Abstract
Mesenteric vein thrombosis is increasingly recognized as a cause of mesenteric ischemia. Acute thrombosis commonly presents with abdominal pain and chronic type with features of portal hypertension. Contrast enhanced CT scan of abdomen is quite accurate for diagnosing and differentiating two types of mesenteric venous thrombosis. Prothrombotic state, hematological malignancy, and local abdominal inflammatory conditions are common predisposing conditions. Over the last decade, JAK-2 (janus kinase 2) mutation has emerged as an accurate biomarker for diagnosis of myeloproliferative neoplasm, an important cause for mesenteric venous thrombosis. Anticoagulation is the treatment of choice for acute mesenteric venous thrombosis. Thrombolysis using systemic or transcatheter route is another option. Patients with peritoneal signs or refractory to initial measures require surgical exploration. Increasing recognition of mesenteric venous thrombosis and use of anticoagulation for treatment has resulted in reduction in the need for surgery with improvement in survival.
Keywords: mesenteric venous thrombosis, portal hypertension, thrombosis
Abbreviations: MVT, mesenteric venous thrombosis; TIPS, transjugular intrahepatic portosystemic shunt; PVT, portal vein thrombosis; JAK2, janus kinase 2
Mesenteric venous thrombosis (MVT) is an uncommon cause of mesenteric ischemia accounting for 5–15% of the cases.1 It was first described as a distinct cause of mesenteric ischemia by Warren and Eberhard. It can be either acute presenting commonly with abdominal pain or chronic presenting with features of portal hypertension. Rarely, it can be diagnosed as an incidental finding on abdominal CT scan. Causes of MVT include prothrombotic states, trauma and intra-abdominal infections. Advances in the radiology techniques and anticoagulation have led to improved diagnosis and outcomes.2
Mesenteric circulation
Blood flow to the intestines starts from the superior mesenteric artery which arises from the abdominal aorta. It provides branches to the pancreas and duodenum; three branches to the proximal colon, and terminates in the arteriae rectae supplying the jejunum and ilium. Venous blood first drains into the venae rectae which then forms the ileocolic, middle colic and right colic veins which come together to form the superior mesenteric vein that in turn meets the splenic vein to form the portal vein. The lower part of the esophagus and the upper part of the lesser curvature drains through the left gastric vein directly into the portal vein at the point of its formation while blood from the fundus of the stomach drains through short veins into the splenic vein. The left colon venous return comes through the inferior mesenteric vein which joins the splenic vein.
Acute mesenteric venous thrombosis
Incidence and Etiology
The incidence of MVT has increased with improvement in the diagnostic modalities resulting in increasing number of cases diagnosed incidentally. Incidence between 1970 and 1982 was estimated to be 2 per 100,000 compared to 2.7 per 100,000 between 2000 and 2006.3 MVT accounts for 6%–9% of all the cases of acute mesenteric ischemia and 1/1000 of emergency department admissions.4 The mean age of patients at presentation is 45–60 years with a slight male to female predominance.
Prothrombotic states, surgery, inflammatory bowel disease and malignancy are common risk factors for the development of MVT. Thrombosis of the larger distal portions of the mesenteric vein is mostly secondary to local factors, such as malignancy, pancreatitis and infection, and is associated with portal vein thrombosis while thrombosis that originate from the vena rectae, leading to isolated MVT thrombosis is most commonly related to a prothrombotic state.
