
| pCMV-KRAB-rtTA | XhoI |
| pUHT 61-1 | Hind III |
| pUHrT 62-1 | Hind III |
| pBI-EGFP/E7 | Drd I |
| pBI DTAloxP hTert | Drd I |
| SV40 large T | Bgl I |
The enzymes were denatured by addition of the phenol-chloroform extraction. The DNA was purified using the commercial PCR-Purification Kit (Qiagen), and the linearization was validated by TBE (Tris/boric acid/EDTA)-gel electrophoresis (0.8%).
Cell Isolation and Transfection
We have compared seven commercially available systems (Lipofectamine, Effectene, Nanofectin, JetPei-Gal, Metafectene, GenePorter, and GeneJammer) to obtain optimal transfection of hepatocytes with plasmids used in this study.
Adult hepatocytes were isolated according to a method described by Seglen et al, 1973 with little modification.23 Isolated hepatocytes were plated onto collagen-coated 6- and 24- well plates at a density of 10.000–30.000 cell/cm depending on the type of transfection performed. After cultivation in Williams' E medium, the cells were transfected with a plasmid.
Results
We have tested transfection reagents from different companies for their efficiency and feasibility and have found that the greatest discrepancy lies in the efficiency. The reagent Effectene was shown to induce cell death in a vast majority of hepatocytes probably due its toxicity. By contrast, cell toxicity of the reagents Nanofectin (PAA, Cölbe), GenPorter (Invitrogen), Metafectene (Biontex, München) and JetPei-Gal (Qbiogene, Heidelberg) was less than 10%. However, they are not recommended to be used for transfection of hepatocytes because of the low transfection rate which is less than 20%. The efficiency of the reagent GeneJammer (Stratagene) was about 40%–50%, but the best result could be obtained with the reagent Lipofectamine (Invitrogen, Karlsruhe). When using the latter reagent, the apoptosis rate was less than 10%, while the transfection efficiency accounted for more than 70% (Figure 1A and B). Thus, we used lipofectamine to transfect hepatocytes with plasmids. We also used combinations of the Tet-Off system with human telomerase and E7 to immortalize adult primary hepatocytes. Unfortunately, after 25 attempts, none of the transfection systems resulted in production of a stable, proliferating cell line. Although the transfection efficiency was more than 70% on the first day, a vast majority of the transfected hepatocytes lost their signal within the first 5–7 days. The remaining transfected hepatocytes persisted for 2–4 weeks, and divided one or two times without forming a clone.
Figure 1.
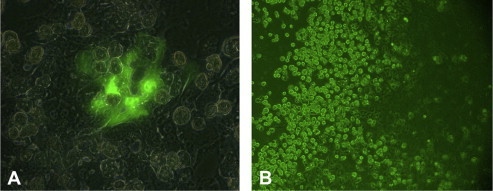
Comparison of the transfection efficiency of A) lipofectamine and B) effectene in primary human hepatocytes to obtain an immortalized cell line. Transfection using lipofectamine as a reagent yielded a higher number of EGFP-expressing cells, when compared with effectene.
We showed that human fetal hepatocytes were capable of proliferating for about 10 passages. We used the passage 4 and all vector combinations (plus SV40 large T, not inchangeable) for the immortalization experiments. After 10 attempts of transfection, we obtained one stable cell line expressing EGFP when SV40 large T and hTert were used as vectors (Figure 2). Since we observed that non-transfected cells were also able to proliferate we isolated EGFP-positive cells by FACS cell sorting (Figure 3A and B). The sorted cells were then characterized by immunohistochemical analyses (Figure 4). Addition of 300 ng/ml doxycycline resulted in down regulation of EGFP expression (Figure 5A–C). Inconsequently, those inductor (Tet-Off) and the responder (hTert-EGFP) were turned off by doxycycline, the cells still further proliferate and in that result the cells were not able to differentiate.
Figure 2.
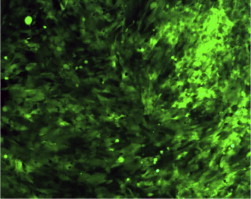
Primary human fetal hepatocytes were transfected with EGFP that was cloned into the hTert vector. Following transfection with the hTert vector using lipofectamine as a transfection reagent, human fetal hepatocytes were highly proliferative. However, these cells were viable for 2–4 weeks (x).
Figure 3.