Primary or idiopathic MVT account for 21–49% of the cases and depends on the depth of evaluation.2,5–7 The proportion of patients with idiopathic cases decreases with more extensive evaluation.8 Malignancies including myeloproliferative neoplasms are the most common form of prothrombotic disorders associated with MVT.4 JAK2 V617 mutation was previously studied and association between the presence of the mutation and MVT was found, 17 patients out of 99 with MVT were found to have the mutation and none of them had other prothrombotic risks, 7 of them had myeloproliferative disease; and of the remaining 10 patients, two were diagnosed with myelofibrosis, and one was diagnosed with polycythemia vera on the follow up after the study.9 Oral contraceptive use accounts for 9–18% of cases in young women.10,11 In a retrospective analysis, 4 out of 12 patients with no identifiable hypercoagulable state were using oral contraceptives.12 Antithrombin III, Protein S and Protein C deficiencies are associated with MVT. It is estimated that 4–10% of these patients will develop this disorder.13 In another study on patients with MVT compared to healthy people, a significantly higher thrombophilic genotype was found. Methylene tetrahydrofolate reductase TT677 genotype was present in 6 (50%), the factor V Leiden in 3 (25%), and the prothrombin transition G20210A in 3 (25%). Combined mutations were present in 4 (33%) patients. Intra-abdominal surgery, especially splenectomy is a common risk factor.4,6 MVT and portal vein thrombosis are more common in patients with cirrhosis, especially with advanced disease. Prevalence of portal vein thrombosis in patients listed for liver transplantation has been reported to be as high as 15%. This can be in part related to the disturbed flow through the portal vein and a procoagulant imbalance related to the cirrhosis.14 Hyperhomocysteinaemia along with the methylene tetrahydrofolate reductase C677T mutation causes general predisposition to both arterial and venous thrombosis; its role in MVT was investigated in a case control study previously and association with MVT was found.15
The size and extent of venous thrombosis largely affect the outcome, clinical presentation and probability of bowel infarction. Infarction of the bowel mostly requires involvement of the venous arcades and vasa recta which in turn causes complete venous occlusion.3,8,16 Arterial vasospasm and thrombosis can also be a major factor leading to propagation of the ischemia and bowel infarction.17
Clinical Presentation
The most common presenting symptom in acute MVT is abdominal pain which occurs in 91–100% of the cases, nausea, vomiting and melena are also common symptoms.16 The duration of symptoms is more than 1.5–2 days in more than 75% of the cases.3,16,18 Usually the abdominal pain is out of proportion to the physical exam findings.3,8 Although melena, hematemesis or hematochezia occur in only about 15% of the cases, occult blood is present in 50% of the cases.8,17 Fever and peritoneal signs are suggestive of progression of the infarction, and hypotension with systolic blood pressure of less than 90 mmHg along with ascites formation are associated with poor prognosis.17
Diagnosis
Although, there are subtle differences between arterial and venous mesenteric ischemia, it is possible to clinically differentiate the two conditions (Table 1). Patients with history of atrial fibrillation or heart disease are more likely to have arterial ischemia while personal or family history of deep venous thrombosis increases the suspicion of MVT. Physical findings and severity of ischemia do not correlate well. Rebound tenderness has not been shown to be an accurate sign for diagnosis of bowel infarction and also did not correlate with the severity of the ischemia.5,19,20 Laboratory testing is usually not helpful in the diagnosis of MVT. Serum lactate level often does not correlate with intestinal infarction initially; and at the time lactic acidosis is present it is late in the course of illness and, at that point, mortality is already 75%. Leukocytosis and hemoconcentration are common findings in MVT.4 Plain films are often negative and mainly used to rule out other causes of abdominal pain; findings suggesting bowel ischemia caused by MVT include thumbprinting which represents semi opaque indentations in the bowel lumen secondary to edema, air in the intestines (Pneumatosis Intestinalis), air in the portal vein and free peritoneal air can be seen but usually are late findings.
Table 1.
Comparison of Acute Mesenteric Venous Thrombosis and Acute Arterial Mesenteric Ischemia.
| Risk factor | Acute intra-abdominal process | Atrial fibrillation |
| Thrombophilia | Cardiomyopathy and CHF | |
| Malignancy | Valvular heart disease | |
| Cirrhosis | ||
| History of DVT | Absent | Present in 20–40% |
| CT | Diagnostic in 40–50% only | In more than 90% |
| Angiogram | Diagnostic in most | In 50–60% only |
| Acute presentation | Most of the time | Can be subacute or chronic |
| Bowel infarction | Likely if not relieved in 12 h | Not usual if diagnosed and AC started |
| Treatment | Embolectomy or IA papaverine | Anticoagulation, possible thrombolysis, systemic or directed |
| Need for surgery | Frequent | Less likely if AC started early |
| Ischemic to normal transition | Abrupt | Gradual |
| Mortality | More common | Less common with CT and AC availability |
| Chronicity | Rare | Can occur with portal hypertension and varices |
CHF: Congestive Heart Failure; AC: Anticoagulation; DVT: Deep Venous Thrombosis.
Contrast enhanced CT scan is the diagnostic modality of choice. Increasing use of CT scan for abdominal pain in the emergency department is associated with decrease in the time to diagnosis from 1 week to 1 day.2 A filling defect in the mesenteric vein is the most common finding in patients with MVT. Bowel wall thickening, pneumatosis intestinalis, portal vein gas and persistent enhancement of the bowel wall suggest bowel wall ischemia.11,21,22 Although, these findings are specific, but their sensitivity is low in diagnosis of bowel infarction and transmural necrosis.23 Doppler ultrasound can be used bedside, and may demonstrate the thrombus and although it is specific, it is operator dependent and not as sensitive as CT and MRI. Isolated MVT with no portal vein thrombosis is more difficult diagnose on CT with only 67% sensitivity.16 Nuclear scintiangiography is diagnostic in 75% of the cases but is not widely performed. Angiography is an invasive diagnostic and therapeutic measure that is reserved for cases with high pretest probability and a non-diagnostic CT/MRI, or cases where invasive therapeutic measures are planned.24
Once MVT is confirmed, work up to diagnose the underlying etiology is indicated. Care should be given to draw blood samples before initiation of anticoagulation especially for diagnosis of prothrombotic states. Testing for JAK2 mutation is important for diagnosis of myeloproliferative neoplasms including polycythemia Vera, essential thrombocythemia and myelofibrosis. Testing for JAK2 mutation has replaced bone marrow biopsy as the initial screening method for diagnosis of myeloproliferative neoplasms.25 Hyperhomocysteinemia, Protein C or S deficiency, prothrombin gene mutation, and antithrombin III deficiency are other disorders to consider.
Treatment
Goals of treatment are recanalization, prevention of propagation of the thrombosis which can lead to worsening of intestinal ischemia and on the long term, to prevent recurrences.
Supportive Treatment
Pain control, fluid and electrolyte replacement and bowel rest should be initiated with the acute presentation. Nasogastric intubation can be initiated on presentation as needed in cases of ileus, abdominal distension and intractable nausea and vomiting. Supportive treatment also includes blood transfusion for patients presenting with bleeding. The use of antibiotics was not associated with improvement in mortality or shorter hospital stay though if the patient has perforation, sepsis secondary to bacterial translocation or septic thrombophlebitis appropriate antibiotics should be initiated.24
Anticoagulation
Anticoagulation with unfractionated or low molecular weight heparin should be initiated as soon as the diagnosis is made even intraoperatively or in the presence of bleeding as it has shown to significantly improve survival.4,8,10,26 Anticoagulation resulted in recanalization in most of the patients in one study (more than 80%) if started in the early stages and that complete recanalization was associated with less extensive disease.12 In another study, recanalization rates on anticoagulation were lower at about 30% in the portal vein and up to 61% in the superior mesenteric vein thrombosis.27 After the acute phase, and excluding the need of an immediate surgical intervention, anticoagulation should be maintained to prevent recurrence of thrombosis.10 Duration of 3–6 months for patients with reversible causes such as trauma, infection or pancreatitis is recommended with case by case evaluation of risks and benefits. Lifelong anticoagulation should be considered in cases with persistent hypercoagulable state, irreversible systemic condition or idiopathic cases. Maintenance of anticoagulation can be achieved by warfarin targeting an international normalized ratio of 2–3, though that requires frequent monitoring and attention should be given to drug–drug interactions that are related to the P450 pathway.28–30 Another option is the use of fixed dose of newer direct thrombin and factor Xa inhibitors. Advantages include less interactions and lack of need for frequent monitoring.
Bleeding from anticoagulation is still a consideration on the long term although the risk is low (10%). The most common site of bleeding is gastrointestinal,16,31 and for patients who develop varices, prevention of bleeding with beta blockers is preferred as ligation might cause ulceration and bleeding.
Interventional Radiological Options
Patients with persisting symptoms, worsening abdominal pain 48–72 h after initiation of anticoagulation, or development of signs of peritonitis and who are poor surgical candidates may be considered for interventional radiological options (Figure 1). Most published data on interventional radiological treatments for MVT are from case reports and small case series. Although, systemic intravenous tPA was successfully used, many recent studies implement transcatheter routes.32,33 In one retrospective study, 20 patients of acute MVT received interventional radiological treatment via femoral artery to access mesenteric artery, or femoral vein to access the mesenteric vein or transjugular vein or a combined approach with a good treatment response, but, at a higher risk for complications, mainly bleeding, which often required blood transfusion.34 Similarly, in another study intravascular thrombolytic therapy was used in 12 patients with complete response in 3, partial response in4, and major procedure related bleeding in 6 patients with fatal outcome in two patients.35 Another study reported its use on 11 patients (6 post-surgical) and a mean of 10 days after the onset of MVT. In patients with high risk of bleeding, especially patients who are in the immediate post-surgical period and those with malignancies, mechanical thrombectomy was done in an attempt to decrease the duration of thrombolysis. This study among others along with case reports suggested good outcomes using the transcatheter thrombolysis.36–38
Figure 1.