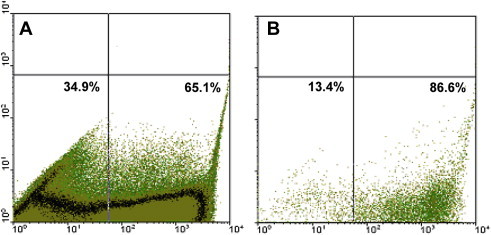
FACS cell sorting of EGFP-expressing human fetal hepatocytes. Human fetal hepatocytes were transfected with EGFP cloned into the hTert vector and sorted by FACS. A) Hepatocytes before cell sorting. About 35% and 65% of the cells were not transfected or EGFP-transfected, respectively. B) Hepatocytes after cell sorting. A shift to EGFP-transfected cells is observed after cell sorting (from 65% to 87%).
Figure 4.
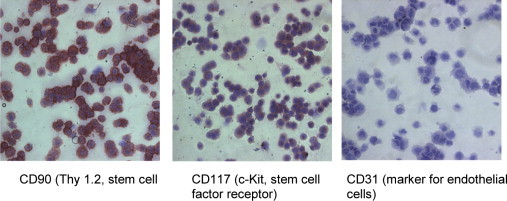
Immunohistochemical analysis of immortalized human fetal hepatocytes. The cells were strongly and moderate positive for CD90 and CD117, respectively. Negative cell staining was obtained for CD34 (not shown) and CD31. These and further (not shown) stainings of the cells confirm the establishment of a fetal hepatocyte cell line.
Figure 5.
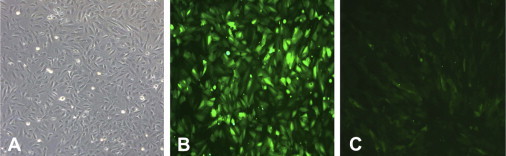
Images of cultured immortalized human fetal hepatocytes. A) Light microscopy; B) fluorescence image of EGFP-expressing cells transfected with the hTert vector; C) Tet off response to doxcycline (300 ng/ml), leading to downregulation of EGFP-expression.
We used an inducible system containing human telomerase and E7 (human papilloma virus gene), both of which are cloned into responder constructs controlled by doxycyclin transactivator construct. Additionally, for the immortalization of human fetal hepatocytes we also used the vector SV40 large T which is not influenced by doxycyclin. Since it was observed that non-transfected cells are also able to proliferate GFP-positive cells were isolated by FACS cell sorting. However, it was not possible to get a pure GFP-positive cell population. Instead, there was a constant decrease in the number of GFP-positive cells after several passages. Therefore, cell cloning was performed in 96-well plates, and several GFP-positive clones were obtained and expanded in 75 cm² flasks (Figure 6). Green fluorescent cells detected by FACS were chosen, and these cells were cultured in the presence or absence of doxycyclin (300 ng/ml) for 11 passages to observe any changes in their morphology, proliferation rate and antigen expression by immunohistochemical analysis. Due to the control of the human telomerase and E7 by doxycyclin a difference between the cell culture with (−doxycyclin) and without additional expressed human telomerase (+doxycyclin) was expected. Because of the negative detection of the vector pBI-EGFP/E7 by PCR (Figure 7) it was assumed that this vector is not inserted in the genome, and therefore, it was negligible.
Figure 6.
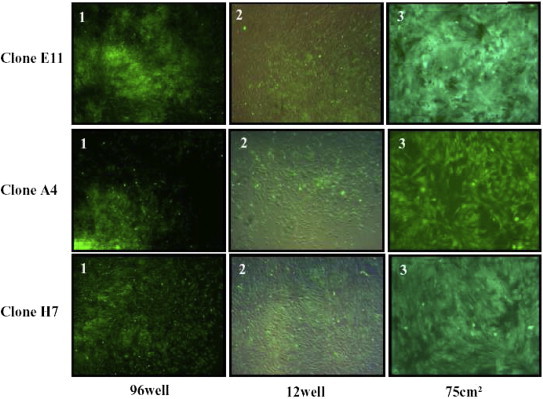
Morphological overview of different cell clone expansion cultures under green fluorescence light 100x. 1—Day 10 after cloning, 2—Day 14 after cloning, 3—Day 38 after cloning.
Figure 7.

Detection of the 3 immortalization vectors in the cell clones by PCR analysis.1—pBI-EGFP/E7 (250 bp), 2—pBI DTAloxP hTert (396 bp), 3—SV40 large T (419 bp).