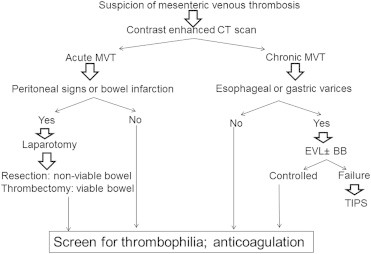
Algorithm for management of acute and chronic mesenteric venous thrombosis. MVT: Mesenteric venous thrombosis; EVL: Endoscopic variceal ligation; BB: Beta blockers; TIPS: Transjugular intrahepatic portosystemic shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) can be used for MVT with a rationale of creating low pressure system which works as a vacuum of clot fragments and improve the effectiveness of thrombolysis in the case of acute thrombosis. The use of TIPS was associated with immediate symptomatic improvement and successful recanalization in 83% of patients treated.39 Two cases, one with extensive portal, mesenteric and splenic vein thrombosis and another with mesenteric and portal vein thrombosis have been reported to have favorable outcomes with TIPS.40 A retrospective study on 20 patients with portal vein thrombosis (PVT) suggested a high success rate in cases of non-cavernous PVT and it was effective in prevention of variceal bleeding and recurrent ascites.41 Another prospective analysis of 9 patients with PVT reported a complication rate of up to 22% with 11% mortality.42 Pulmonary embolism is a concern; however, no clinically significant pulmonary embolisms were witnessed in one center experience.40
Surgery
Patients with persisting or worsening symptoms and those with development of frank perforation or signs of peritonitis may require surgical intervention. Surgical resection of necrotic bowel and anastomosis is the standard procedure. Heparin should be given intraoperatively, and as arterial vasospasm is an important mechanism of ischemia, giving papaverin during the operation should be considered.17 Infusion of tPA through operatively placed catheters was also reported and could, along with heparinization and papaverin, prevent the extension of the infarction.43 Two methods helped to limit the extent of initial resection and the short bowel syndrome as a complication of a wide resection; one was the second look approach,18 the other was the use of Doppler method and fluorescein to estimate bowel viability. In a previous prospective study, fluorescein was the best method of determination of viability followed by clinical judgment which was better than Doppler.44
Outcomes
In general, outcomes in mesenteric venous thrombosis are better when compared to arterial thrombosis with mortality of 44% as compared to 66–89% respectively.45 Improvement in the diagnostic modalities and therapy has led to improved outcomes and recent reports suggest mortality rates of 10–20% (Table 2). Underlying cause of thrombosis, short bowel syndrome secondary to surgery and recurrence of thrombosis are three main factors affecting outcomes. Recurrence occurs most commonly in the first 30 days after presentation. Rates of 0–25% reported which can be decreased to 0–3% in patients who continue on anticoagulation.46,47 Bleeding from anticoagulation is uncommon, occurs in 10% of the cases and the most common site is gastrointestinal. Unless the bleeding is intracranial, anticoagulation rarely causes death.
Table 2.
Treatment and Outcome of Patients with Mesenteric Venous Thrombosis: Data from Reported Observational Studies.
| Author (year) | N | FU (years.) | Treatment (%) | Survival (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Surgery | Infarction | Anticoagulation | Bleed | Recurrenced | Short-term | Overall | |||
| Abdu (1986) | 372 | NA | NA | NA | NA | NA | NA | NA | NA |
| Harward (1989) | 16 | 2.75 | 31 | 25 | 100 | NA | NA | 81 | NA |
| Rhee (1994) | 72 | 1.6 | 64 | NA | 62 | NA | 36 (25) | ||
| Condat (2000)a | 33 | 2.5 | 6 | 12 | 93 | 6 | 13 (0) | 100 | 97 |
| Morasch (2001) | 31 | 4.75 | 32 | NA | 72 | NA | NA | 77 | 68 |
| Brunaud (2001)b | 26 | 4.25 | 50 | NA | 50 | NA | NA | 81 | 77 |
| Kumar (2003)a | 69 | NA | 44 | NA | 91 | 1 | 6 | NA | NA |
| Zhang (2004)b | 41 | 2.75 | NA | NA | NA | NA | 3 (0) | 78 | 73 |
| Grisham (2005) | 23 | NA | 17 | NA | 100 | NA | NA | NA | 93 |
| Amarapurkar (2007) | 28 | 4.2 | 36 | 29 | 75 | 13 | 22 (22) | 84 | 81 |
| Amitrano (2007)c | 121 | 3.5 | 26 | 28 | NA | 15 | 11 | 95 | 90 |
| Acosta (2008) | 51 | NA | 25 | NA | NA | NA | NA | 80 | 67 |
| Abu-Daff (2009) | 31 | 5 | 77 | NA | NA | NA | NA | 87 | 68 |
| Alvi (2009) | 20 | 23 | 40 | NA | 85 | NA | NA | 80 | NA |
| Dentali (2009) | 77 | 3 | NA | NA | 46 | NA | 6 | 100 | 94 |
| Cendese (2009) | 9 | 2.25 | 55 | 5 | 100 | NA | NA | 100 | 89 |
| Thatipelli (2010) | 76 | 2.25 | NA | NA | 54 | 18 | 40 | NA | 75 |
| Plessier (2010)a | 55 | 1 | NA | 2 | 95 | 8 | NA | 100 | 98 |
Portal and mesenteric thrombosis included.
Mesenteric venous thrombosis with symptoms of <4 weeks duration.
Splanchnic venous thrombosis without cancer or cirrhosis.
Figures in parentheses represent proportion of recurrent patients with recurrence in the mesenteric vein.
Chronic mesenteric venous thrombosis
Chronic MVT is a rare disorder that accounts for 20–40% of the cases of MVT.16,46 Presentation is usually different from acute MVT as patients present with vague abdominal symptoms or features of portal hypertension and variceal hemorrhage if the thrombosis extends to involve the portal vein.
Clinical Features
Extensive involvement of the superior mesenteric vein, portal vein and splenic vein often cause persistent abdominal pain related to significant bowel edema, sometimes also leading to malabsorption. A proportion of these patients are on long term total parenteral nutrition. Some patients develop portal hypertensive cholangiopathy mimicking primary sclerosing cholangitis. This is caused by edema and the presence of collateral vessels around the bile ducts leading to obstruction and cholangitis. MRCP is diagnostic though ERCP and treatment holds high risk of bleeding given the risk of injuring one of the collateral blood vessels.
Management
Commonly, the disease is diagnosed as an incidental finding on CT scan of the abdomen. CT scan is diagnostic with sensitivity of >90% though estimates on Doppler ultrasound and MRI is lacking. Differentiation from acute MVT is made based on presence of collaterals and cavernoma in patients with chronic disease. Treatment is focused on anticoagulation and prevention of bleeding.48 Patients with isolated chronic MVT are treated with anticoagulation. Patients with concomitant PVT are at risk for development of portal hypertension and variceal bleeding. This is treated as any patient with esophageal varices including primary prophylaxis, control of active bleeding, and prevention of recurrent bleeding (Figure 1). Patients with cholangiopathy may be complicated with strictures and/or biliary stones. ERCP for diagnosis or for therapeutic purposes including sphincterotomy or endoscopic stenting should be carefully done for risk of bleeding secondary to venous collaterals around the bile ducts. Some patients may require surgical hepaticojejunostomy for management of biliary complications.49
Chronic MVT in general has a favorable prognosis with 1–5 year survival ranging from 78 to 83%. The main cause of death in this population was variceal hemorrhage, underlying malignance, sepsis, decompensated liver disease and then ischemic bowel.
Summary
MVT is an uncommon, yet important cause of intestinal ischemia. Causes include local factors and prothrombotic states. The most common presentation is abdominal pain, and, with enough suspicion, CT of the abdomen is diagnostic. Treatment is mainly medical using anticoagulation as soon as the diagnosis is made even in the presence of bleeding or the need for surgical intervention. The duration of treatment is based on the presence of identifiable reversible risk factor in which case anticoagulation can be given for 3–6 months. Otherwise, indefinite anticoagulation should be considered. Surgery is required in cases of transmural infarction and with the presence of peritoneal signs. Outcomes are mainly determined by underlying prothrombotic state, recurrence of the thrombosis and developing short bowel syndrome.