We used both human telomerese and human papilloma virus gene which are cloned into responder constracts controlled by doxycyclin and also SV40 large T which is not influenced by doxycyclin. So three GFP-positive clones (A4, E11, H7) were developed in our experiment. The clone H7 was chosen because of its highest percentage of GFP-positive cells (Figure 8). From this clone two cell populations were treated with or without doxycyclin for 11 passages. After 1 or 2 passages, the green fluorescence completely decreased in the presence of doxycyclin (Figure 9). As the non-regulated vector SV40 large T also contains an oncogene-like vector there were no differences in the growth for both cultures within the 11 passages (Figure 10). This was confirmed by determining the proliferation rate in both cell cultures by FACS using Ki67 antibody (Figure 11). Due to their positive staining for vimentin and CD71 they possessed the mesenchymal characteristics (Table 1). Furthermore, metabolic activities of the two different cultures were analyzed for lactate formation, glucose consumption, albumin secretion and urea production (Figure 12).
Figure 8.
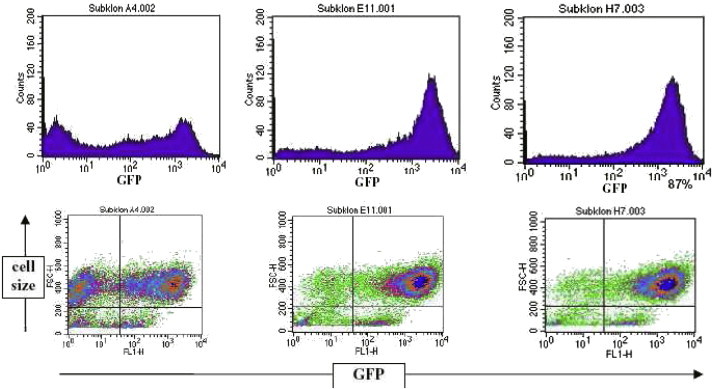
Measurement of the green fluorescence capacity of the different cell clones by FACS analysis. The purity of GFP-positive cells is given in percent.
Figure 9.
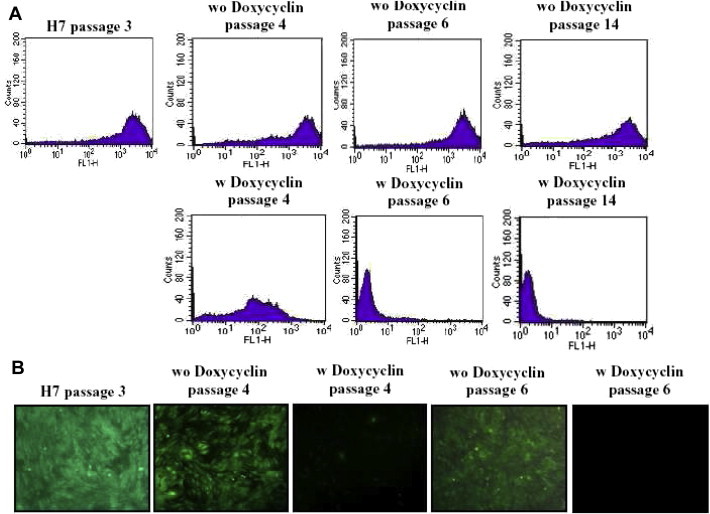
A) Comparison of the distribution of the green fluorescence (GFP = FL1-H) from both cell cultures (without (wo) and with (w) doxycyclin) during several passages. B) Morphological overview of both cell cultures under green fluorescence light 100x.
Figure 10.
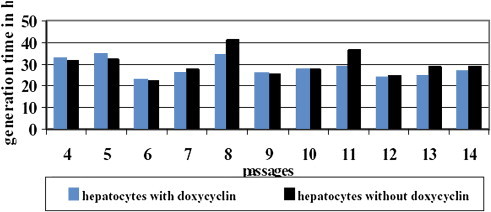
Comparison of the generation time of both cell cultures during all passages.
Figure 11.
Comparison of the proliferation rate of both cell cultures by Ki-67 FACS analysis without doxycyclin with doxycyclin.
Table 1.