Conflicts of interest
All authors have none to declare.
References
- 1.Grendell J.H., Ockner R.K. Mesenteric venous thrombosis. Gastroenterology. 1982;82:358–372. [PubMed] [Google Scholar]
- 2.Zhang J., Duan Z.Q., Song Q.B., Luo Y.W., Xin S.J., Zhang Q. Acute mesenteric venous thrombosis: a better outcome achieved through improved imaging techniques and a changed policy of clinical management. Eur J Vasc Endovasc Surg. 2004;28:329–334. doi: 10.1016/j.ejvs.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Harnik I.G., Brandt L.J. Mesenteric venous thrombosis. Vasc Med. 2010;15:407–418. doi: 10.1177/1358863X10379673. [DOI] [PubMed] [Google Scholar]
- 4.Singal A.K., Kamath P.S., Tefferi A. Mesenteric venous thrombosis. Mayo Clin Proc Mayo Clin. 2013;88:285–294. doi: 10.1016/j.mayocp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Brunaud L., Antunes L., Collinet-Adler S. Acute mesenteric venous thrombosis: case for nonoperative management. J Vasc Surg. 2001;34:673–679. doi: 10.1067/mva.2001.117331. [DOI] [PubMed] [Google Scholar]
- 6.Morasch M.D., Ebaugh J.L., Chiou A.C., Matsumura J.S., Pearce W.H., Yao J.S. Mesenteric venous thrombosis: a changing clinical entity. J Vasc Surg. 2001;34:680–684. doi: 10.1067/mva.2001.116965. [DOI] [PubMed] [Google Scholar]
- 7.Acosta S., Alhadad A., Svensson P., Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg. 2008;95:1245–1251. doi: 10.1002/bjs.6319. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S., Sarr M.G., Kamath P.S. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683–1688. doi: 10.1056/NEJMra010076. [DOI] [PubMed] [Google Scholar]
- 9.Colaizzo D., Amitrano L., Tiscia G.L. The JAK2 V617F mutation frequently occurs in patients with portal and mesenteric venous thrombosis. J Thromb Haemost. 2007;5:55–61. doi: 10.1111/j.1538-7836.2006.02277.x. [DOI] [PubMed] [Google Scholar]
- 10.Abdu R.A., Zakhour B.J., Dallis D.J. Mesenteric venous thrombosis–1911 to 1984. Surgery. 1987;101:383–388. [PubMed] [Google Scholar]
- 11.Harward T.R., Green D., Bergan J.J., Rizzo R.J., Yao J.S. Mesenteric venous thrombosis. J Vasc Surg. 1989;9:328–333. [PubMed] [Google Scholar]
- 12.Condat B., Pessione F., Helene Denninger M., Hillaire S., Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000;32:466–470. doi: 10.1053/jhep.2000.16597. [DOI] [PubMed] [Google Scholar]
- 13.Pabinger I., Schneider B. Thrombotic risk in hereditary antithrombin III, protein C, or protein S deficiency. A cooperative, retrospective study. Gesellschaft fur Thrombose- und Hamostaseforschung (GTH) study group on natural inhibitors. Arterioscler Thromb Vasc Biol. 1996;16:742–748. doi: 10.1161/01.atv.16.6.742. [DOI] [PubMed] [Google Scholar]
- 14.Tripodi A., Mannucci P.M. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 15.He J.A., Hu X.H., Fan Y.Y. Hyperhomocysteinaemia, low folate concentrations and methylene tetrahydrofolate reductase C677T mutation in acute mesenteric venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39:508–513. doi: 10.1016/j.ejvs.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S., Kamath P.S. Acute superior mesenteric venous thrombosis: one disease or two? Am J gastroenterology. 2003;98:1299–1304. doi: 10.1111/j.1572-0241.2003.07338.x. [DOI] [PubMed] [Google Scholar]
- 17.Boley S.J., Kaleya R.N., Brandt L.J. Mesenteric venous thrombosis. Surg Clin North Am. 1992;72:183–201. doi: 10.1016/s0039-6109(16)45634-3. [DOI] [PubMed] [Google Scholar]
- 18.Rhee R.Y., Gloviczki P., Mendonca C.T. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg. 1994;20:688–697. doi: 10.1016/s0741-5214(94)70155-5. [DOI] [PubMed] [Google Scholar]