Immunohistochemical Analysis of Both Cell Cultures for Several Passages.
| Antibody | 3.passage | 7.p wo doxy | 7.p w doxy | 10.p wo doxy | 10.p w doxy | 14.p wo doxy | 14.p w doxy |
|---|---|---|---|---|---|---|---|
| Albumin | + | + | + | + | + | + | + |
| CD90 | + | + | + | + | + | + | + |
| CD105 | + | + | + | + | + | + | + |
| HLA | + | + | + | + | + | + | + |
| AFP | + | + | + | + | + | + | + |
| CD34 | − | − | − | − | − | − | − |
| CD166 | + | + | + | − | − | − | − |
| CD45 | − | − | − | − | − | − | − |
| Desmin | + | − | + | − | − | − | − |
| Connexin 43 | + | + | + | + | + | + | + |
| Van Willebrandt | − | − | − | − | − | − | − |
| CK19 | − | − | − | − | − | − | − |
| CD71 | + | + | + | + | + | + | + |
| Vimentin | + | + | + | + | + | + | + |
| CD117 | − | − | − | − | − | − | − |
| CD31 | − | − | − | − | − | − | − |
| Fibroblast | − | − | − | − | − | − | − |
Figure 12.
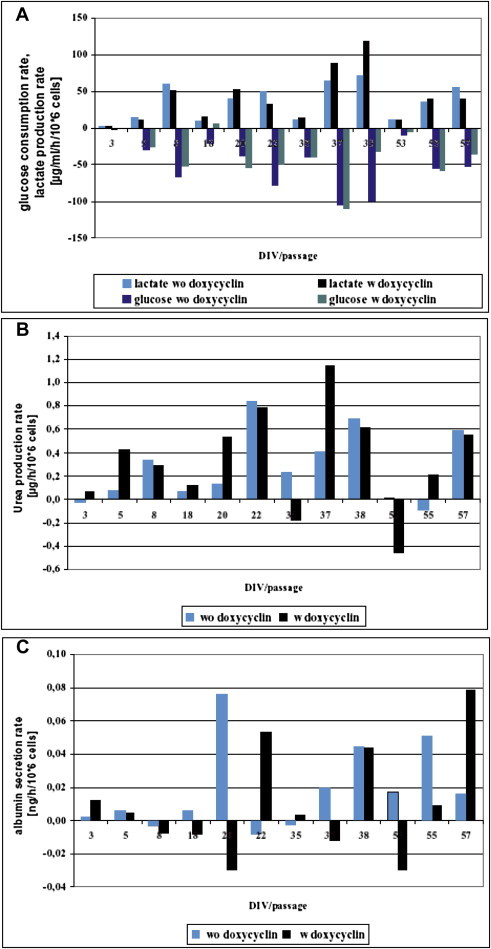
Metabolic activity of both cell cultures treated with and without doxycyclin A—glucose consumption, lactat formation, B—urea production, C—albumin synthesis. Rates are related to the days in vitro per passage. Day 3–8 = passage 4, Day 18–22 = passage 7, Day 35–38 = passage 10, day 53–57 = passage 14.
For immortalization of human fetal hepatocytes, we used both human telomerese and human papilloma virus gene which are cloned into responder constructs controlled by doxycyclin and also SV40 large T which is not influenced by doxycyclin. So three GFP-positive clones (A4, E11, H7) were developed in our experiment. The clone H7 was chosen because of its highest percentage of GFP-positive cells (Figure 2). From this clone two cell populations were treated with or without doxycyclin for 11 passages. It has been reported that the doxycycline inducible system is non-toxic and is compatible with self regulatory growth-control during fermentation. After 1 or 2 passages, the green fluorescence completely decreased in the presence of doxycyclin (Figure 2). As the non-regulated vector SV40 large T also contains an oncogene-like vector there were no differences in the growth for both cultures within the 11 passages (Figure 2). This was confirmed by determining the proliferation rate in both cell cultures by FACS using Ki67 antibody (Figure 3). Due to their positive staining for vimentin and CD71 they possessed the mesenchymal characteristics (Table 1). Hepatocytes did not simultaneously produce alpha-fetoprotein (AFP) and albumin. The addition of doxycycline (300 ng/ml) resulted in down-regulation of enhanced green fluorescent protein (EGFP) expression. Inductor (Tet-Off) and responder (hTert-EGFP) were controlled by doxycycline, still proliferation persisted and cells could not differentiate. This observation may be explained by the fact that 1) proliferation of the immortalized fetal hepatocytes is not due to the vectors used but to the secondary effect of the immortalization process, e.g. mutation; or 2) the not controllable vector SV40 large T was used and is for the uncontrolled proliferation.
Discussion and conclusion
Bioartificial liver device based cell therapy is a promising novel therapy for liver patients but it needs adequate amount of viable and actively functioning adult mature hepatocytes.24–26 Primary hepatocytes is ideal source for bioartificial liver device but not practically suitable due such limitations like the availability of these cells is limited, and expansion of human hepatocytes in culture is difficult, even advanced culture condition, still finite number of cell division and then stop. Although the cryopreservation techniques has been suggested as a standard protocol for long term storage liver cell retaining their function.27–29 Cryopreserved primary hepatocytes alter the metabolic function as compared with fresh hepatocytes. Cell obtained from animal like mice, which have only 15–30 population doubling and life span also vary i.e 2 year life span (mice) and 75–80 years (human). It is roughly estimated that 25–50 billion hepatocytes, contribute 10%–20% of the liver mass, are necessary to support of life of patient.30 The large quantities of cells is require for hepatocyte-based therapies The immortalized human fetal hepatocytes can meet this requirement because these cells exhibit significant proliferative capacity with the ability to differentiate into mature hepatocytes. Further the proliferation capacity of fetal hepatocytes is better than adult hepatocytes and two times smaller in size than adult which suggest these cells should cross the sinusoidal barrier and integrate with the liver parenchyma more efficiently than adult hepatocytes. So less number of transplanted cells should be needed for the same therapeutic benefit and less risk of thrombosis. Taken together, immortalization of human fetal hepatocytes should be best ideal source for liver direct therapies by using the current advances of cell immortalization technology because human fetal hepatocytes possess significant spontaneous proliferative capacity, but the proliferative activity of cultured human fetal hepatocytes (FH) begins to decrease over several months.31 The ideal source for bioartificial support device would be an immortalized, non-tumorigenic, differentiate human fetal hepatocytes cell line by immortalization methods based on recent advances of telomere biology and oncogene. The cell line HepG2 derived from adult tumor is widely used and grows infinitely but its genetic constitution and precise genomic alternation remain unknown. Currently available malignant cell lines including HepG2 or C3A cells,32 or xenogeneic (porcine) hepatocytes33 for BLD but these carry potential risks to recipients, e.g., inoculation of tumor cells34 or zoonotic diseases, notably transmission of porcine endogenous retrovirus.35–37 Previously, we reported that porcine endogenous retrovirus infects primary human cells in a bioartificial liver system.37 These situation stimulate generate human hepatocyte-derived cell lines and to explore various strategies based on telomere biology for immortalizing and expanding the large number of human hepatocytes towards bioartificial liver supports.
For a successful long-term stable hepatocytes culture, immortalized adult hepatocytes were thought to serve as an alternative cell source. We established a conditionally immortalized hepatic cell line. Since the production of a stable cell line from adult primary hepatocytes could not be achieved using the conditional immortalization technique we used human fetal hepatocytes, which could be immortalized. We proposed that following cell transfection and differentiation using certain growth factors, a hepatic cell line express liver-specific functions at levels closely to those in vivo. Fetal liver cell transplantation has been considered as an alternative to whole liver transplantation.38
We are in process for further experiments to determine phase I (cytochrome P450) enzyme activities. It is possible to induce differentiation of human fetal hepatocytes into adult hepatocyte-like cells by changing the environment and using growth factors. To date, however, we have not been able to induce differentiation of these cells into adult hepatocyte-like cells. But we have established a conditional immortalized human fetal hepatocyte cell line with the mesenchymal characteristics.
Recently, Nobel reprogramming discovery was invented39 in 2007 to reprogram the skin fibroblast cells for convert the pluripotent stem cell (iPS) like human embryonic stem cells using four reprogramming plasmid factors. This technology could be alternative option for option for immortalization process of adult primary cells but reprogramming utilizes the retro viral gene delivery processes for transfection that main limitation for clinical application. The high oncogenic risks are always associated with such iPS cells.40,41 The iPS cells and cancer stem cell share several properties.40,41 In 2013, some research created mouse iPS cells were created using the combination of some specific small molecules42 only without using of any such reprogramming viral factors. However, until now, there is no report about creation of human iPS cells using chemical alone. There are several groups generated iPS cell derived hepatocytes43 but none of such iPS derived hepatocytes cells were used clinically foe liver diseases.