- 19.Liddington M.I., Thomson W.H. Rebound tenderness test. Br J Surg. 1991;78:795–796. doi: 10.1002/bjs.1800780710. [DOI] [PubMed] [Google Scholar]
- 20.Prout W.G. The significance of rebound tenderness in the acute abdomen. Br J Surg. 1970;57:508–510. doi: 10.1002/bjs.1800570706. [DOI] [PubMed] [Google Scholar]
- 21.Gehl H.B., Bohndorf K., Klose K.C., Günther R.W. Two-dimensional MR angiography in the evaluation of abdominal veins with gradient refocused sequences. J Comput Assist Tomogr. 1990;14:619–624. doi: 10.1097/00004728-199007000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Haddad M.C., Clark D.C., Sharif H.S., al Shahed M., Aideyan O., Sammak B.M. MR, CT, and ultrasonography of splanchnic venous thrombosis. Gastrointest Radiol. 1992;17:34–40. doi: 10.1007/BF01888505. [DOI] [PubMed] [Google Scholar]
- 23.Milone M., Di Minno M.N., Musella M. Computed tomography findings of pneumatosis and portomesenteric venous gas in acute bowel ischemia. World J Gastroenterol: WJG. 2013;19:6579–6584. doi: 10.3748/wjg.v19.i39.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grisham A., Lohr J., Guenther J.M., Engel A.M. Deciphering mesenteric venous thrombosis: imaging and treatment. Vasc Endovascular Surg. 2005;39:473–479. doi: 10.1177/153857440503900603. [DOI] [PubMed] [Google Scholar]
- 25.Primignani M., Barosi G., Bergamaschi G. Role of the JAK2 mutation in the diagnosis of chronic myeloproliferative disorders in splanchnic vein thrombosis. Hepatology. 2006;44:1528–1534. doi: 10.1002/hep.21435. [DOI] [PubMed] [Google Scholar]
- 26.Naitove A., Weismann R.E. Primary mesenteric venous thrombosis. Ann Surg. 1965;161:516–523. doi: 10.1097/00000658-196504000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plessier A., Darwish-Murad S., Hernandez-Guerra M. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51:210–218. doi: 10.1002/hep.23259. [DOI] [PubMed] [Google Scholar]
- 28.Clark N.P., Delate T., Riggs C.S. Warfarin interactions with antibiotics in the Ambulatory Care Setting. JAMA Intern Med. 2014;174:409–416. doi: 10.1001/jamainternmed.2013.13957. [DOI] [PubMed] [Google Scholar]
- 29.Daly A.K. Optimal dosing of warfarin and other coumarin anticoagulants: the role of genetic polymorphisms. Arch Toxicol. 2013;87:407–420. doi: 10.1007/s00204-013-1013-9. [DOI] [PubMed] [Google Scholar]
- 30.Garcia D.A. Patients with stable, therapeutic INR values should remain on warfarin. J Thromb Thrombolysis. 2013;35:336–338. doi: 10.1007/s11239-013-0867-2. [DOI] [PubMed] [Google Scholar]
- 31.Thatipelli M.R., McBane R.D., Hodge D.O., Wysokinski W.E. Survival and recurrence in patients with splanchnic vein thromboses. Clin Gastroenterol Hepatol. 2010;8:200–205. doi: 10.1016/j.cgh.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 32.al Karawi M.A., Quaiz M., Clark D., Hilali A., Mohamed A.E., Jawdat M. Mesenteric vein thrombosis, non-invasive diagnosis and follow-up (US + MRI), and non-invasive therapy by streptokinase and anticoagulants. Hepatogastroenterology. 1990;37:507–509. [PubMed] [Google Scholar]
- 33.Robin P., Gruel Y., Lang M., Lagarrigue F., Scotto J.M. Complete thrombolysis of mesenteric vein occlusion with recombinant tissue-type plasminogen activator. Lancet. 1988;1:1391. doi: 10.1016/s0140-6736(88)92198-8. [DOI] [PubMed] [Google Scholar]
- 34.Hollingshead M., Burke C.T., Mauro M.A., Weeks S.M., Dixon R.G., Jaques P.F. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:651–661. doi: 10.1097/01.RVI.0000156265.79960.86. [DOI] [PubMed] [Google Scholar]
- 35.Smalberg J.H., Spaander M.V., Jie K.S. Risks and benefits of transcatheter thrombolytic therapy in patients with splanchnic venous thrombosis. Thromb Haemost. 2008;100:1084–1088. [PubMed] [Google Scholar]