In conclusion, we developed conditional immortalized human fetal hepatocytes cell line successfully with mesenchymal characteristics and other specific hepatic mechanism like lactate formation, glucose consumption, albumin secretion and urea production. Unlike primary hepatocytes these cell have potential to grow unlimited in vitro, as a possible source for BLD. Although the differentiation pattern and fate of this cell line need to further research but our present result provides that our conditional immortalized human fetal hepatocytes cell line would permit various basic studies, preclinical protocol, in this cell line closely related to human and overcome the limitation of worldwide shortage of human liver for liver transplantation.
Conflicts of interest
All authors have none to declare.
Acknowledgment
Special thanks to Prof. Jan G. Hengstler for supplying human fetal hepatocyte to us for experiment. Thanks to Isabell Schulz and Dr. Bockamp for their experimental help.
References
- 1.Pfeifer A.M., Cole K.E., Smoot D.T. Simian virus 40 large tumor antigen-immortalized normal human liver epithelial cells express hepatocyte characteristics and metabolize chemical carcinogens. Proc Natl Acad Sci U S A. 1993;90(11):5123–5127. doi: 10.1073/pnas.90.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amicone L., Spagnoli F.M., Spath G. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO J. 1997;16(3):495–503. doi: 10.1093/emboj/16.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryan T.M., Reddel R.R. SV40-induced immortalization of human cells. Crit Rev Oncog. 1994;5(4):331–357. doi: 10.1615/critrevoncog.v5.i4.10. [DOI] [PubMed] [Google Scholar]
- 4.Lustig A.J. Crisis intervention: the role of telomerase. Proc Natl Acad Sci U S A. 1999;96(7):3339–3341. doi: 10.1073/pnas.96.7.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macera-Bloch L., Houghton J., Lenahan M., Jha K.K., Ozer H.L. Termination of lifespan of SV40-transformed human fibroblasts in crisis is due to apoptosis. J Cell Physiol. 2002;190(3):332–344. doi: 10.1002/jcp.10062. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg A.S., Randell S.H., Stewart S.A. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene. 2002;21(29):4577–4586. doi: 10.1038/sj.onc.1205550. [DOI] [PubMed] [Google Scholar]
- 7.Davies B.R., Steele I.A., Edmondson R.J. Immortalisation of human ovarian surface epithelium with telomerase and temperature-sensitive SV40 large T antigen. Exp Cell Res. 2003;288(2):390–402. doi: 10.1016/s0014-4827(03)00218-0. [DOI] [PubMed] [Google Scholar]
- 8.Kudo Y., Hiraoka M., Kitagawa S. Establishment of human cementifying fibroma cell lines by transfection with temperature-sensitive simian virus-40 T-antigen gene and hTERT gene. Bone. 2002;30(5):712–717. doi: 10.1016/s8756-3282(02)00689-0. [DOI] [PubMed] [Google Scholar]
- 9.Feng J., Funk W.D., Wang S.S. The RNA component of human telomerase. Science. 1995;269(5228):1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 10.Harrington L., Zhou W., McPhail T. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11(23):3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilian A., Bowtell D.D., Abud H.E. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6(12):20. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 12.Lingner J., Hughes T.R., Shevchenko A., Mann M., Lundblad V., Cech T.R. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276(5312):561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 13.Meyerson M., Counter C.M., Eaton E.N. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90(4):785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 14.Yi X., Tesmer V.M., Savre-Train I., Shay J.W., Wright W.E. Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol Cell Biol. 1999;19(6):3989–3997. doi: 10.1128/mcb.19.6.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai C.C., Chen C.L., Liu H.C. Overexpression of hTERT increases stem-like properties and decreases spontaneous differentiation in human mesenchymal stem cell lines. J Biomed Sci. 2010;17:64. doi: 10.1186/1423-0127-17-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodnar A.G., Ouellette M., Frolkis M. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 17.Ouellette M.M., Aisner D.L., Savre-Train I., Wright W.E., Shay J.W. Telomerase activity does not always imply telomere maintenance. Biochem Biophys Res Commun. 1999 Jan 27;254(3):795–803. doi: 10.1006/bbrc.1998.0114. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez R.D., Morales C.P., Herbert B.S. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 2001;15(4):398–403. doi: 10.1101/gad.859201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Chang E., Cherry A.M. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274(37):26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 20.Soloff M.S., Jeng Y.J., Ilies M. Immortalization and characterization of human myometrial cells from term-pregnant patients using a telomerase expression vector. Mol Hum Reprod. 2004;10(9):685–695. doi: 10.1093/molehr/gah086. [DOI] [PubMed] [Google Scholar]