- 36.Kim H.S., Patra A., Khan J., Arepally A., Streiff M.B. Transhepatic catheter-directed thrombectomy and thrombolysis of acute superior mesenteric venous thrombosis. J Vasc Interv Radiol. 2005;16:1685–1691. doi: 10.1097/01.RVI.0000182156.71059.B7. [DOI] [PubMed] [Google Scholar]
- 37.Yankes J.R., Uglietta J.P., Grant J., Braun S.D. Percutaneous transhepatic recanalization and thrombolysis of the superior mesenteric vein. AJR Am J Roentgenol. 1988;151:289–290. doi: 10.2214/ajr.151.2.289. [DOI] [PubMed] [Google Scholar]
- 38.Wang M.Q., Liu F.Y., Duan F., Wang Z.J., Song P., Fan Q.S. Acute symptomatic mesenteric venous thrombosis: treatment by catheter-directed thrombolysis with transjugular intrahepatic route. Abdom Imaging. 2011;36:390–398. doi: 10.1007/s00261-010-9637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semiz-Oysu A., Keussen I., Cwikiel W. Interventional radiological management of prehepatic obstruction of [corrected] the splanchnic venous system. Cardiovasc Intervent Radiol. 2007;30:688–695. doi: 10.1007/s00270-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 40.Sehgal M., Haskal Z.J. Use of transjugular intrahepatic portosystemic shunts during lytic therapy of extensive portal splenic and mesenteric venous thrombosis: long-term follow-up. J Vasc Interv Radiol. 2000;11:61–65. doi: 10.1016/s1051-0443(07)61283-4. [DOI] [PubMed] [Google Scholar]
- 41.Walser E.M., NcNees S.W., DeLa Pena O. Portal venous thrombosis: percutaneous therapy and outcome. J Vasc Interv Radiol. 1998;9:119–127. doi: 10.1016/s1051-0443(98)70493-2. [DOI] [PubMed] [Google Scholar]
- 42.Ganger D.R., Klapman J.B., McDonald V. Transjugular intrahepatic portosystemic shunt (TIPS) for Budd-Chiari syndrome or portal vein thrombosis: review of indications and problems. Am J Gastroenterol. 1999;94:603–608. doi: 10.1111/j.1572-0241.1999.00921.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan J.L., Weintraub S.L., Hunt J.P., Gonzalez A., Lopera J., Brazzini A. Treatment of superior mesenteric and portal vein thrombosis with direct thrombolytic infusion via an operatively placed mesenteric catheter. Am Surg. 2004;70:600–604. [PubMed] [Google Scholar]
- 44.Bulkley G.B., Zuidema G.D., Hamilton S.R., O'Mara C.S., Klacsmann P.G., Horn S.D. Intraoperative determination of small intestinal viability following ischemic injury: a prospective, controlled trial of two adjuvant methods (Doppler and fluorescein) compared with standard clinical judgment. Ann Surg. 1981;193:628–637. doi: 10.1097/00000658-198105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoots I.G., Koffeman G.I., Legemate D.A., Levi M., van Gulik T.M. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91:17–27. doi: 10.1002/bjs.4459. [DOI] [PubMed] [Google Scholar]
- 46.Amitrano L., Guardascione M.A., Scaglione M. Prognostic factors in noncirrhotic patients with splanchnic vein thromboses. Am J Gastroenterol. 2007;102:2464–2470. doi: 10.1111/j.1572-0241.2007.01477.x. [DOI] [PubMed] [Google Scholar]
- 47.Dentali F., Ageno W., Witt D. Natural history of mesenteric venous thrombosis in patients treated with vitamin K antagonists: a multi-centre, retrospective cohort study. Thromb Haemost. 2009;102:501–504. doi: 10.1160/TH08-12-0842. [DOI] [PubMed] [Google Scholar]
- 48.Orr D.W., Harrison P.M., Devlin J. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol. 2007;5:80–86. doi: 10.1016/j.cgh.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 49.Diman R.K., Behera A., Chawla Y.K. Portal hypertensive biliopathy. Gut. 2007;56:1001–1008. doi: 10.1136/gut.2006.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]