- 21.Woodworth C.D., Kreider J.W., Mengel L., Miller T., Meng Y.L., Isom H.C. Tumorigenicity of simian virus 40-hepatocyte cell lines: effect of in vitro and in vivo passage on expression of liver-specific genes and oncogenes. Mol Cell Biol. 1988;8(10):4492–4501. doi: 10.1128/mcb.8.10.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada H., Nakagawa H., Oyama K. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res. 2003;1(10):729–738. [PubMed] [Google Scholar]
- 23.Seglen P.O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- 24.Selden C., Spearman C.W., Kahn D. Evaluation of encapsulated liver cell spheroids in a fluidised-bed bioartificial liver for treatment of ischaemic acute liver failure in pigs in a translational setting. PLoS One. 2013;8(12):e82312. doi: 10.1371/journal.pone.0082312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuzil D.F., Rozga J., Moscioni A.D. Use of a novel bioartificial liver in a patient with acute liver insufficiency. Surgery. 1993;113(3):340–343. [PubMed] [Google Scholar]
- 26.Rozga J., Podesta L., LePage E. A bioartificial liver to treat severe acute liver failure. Ann Surg. 1994;219(5):538–544. doi: 10.1097/00000658-199405000-00012. discussion 44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillouzo A., Rialland L., Fautrel A., Guyomard C. Survival and function of isolated hepatocytes after cryopreservation. Chem Biol Interact. 1999;121(1):7–16. doi: 10.1016/s0009-2797(99)00087-3. [DOI] [PubMed] [Google Scholar]
- 28.Alexandre E., Viollon-Abadie C., David P. Cryopreservation of adult human hepatocytes obtained from resected liver biopsies. Cryobiology. 2002;44(2):103–113. doi: 10.1016/s0011-2240(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd T.D., Orr S., Skett P., Berry D.P., Dennison A.R. Cryopreservation of hepatocytes: a review of current methods for banking. Cell Tissue Bank. 2003;4(1):3–15. doi: 10.1023/A:1026392216017. [DOI] [PubMed] [Google Scholar]
- 30.Sussman N.L., Gislason G.T., Kelly J.H. Extracorporeal liver support. Application to fulminant hepatic failure. J Clin Gastroenterol. 1994;18(4):320–324. [PubMed] [Google Scholar]
- 31.Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci;115(Pt 13):2679–2688. [DOI] [PubMed]
- 32.Ellis A.J., Hughes R.D., Wendon J.A. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24(6):1446–1451. doi: 10.1002/hep.510240625. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe F.D., Mullon C.J., Hewitt W.R. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann Surg. 1997;225(5):484–491. doi: 10.1097/00000658-199705000-00005. discussion 91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patience C., Takeuchi Y., Weiss R.A. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3(3):282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 35.Nyberg S.L., Remmel R.P., Mann H.J., Peshwa M.V., Hu W.S., Cerra F.B. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg. 1994;220(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- 36.Nyberg S.L., Mann H.J., Hu M.Y. Extrahepatic metabolism of 4-methylumbelliferone and lidocaine in the anhepatic rabbit. Drug Metab Dispos. 1996;24(6):643–648. [PubMed] [Google Scholar]
- 37.Frühauf J.H., Mertsching H., Giri S., Frühauf N.R., Bader A. Porcine endogenous retrovirus released by a bioartificial liver infects primary human cells. Liver Int. 2009 Nov;29(10):1553–1561. doi: 10.1111/j.1478-3231.2009.02087.x. [DOI] [PubMed] [Google Scholar]
- 38.Oertel M. Fetal liver cell transplantation as a potential alternative to whole liver transplantation? J Gastroenterol. 2011;46:953–965. doi: 10.1007/s00535-011-0427-5. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Lee A.S., Tang C., Rao M.S., Weissman I.L., Wu J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19(8):998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suva M.L., Riggi N., Bernstein B.E. Epigenetic reprogramming in cancer. Science. 2013;339(6127):1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou P., Li Y., Zhang X. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz R.E., Fleming H.E., Khetani S.R., Bhatia S.N. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv. 2014;32(2):504–513. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


